Skeletal muscle, the most widely distributed and rapidly growing tissue of the vertebrate body, plays major roles in different biological functions( Reference Fanzani, Conraads and Penna 1 ). However, infection and inflammation results in the rapid loss of muscle mass and myofibrillar proteins (muscle atrophy), which results in muscle weakness and increased morbidity during acute illness or poor quality of life( Reference Fanzani, Conraads and Penna 1 , Reference Orellana, Suryawan and Wilson 2 ). Multiple lines of evidence suggest that pro-inflammatory cytokines may contribute to muscle atrophy( Reference Philippou, Maridaki and Theos 3 , Reference Frost and Lang 4 ). Pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, have been implicated in the regulation of muscle protein degradation( Reference Zoico and Roubenoff 5 ). In addition, pro-inflammatory cytokines are also responsible for increased muscle atrophy F-box (MAFbx) and muscle RING finger 1 (MuRF1) expression( Reference Fanzani, Conraads and Penna 1 ), which are considered as accurate markers of the atrophy process( Reference Jamart, Gomes and Dewey 6 ). Thus, nutritional regulation targeting the suppression of pro-inflammatory cytokine expression may hold great promise for attenuating muscle atrophy and improving health of animals and humans.
Asparagine (Asn), a neutral amino acid, can be synthesised from aspartate and glutamine( Reference Wu, Bazer and Davis 7 ). Thus, traditionally, it is thought as a nutritionally non-essential amino acid in mammals( Reference Wu, Bazer and Davis 7 ). However, increasing evidence has shown that Asn plays an important role in many physiological and biological processes. First, Asn is necessary for the synthesis of many proteins in mammalian cells( Reference Zhang, Fan and Venneti 8 ). In addition, Asn has evolved to be a metabolic regulator of cell proliferation and apoptosis( Reference Zhang, Fan and Venneti 8 ). Moreover, through the reaction catalysed by asparginase, Asn can be degraded into aspartate, which is a precursor for gluconeogenesis or tricarboxylic acid cycle( Reference Xu, Dai and Graf 9 ). Of particular interest, Lancha et al.( Reference Lancha, Poortmans and Pereira 10 ) reported that Asn and aspartate could be metabolised by skeletal muscle. They have demonstrated that Asn and aspartate supplementation increased glycogen concentration and modulated the glucose uptake in muscle( Reference Lancha, Poortmans and Pereira 10 ). However, to our knowledge, the research on Asn modulating muscle atrophy and its mechanism(s) are lacking.
Pattern-recognition receptors, including toll-like receptor (TLR) and nucleotide-binding oligomerisation domain protein (NOD), activate downstream signalling pathways that induce innate immune responses via recognising pathogen-associated molecular patterns( Reference Ahn, Yoon and Park 11 ). Several lines of evidence indicate that TLR and NOD are functionally expressed in skeletal muscles( Reference Frost and Lang 4 , Reference Tamrakar, Schertzer and Chiu 12 ). Both TLR and NOD mediate the activation of NF-κB pathway, which induces the expression of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α ( Reference Prajapati, Jena and Rajput 13 ). These pro-inflammatory cytokines are critical regulators of muscle protein balance( Reference Frost, Nystrom and Lang 14 ). In addition, the pro-inflammatory cytokines have been demonstrated to affect protein kinase B (Akt)( Reference Crossland, Constantin-Teodosiu and Gardiner 15 ) and AMP-activated protein kinase (AMPK) pathways( Reference Steinberg, Michell and van Denderen 16 , Reference Ko, Zhang and Jung 17 ). The activation of Akt and AMPK regulate muscle protein degradation through the nuclear transcription factors termed Forkhead Box O (FOXO) and FOXO target genes (i.e. MAFbx and MuRF1)( Reference Fanzani, Conraads and Penna 1 , Reference Nakashima and Yakabe 18 ).
On the basis of the findings cited above, we hypothesised that Asn supplementation would suppress the production of muscle pro-inflammatory cytokines through influencing TLR4 and NOD signalling pathways, and protect against muscle atrophy, partially via regulating Akt and AMPK signalling. In this study, administration of Escherichia coli lipopolysaccharide (LPS) to animals was used to mimic endotoxaemia( Reference Crossland, Constantin-Teodosiu and Gardiner 15 ). Besides, we used a piglet model, which is a well-characterised animal model for nutrition research of humans, specifically children and adolescents with rapid muscle growth( Reference Merrifield, Lewis and Claus 19 , Reference Dunshea and Cox 20 ). The aim of this experiment was to investigate whether Asn could attenuate muscle atrophy caused by LPS challenge, and to elaborate its molecular mechanism(s).
