In the Bangladesh and India Sundarbans estimation of tiger Panthera tigris home range size is needed to infer habitat quality and thus estimate the number of tigers this mangrove habitat could support. Information on tiger movement is also required to help design monitoring programmes for the species. Radio or global positioning system (GPS) collars are useful for collecting such information but the difficulty of capturing tigers for collaring in the mangrove habitat of the Sundarbans has previously prevented the application of this approach. The objectives of this study were therefore to use GPS collars to acquire preliminary estimates of female tiger home range size and movement in the Bangladesh Sundarbans.
The 6,017 km2 Sundarbans Reserved Forest of Bangladesh comprises densely vegetated islands that are periodically inundated by the tide (Gopal & Chauhan, Reference Gopal and Chauhan2006). The tiger is the only large terrestrial carnivore in the Sundarbans, where its principal prey are chital Axis axis and wild boar Sus scrofa (Reza et al., Reference Reza, Feeroz and Islam2001).
Between 2004 and 2006 two adult female tigers were captured in the south-east of the Sundarbans Reserved Forest using modified leg snares (Goodrich et al., Reference Goodrich, Kerley, Schleyer, Miquelle, Quigley and Smirnov2001) and established animal handling guidelines (Gannon & Sikes, Reference Gannon and Sikes2007). The tigers were immobilized with 6–8 mg kg-1 of Telazol, administered using a projector and dart (Palmer Cap-Chur Inc., Powder Springs, USA). Tiger F1 weighed 75 kg and was estimated to be 12–14 years old (based on discoloration, damage and general wear of teeth). Tiger F2 weighed 80 kg and was estimated to be 10–14 years old (based on teeth condition). There was no evidence to suggest that either female had any dependent offspring. Both tigers were fitted with GPS collars (Advanced Telemetry Systems, Isanti, USA). F1 was tracked for 5.5 months until she died of unknown causes. F2 was tracked for 2.5 months until the GPS collar batteries expired. F2 was then recaptured and released at the capture site after the collar was removed. The GPS locations from the collars were used to construct minimum convex polygon (MCP) and fixed kernel (FK) home ranges using the geographical information system ArcView v. 3.3 (ESRI, Redlands, California) and the ArcView extension Animal Movement v. 1.1 (Hooge & Eichenlaub, Reference Hooge and Eichenlaub1997). The smoothing factor for FK isopleths was determined by least squares cross validation (Seaman et al., Reference Seaman, Millspaugh, Kernohan, Brundige, Raedeke and Gitzen1999). Water bodies that were never crossed by the two tigers, or land that lay across from these water bodies, were discounted from the home range estimates. The high frequency of location acquisition by the GPS collars (one location per 4 hours for F1 and one location per 30 minutes for F2) made it unlikely that the tigers could have crossed these water bodies and returned without recording a location. BIOTAS v. 1.03 (Ecological Software Solutions, Hegymagas, Hungary) was used to construct location-area curves, to determine if and when the 95% home range size had been reached, and to determine mean and maximum distance moved per day. Location data were also used to identify points at which water bodies were crossed and the mean frequency of such crossing.
The GPS collars recorded 679 locations for F1 during April–October 2004 and 1,528 for F2 during March–May 2006. After 4 months of monitoring F1 made a foray to the east of her normal home range, returning after 3 days. She moved to the same area 6 weeks later, and died c. 9 km from her normal home range. Tracks, judged by their size to be that of a new female, were observed in F1’s former home range within days of F1 moving out. Female tracks together with those of a large cub were observed in the same area 1 year later. The poor condition of F1’s teeth, her movement pattern and the appearance of a new female, suggests that F1 may have been unable to defend her territory from a rival. Therefore the forays to the east were discounted from the calculation of home range size, as they were not representative of F1’s normal movement pattern.
Location-area curves indicated that 95% MCP home ranges were acquired after c. 275 and 910 locations (c. 2 months) for F1 and F2 respectively. The mean 95 and 50% MCP home ranges were 12.3 km2 (F1 = 14.1 km2, F2 = 10.6 km2) and 4.23 km2 (F1 = 4.2 km2, F2 = 4.3 km2), respectively. The mean 95 and 50% FK home ranges sizes were 14.2 km2 (F1 = 16.2 km2, F2 = 12.2 km2) and 3.0 km2 (F1 = 3.5, F2 = 2.5 km2) respectively (Fig. 1). A mean female home range size of 14.2 km2 would indicate a density for the south-east Sundarbans of seven adult females per 100 km2.