Methods
Animal care and experimental design
This study was approved by the Animal Care and Use Committee of Hubei Province, People’s Republic of China. A total of twenty-four weaned castrated barrows (Duroc×Large White×Landrace, 8·9 (sem 0·7) kg initial body weight (BW)) were acquired and randomly divided into four treatments. There were six replicate pens per treatment. To keep animal uniformity, the piglets were of the same sex. The piglet was individually caged in 1·80×1·10 m pen with a feeder and a nipple waterer, and housed in a controlled-environment chamber. The basal diet (online Supplementary Table S1) was prepared according to the nutrient requirements of the National Research Council( 21 ).
The experiment consisted of four treatment groups: (1) non-challenged control (CONTR; piglets fed a control diet and injected with 0·9 % NaCl solution); (2) LPS-challenged control (LPS; piglets fed the same control diet and injected with E. coli LPS (Escherichia coli serotype 055: B5; Sigma Chemical Inc.)); (3) LPS+0·5 % Asn treatment (piglets fed a 0·5 % Asn diet and injected with LPS); and (4) LPS+1·0 % Asn treatment (piglets fed a 1·0 % Asn diet and injected with LPS). The Asn doses (purity >99 %; Amino Acid Bio-Chemical Co.) were selected according to our previous studies( Reference Li, Liu and Shi 22 ). Our previous studies showed that, before LPS challenge 0·5 and 1·0 % Asn addition did not affect growth performance, total and differential leucocyte counts and serum biochemical parameters of weaning piglets (Xiuying Wang, Yulan Liu, Dingan Pi, Weibo Leng, Huiling Zhu, Shuang Li and Haifeng Shi, unpublished results), indicating that the Asn level in basal diet was enough to meet the requirements of weanling piglets’ growth and physiological function in normal physiological condition. However, our previous studies also showed that, after LPS challenge, 0·5 % Asn attenuated weight loss, and both 0·5 and 1·0 % Asn attenuated the changes of total and differential leucocyte counts and serum biochemical parameters induced by LPS in weaning piglets( Reference Li, Liu and Shi 22 ), suggesting the importance of exogenous Asn supply under pathological conditions. Thus, in the current experiment, we focused on investigating the effect of dietary 0·5 and 1·0 % Asn supplementation on muscle variables in LPS-challenged pigs, and did not investigate the effect of Asn in pigs without LPS challenge. We added 1·35, 0·68 and 0 % alanine (purity >99 %; Amino Acid Bio-Chemical Co.) to the control, 0·5 % Asn and 1·0 % Asn diets, respectively, to get isonitrogenous diets. After 19-d feeding with the control, 0·5 % Asn and 1·0 % Asn diets, the challenged groups were treated with intraperitoneal injection of LPS at 100 μg/kg BW, and the non-challenged group was treated with the same volume of 0·9 % NaCl solution. The LPS dose was chosen in accordance with our previous experiments( Reference Liu, Huang and Hou 23 , Reference Liu, Chen and Odle 24 ), which demonstrated that this dose of LPS caused tissue damage in weaning piglets.
Plasma and muscle sample collections
At 4 h after administration with saline or LPS, blood samples were collected into heparinised vacuum tubes and centrifuged (3500 g for 10 min) to separate plasma. Plasma was kept at −80°C for further measurement of TNF-α, cortisol, glucagon and glucose concentrations. Following blood collection at 4 h, the piglets were humanely euthanised with pentobarbitone. The gastrocnemius and longissimus dorsi (LD) muscles were collected rapidly, frozen immediately in liquid N2 and then stored at −80°C for further measurement. In many experiments, gastrocnemius and LD muscles were used for studying muscle atrophy( Reference Drew, Phaneuf and Dirks 25 , Reference Ooi, da Costa and Edgar 26 ). MAFbx was highly up-regulated in the gastrocnemius and LD muscles in piglets with porcine congenital splayleg, which is characterised by muscle fibre atrophy( Reference Ooi, da Costa and Edgar 26 ). Thus, we were determined to choose these two muscles to study sepsis-induced atrophy. In addition, previous studies have found that, within 3–6 h post injection, LPS increased the mRNA or protein expression of pro-inflammatory cytokines and caused tissue damage( Reference Liu, Huang and Hou 23 , Reference Liu, Chen and Odle 24 , Reference Touchette, Carroll and Allee 27 – Reference Alscher, Phang and McDonald 29 ). Besides, during the time frame, the mRNA and protein level of TLR4 was also up-regulated( Reference Liu, Chen and Odle 24 , Reference Xu, You and Li 30 ). Therefore, the time point of 4 h after LPS or saline injection was selected for experimental measurements.
Plasma TNF-α, cortisol, glucagon and glucose concentrations
Plasma TNF-α concentration was analysed by using a commercially available porcine ELISA assay kit (R&D Systems). Plasma cortisol and glucagon concentrations were measured with 125I RIA assay kits (Beijing North Institute of Biological Technology). Plasma glucose concentration was determined by the glucose GOD-PAP assay kit (DiaSys Diagnostic Systems GmbH). All experimental procedures and data analyses were performed according to the manufacturer’s instructions.
Muscle protein, DNA and RNA contents
Muscle protein, DNA and RNA contents were analysed using the method of Liu et al.( Reference Liu, Huang and Hou 23 ).
mRNA abundance analysis by real-time PCR
Total RNA extraction, quantification, complementary DNA synthesis and real-time PCR were in accordance with the method of Liu et al.(
Reference Liu, Chen and Odle
24
). The primer pairs used are presented in the online Supplementary Table S2. The expression of target genes v. housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase; GAPDH) was computed using the formula
![]() $2^{{{\minus}\Delta \Delta C_{T} }} $
of Livak and Schmittgen(
Reference Livak and Schmittgen
31
). The results of the present study suggested that there was no difference in the expression of GAPDH among four treatments. Relative mRNA abundance of every target gene was normalised to the control group.
$2^{{{\minus}\Delta \Delta C_{T} }} $
of Livak and Schmittgen(
Reference Livak and Schmittgen
31
). The results of the present study suggested that there was no difference in the expression of GAPDH among four treatments. Relative mRNA abundance of every target gene was normalised to the control group.
Protein abundance analysis by Western blot
Protein immunoblot analysis was measured according to the previously described method( Reference Liu, Chen and Odle 24 ). In brief, the muscle samples were homogenised and centrifuged, and the supernatants were collected. The protein contents of the supernatants were measured using the bicinchoninic acid (BCA) reagent( Reference Liu, Chen and Odle 24 ). An equal amount of muscle proteins was loaded onto 10 % polyacrylamide gels, separated through SDS-PAGE, transferred to blotting membranes and then incubated with the primary antibodies( Reference Liu, Chen and Odle 24 ). After that, the membranes were incubated with the secondary antibody( Reference Liu, Chen and Odle 24 ). Specific primary antibodies included total AMPKα (tAMPKα; 1:1000; no. 2532), phosphorylated AMPKα (pAMPKα, Thr172; 1:1000; no. 2535), total Akt (tAkt, 1:1000; no. 9272), phosphorylated Akt (pAkt, serine 473; 1:1000; no. 9271), total FOXO 1 (tFOXO1; 1:1000; no. 9454) and phosphorylated FOXO 1 (pFOXO1, serine256; 1:1000; no. 9461) from Cell Signaling; MAFbx (1:1000; no. ab74023) from Abcam; MuRF1 (1:1000; no. 55456-1-AP) from Proteintech Group; and GAPDH (1:1000; no. ANT011) from Antgene Biotech. Blots were developed using an Enhanced Chemiluminescence Western blotting kit (Amersham), and visualised using a Gene Genome bioimaging system. Bands were analysed by densitometry using GeneTools software (Syngene). The relative abundance of target proteins (MAFbx and MuRF1) was expressed as the target protein:GAPDH protein ratio. The phosphorylated forms of AMPKα, Akt and FOXO1 were normalised with the total protein content.
Statistical analysis
All experimental data were analysed by variance specific for repeated measurements using mixed procedure of SAS (SAS Institute Inc.), with treatments (CONTR, LPS, LPS+0·5 % Asn, LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and LD muscle) as the within-animal effect. Only when a significant treatment×muscle interaction occurred, comparisons among treatments in each muscle was performed. The LPS piglets (0 % Asn) were compared with CONTR piglets to determine the effect of LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to dietary Asn supplementation among LPS-challenged piglets. Results were expressed as means values with their pooled standard errors. Differences were considered as significant when P≤0·05. Instances in which 0·05<P<0·10 were taken to indicate trends.