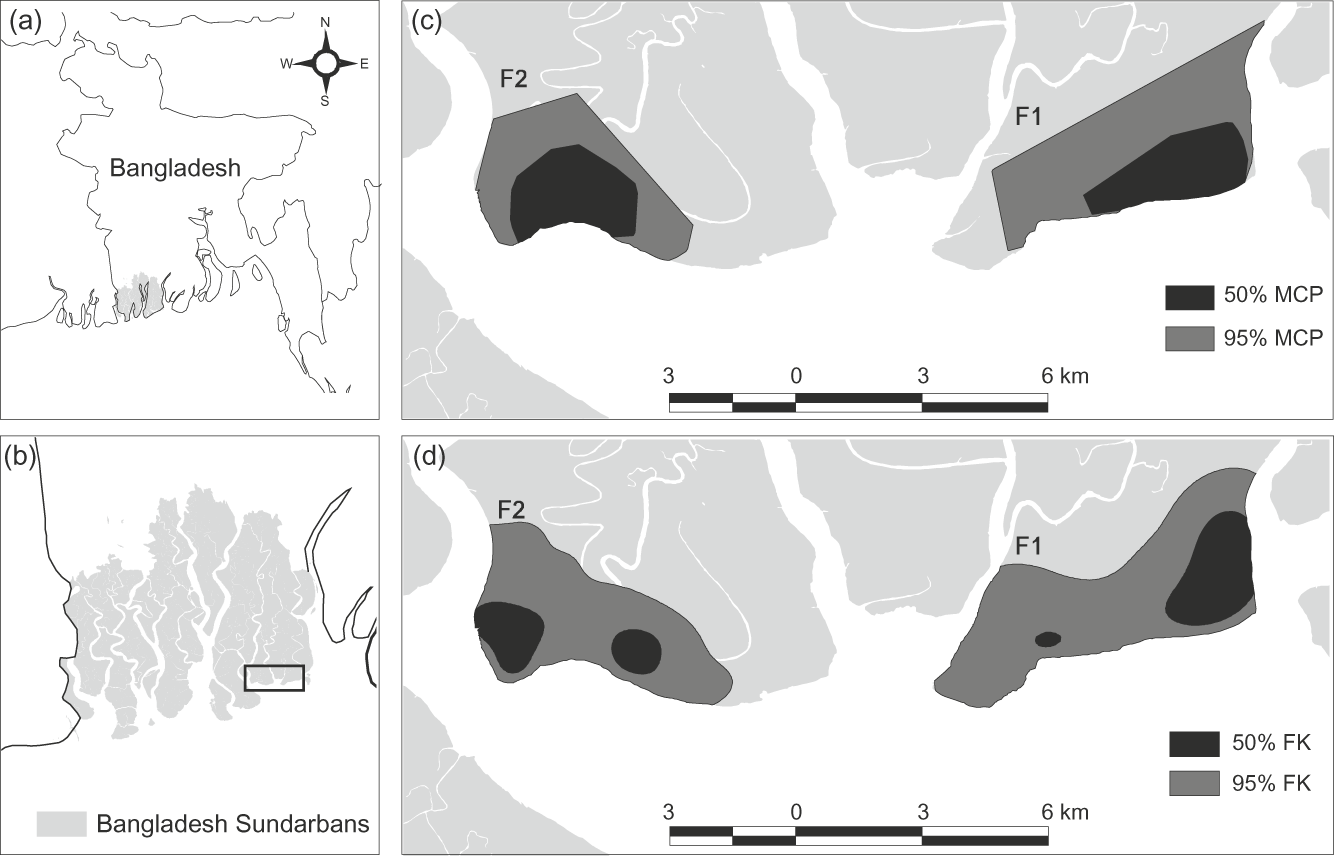
Fig. 1 (a) The location of the Sundarbans (shaded grey) in southern Bangladesh, (b) location of the main figures in the Bangladesh Sundarbans (rectangle), and estimates of home ranges of two adult female tigers (F1 and F2) constructed by (c) 50 and 95% minimum convex polygon (MCP) and (d) 50 and 95% fixed kernel (FK) methods (see text for further details).
Using one location per 4 hours (F1) the estimated mean straight line distance moved was 2.25 km day-1 (range 2.16–2.34 km day-1) and with one location per 30 minutes (F2) the mean daily travel was 3.6 km day-1 (range 0.02–10.0 km day-1). Maximum distance moved per day was 11.3 km for F1 and 10.0 km for F2. Both crossed water bodies at a mean frequency of 17 times per month (range 12–21), equivalent to approximately one crossing every 2 days.
Based on the location-area curves, which showed negligible increase in area with further addition of locations, and the large number of locations for each tiger, it is likely that the home range sizes were not underestimated. The relatively old age of the study tigers is unlikely to have influenced the estimation of home range sizes; other studies suggest that adult female tiger territory size does not change with age and that tigers are displaced when no longer capable of defending their territory (Sunquist, Reference Sunquist1981).