Results
Plasma glucose, cortisol, glucagon and TNF-α concentrations
Relative to CONTR piglets, LPS challenge increased the concentrations of TNF-α, cortisol and glucagon, and decreased glucose concentration in plasma (P<0·01; Table 1). Among the LPS-challenged piglets, Asn supplementation decreased the concentrations of TNF-α, cortisol and glucagon in plasma (linear, P<0·01; quadratic, P<0·05).
Table 1 Effects of asparagine (Asn) supplementation on plasma TNF-α, cortisol, glucagon and glucose concentrations in weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors; n 6 (one piglet per pen))
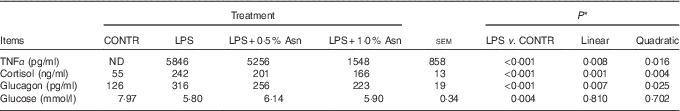
CONTR, non-challenged control; ND, not detectable.
* The LPS pigs were compared with CONTR pigs to determine the effect of LPS. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs.
Muscle protein, DNA and RNA contents
The protein, DNA and RNA contents in LD muscle were higher than those in gastrocnemius muscle (P<0·05; Table 2). No significant treatment×segment interaction was observed for protein and DNA contents. Overall, compared with CONTR piglets, LPS challenge decreased DNA content (P<0·001). Among the LPS-challenged piglets, Asn supplementation increased protein content (linear, P=0·084; quadratic, P<0·05).
Table 2 Effects of asparagine (Asn) supplementation on muscle protein, DNA and RNA contents in weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors; n 6 (one piglet per pen))
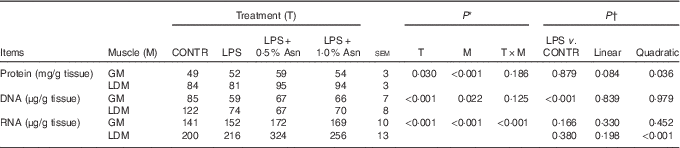
CONTR, non-challenged control; GM, gastrocnemius muscle; LDM, longissimus dorsi muscle.
* P values were obtained using treatment as the main effect and by analysing data from the GM and LDM as repeated measures.
† The LPS pigs were compared with CONTR pigs to determine the effect of LPS. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs.
There was significant treatment×segment interaction observed for RNA content (P<0·001). Among the LPS-challenged piglets, Asn supplementation increased RNA content in LD muscle (quadratic, P<0·001).
Muscle mRNA and protein abundance of muscle atrophy F-box and muscle RING finger 1
The mRNA abundance of MAFbx in gastrocnemius muscle was higher than that in LD muscle (P<0·05; Table 3), and the mRNA abundance of MuRF1 in gastrocnemius muscle tended to be higher than that in LD muscle (P=0·071). There was treatment×segment interactions observed for the mRNA abundance of MAFbx (P=0·05). Relative to CONTR piglets, LPS challenge increased mRNA abundance of MAFbx in gastrocnemius muscle (P<0·01). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of MAFbx in gastrocnemius muscle (linear, P<0·05; quadratic, P<0·05). No significant treatment×segment interaction was found for the mRNA abundance of MuRF1. Overall, compared with CONTR pigs, LPS challenge resulted in an increase in the mRNA abundance of MuRF1 (P<0·001). Among the LPS-challenged pigs, Asn supplementation decreased the mRNA abundance of MuRF1 (linear, P=0·001; quadratic, P<0·01).
Table 3 Effects of asparagine (Asn) supplementation on muscle mRNA expression of AMP-activated protein kinase α (AMPKα), protein kinase B (Akt) signals and their target genes in weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors; n 6 (one piglet per pen))

CONTR, non-challenged control; GM, gastrocnemius muscle; LDM, longissimus dorsi muscle; FOXO, Forkhead Box O; MAFbx, muscle atrophy F-box; MuRF1, muscle RING finger 1.
* P values were obtained using treatment as the main effect and by analysing data from the GM and LDM as repeated measures.
† The LPS pigs were compared with CONTR pigs to determine the effect of LPS. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs.
The protein abundance of MuRF1 in gastrocnemius muscle was higher than that in LD muscle (P<0·05; Fig. 1). No significant treatment×segment interaction was found for the protein abundance of MAFbx and MuRF1. Neither LPS nor Asn treatment affected the protein abundance of MAFbx and MuRF1.