The two 95% MCP home ranges are amongst the smallest recorded for female tigers (Table 1) and are indicative of a relatively high tiger density in the Sundarbans Reserved Forest, comparable to the alluvial floodplain in the Terai region of Nepal (Barlow et al., Reference Barlow, McDougal, Smith, Gurung, Bhatta and Kumal2009). This suggests that this Forest is of higher conservation value for the conservation of tigers than previously thought (Barlow, Reference Barlow2009). The high density is probably related to high prey biomass (Smith et al., Reference Smith, McDougal, Sunquist, Tilson and Seal1987) and possibly to the relatively small size of the Forest’s tigers (Barlow et al., Reference Barlow, Mazak, Ahmad and Smith2010). However, considering the small sample size and that both tigers were from the same part of the Forest, these estimates of home range are preliminary. The two tigers were captured in areas of medium relative tiger abundance, as indicated from a study based on tiger track frequencies along creek banks (Barlow et al., Reference Barlow, Ahmed, Rahman, Howlader, Smith and Smith2008). In areas of lower or higher relative tiger abundance home ranges may be larger and smaller, respectively, than those in this study. In the India Sundarbans the home range of one female tiger was estimated by telemetry to be c. 40 km2 (Sharma, pers. comm.) and a camera trap study indicated a relatively low tiger density of 0.8 per 100 km2 (Karanth & Nichols, Reference Karanth and Nichols2000).
Table 1 Estimates of mean home range sizes of adult female tiger Panthera tigris in Nepal, Bangladesh, India and Russia, with the method used, number of tigers (n) and reference.
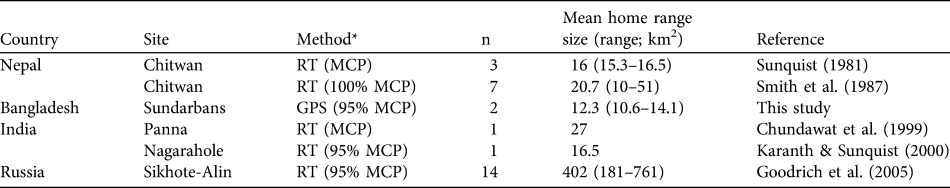
* RT, radio telemetry; MCP, minimum convex polygon; GPS, global positioning system
The mean movement of tigers and the maximum distance moved are the longest recorded movements for a female tiger within her home range but this may be because of the relatively high frequency of locations per day. The high rate of crossing of water bodies supports the assumption of a tiger monitoring survey in the Sundarbans that there is a high chance of detecting tiger presence based on tracks in the muddy banks along water bodies (Barlow et al., Reference Barlow, Ahmed, Rahman, Howlader, Smith and Smith2008).
Further estimates of home range size and movement distances of adult female tigers in both the India and Bangladesh Sundarbans are required to improve inferences of habitat quality, and thus of how many tigers the area can support, and to design monitoring approaches for tigers across the whole landscape. The Wildlife Institute of India is currently conducting a study to collect this information in the India Sundarbans.
Despite being preliminary the findings of this study highlight the conservation value of the Sundarbans tiger population and have provided information for development of a Bangladesh Tiger Action Plan to guide management actions (Ahmad et al., Reference Ahmad, Greenwood, Barlow, Islam, Hossain, Khan and Smith2009) and for a monitoring survey for tigers to evaluate the effectiveness of those actions (Barlow et al., Reference Barlow, Ahmed, Rahman, Howlader, Smith and Smith2008). The Wildlife Trust of Bangladesh and the Zoological Society of London are now working closely with the Bangladesh Forest Department to implement actions in line with the Action Plan.
Acknowledgements
We are grateful to the Bangladesh Ministry of Environment and Forest and the Bangladesh Forest Department for permission to carry out this work, for the assistance of Anwar Hossain, Tariqul Islam, Sk. Mizan Rahman and Md. Mozaharul Islam of the Forest Department, to the US Fish and Wildlife Service and Save the Tiger Fund for financial support, to Guidetours Ltd for logistical support to the field team, and to Tanjilur Rahman, Nasrul Islam Bachchu, Elisabeth Fahrni Mansur, Rubaiyat Mansur Mowgli and Hasan Mansur for their help. The methodology for catching tigers was developed with the help of Bart Schleyer and Pornchai Patumrattanathan. Sha Jamal, Panna Mia and Sundur Ali asisted with fieldwork. Christina Greenwood, Todd Arnold, Dave Garshelis, Donald Siniff and two anonymous reviewers helped to improve this article.
Biographical sketches
Adam Barlow has been researching tigers in Nepal, Thailand and Bangladesh for the last 10 years. Dave Smith has been studying tiger ecology and conservation for 30 years in Nepal, India, Thailand, Cambodia and China. Istiaq Ahmad is currently researching how to monitor tiger prey in the Sundarbans. Abu Hossain is looking at ways to improve the Sundarbans tiger monitoring survey and is also interested in the implementation and evaluation of wildlife law enforcement. Mizan Rahman and Alam Howlader are both villagers from communities close to the Sundarbans and work as wildlife technicians for the Sundarbans Tiger Project. They are both currently helping to design and implement measures to reduce human–tiger conflict in the Sundarbans.