Fig. 1 Effects of asparagine (Asn) supplementation on protein abundance of (a) muscle atrophy F-box (MAFbx) and (b) muscle RING finger 1 (MuRF1) in muscles of weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. The bands shown are the representative Western blot images of MAFbx (42 kDa), MuRF1 (40 kDa) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (37 kDa). The data were analysed as repeated measures with treatments (![]() , non-challenged control (CONTR);
, non-challenged control (CONTR); ![]() , LPS;
, LPS; ![]() , LPS+0·5 % Asn;
, LPS+0·5 % Asn; ![]() , LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n=6; one pig per pen), with standard errors. The protein abundance of MuRF1 in gastrocnemius muscle was higher than that in LD muscle (P=0·011). No significant treatment×segment interaction was found for the protein abundance of MAFbx (P=0·473) and MuRF1 (P=0·630).
, LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n=6; one pig per pen), with standard errors. The protein abundance of MuRF1 in gastrocnemius muscle was higher than that in LD muscle (P=0·011). No significant treatment×segment interaction was found for the protein abundance of MAFbx (P=0·473) and MuRF1 (P=0·630).
Muscle mRNA abundance of AMP-activated protein kinase α, protein kinase B/Forkhead Box O signalling
The mRNA abundance of AMPKα1, AMPKα2 and FOXO4 in gastrocnemius muscle was higher than that in LD muscle (P<0·05; Table 3). The mRNA abundance of FOXO1 in gastrocnemius muscle was lower than that in LD muscle (P<0·001). There were treatment×segment interactions observed for the mRNA abundance of FOXO1 and FOXO4 (P<0·05), and trends for treatment×segment interaction observed for the mRNA abundance of AMPKα1 (P=0·075) and AMPKα2 (P=0·057). Relative to CONTR piglets, LPS challenge increased mRNA abundance of FOXO1 in gastrocnemius and LD muscles (P<0·01). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of AMPKα1 (linear, P<0·05; quadratic, P=0·084) and FOXO4 (linear, P=0·001; quadratic, P=0·001) in LD muscle, and tended to increase mRNA abundance of AMPKα2 in gastrocnemius muscle (linear, P=0·076; quadratic, P=0·079). No significant treatment×segment interaction was found for the mRNA abundance of Akt1. Neither LPS nor Asn treatment affected the mRNA abundance of Akt1.
Muscle protein phosphorylation and abundance of AMP-activated protein kinase α, protein kinase B and Forkhead Box O 1
The ratios of pAMPKα:tAMPKα and pAkt:tAkt and the protein abundance of tAkt and tFOXO1 in gastrocnemius muscle were higher than those in LD muscle, and the protein abundance of tAMPKα and the ratio of pFOXO1:tFOXO1 in gastrocnemius muscle were lower than those in LD muscle (P≤0·001; Fig. 2–4). A trend for treatment×segment interaction was observed for pAMPKα:tAMPKα ratio (P=0·069). Relative to CONTR piglets, LPS challenge increased the ratio of pAMPKα:tAMPKα in gastrocnemius muscle (P<0·01). Among the LPS-challenged piglets, Asn supplementation decreased the ratio of pAMPKα:tAMPKα in gastrocnemius and LD muscles (linear and quadratic, P<0·01).

Fig. 2 Effects of asparagine (Asn) supplementation on the (a) phosphorylated AMP-activated protein kinase (pAMPKα):total AMP-activated protein kinase (tAMPKα) ratio and (b) protein abundance of tAMPKα in muscles of weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. The bands shown are the representative Western blot images of pAMPKα (62 kDa) and tAMPKα (62 kDa). The data were analysed as repeated measures with treatments (![]() , non-challenged control (CONTR);
, non-challenged control (CONTR); ![]() , LPS;
, LPS; ![]() , LPS+0·5 % Asn;
, LPS+0·5 % Asn; ![]() , LPS +1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen) with standard errors. The ratio of pAMPKα:tAMPKα in gastrocnemius muscle was higher than that in LD muscle (P=0·001), and the protein abundance of tAMPKα in gastrocnemius muscle tended to be lower than that in LD muscle (P<0·001). A trend for treatment×segment interaction was observed for pAMPKα:tAMPKα ratio (P=0·069). No significant treatment×segment interaction was found for the protein abundance of tAMPKα (P=0·894). AU, arbitrary units.
, LPS +1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen) with standard errors. The ratio of pAMPKα:tAMPKα in gastrocnemius muscle was higher than that in LD muscle (P=0·001), and the protein abundance of tAMPKα in gastrocnemius muscle tended to be lower than that in LD muscle (P<0·001). A trend for treatment×segment interaction was observed for pAMPKα:tAMPKα ratio (P=0·069). No significant treatment×segment interaction was found for the protein abundance of tAMPKα (P=0·894). AU, arbitrary units.

Fig. 3 Effects of asparagine (Asn) supplementation on the (a) phosphorylated protein kinase B (Akt) (pAkt):total Akt (tAkt) ratio and (b) protein abundance of tAkt in muscles of weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. The bands shown are the representative Western blot images of pAkt (60 kDa) and tAkt (60 kDa). The data were analysed as repeated measures with treatments (![]() , non-challenged control (CONTR);
, non-challenged control (CONTR); ![]() , LPS;
, LPS; ![]() , LPS+0·5 % Asn;
, LPS+0·5 % Asn; ![]() , LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen), with standard errors. The ratio of pAkt:tAkt (P<0·001) and the protein abundance of tAkt (P=0·001) in gastrocnemius muscle were higher than those in LD muscle. No significant treatment×segment interaction was found for the ratio of pAkt:tAkt (P=0·211) and the protein abundance of tAkt (P=0·335). AU, arbitrary units.
, LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen), with standard errors. The ratio of pAkt:tAkt (P<0·001) and the protein abundance of tAkt (P=0·001) in gastrocnemius muscle were higher than those in LD muscle. No significant treatment×segment interaction was found for the ratio of pAkt:tAkt (P=0·211) and the protein abundance of tAkt (P=0·335). AU, arbitrary units.

Fig. 4 Effects of asparagine (Asn) supplementation on the (a) phosphorylated Forkhead Box O (pFOXO):total Forkhead Box O (tFOXO) ratio and (b) protein abundance of tFOXO in muscles of weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge. The bands shown are the representative Western blot images of pFOXO (82 kDa) and tFOXO (82 kDa). The data were analysed as repeated measures with treatments (![]() , non-challenged control (CONTR);
, non-challenged control (CONTR); ![]() , LPS;
, LPS; ![]() , LPS+0·5 % Asn;
, LPS+0·5 % Asn; ![]() , LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen), with standard errors. The ratio of pFOXO1:tFOXO1 in gastrocnemius muscle was lower than that in LD muscle (P<0·001), and the protein abundance of tFOXO1 in gastrocnemius muscle was higher than that in LD muscle (P=0·001). No significant treatment×segment interaction was found for the ratio of pFOXO1:tFOXO1(P=0·159) and the protein abundance of tFOXO1 (P=0·833). AU, arbitrary units.
, LPS+1·0 % Asn) as the between-animal effect and muscle (gastrocnemius muscle and longissimus dorsi (LD) muscle) as the within-animal effect. The LPS (0 % Asn) pigs were compared with CONTR pigs (LPS v. CONTR) to determine the effect of LPS. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs. Values are means (n 6; one pig per pen), with standard errors. The ratio of pFOXO1:tFOXO1 in gastrocnemius muscle was lower than that in LD muscle (P<0·001), and the protein abundance of tFOXO1 in gastrocnemius muscle was higher than that in LD muscle (P=0·001). No significant treatment×segment interaction was found for the ratio of pFOXO1:tFOXO1(P=0·159) and the protein abundance of tFOXO1 (P=0·833). AU, arbitrary units.
No significant treatment×segment interaction was found for the protein abundance of tAMPKα, tAkt and tFOXO1, and the ratios of pAkt:tAkt and pFOXO1:tFOXO1. Overall, relative to CONTR piglets, LPS challenge decreased the ratio of pAkt:tAkt (P<0·001). Among the LPS-challenged piglets, Asn supplementation increased the protein abundance of tAMPKα (linear, P<0·05; quadratic, P=0·075), and the ratios of pAkt:tAkt (linear, P<0·05; quadratic, P<0·05) and pFOXO1:tFOXO1 (linear, P<0·05), and tended to increase the protein abundance of tAkt (linear, P=0·097).
Muscle mRNA abundance of toll-like receptor 4 and nucleotide-binding oligomerisation domain proteins and their downstream signals
The mRNA abundance of TNF receptor-associated factor 6 (TRAF6) in gastrocnemius muscle was higher than that in LD muscle, and the mRNA abundance of TNF-α in gastrocnemius muscle was lower than that in LD muscle (P<0·05; Table 4). No significant treatment×segment interaction was observed for the mRNA abundance of TLR4, myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase 1, TRAF6, NOD1, NOD2, receptor-interacting serine/threonine-protein kinase 2 (RIPK2) and NF-κB p65. Compared with CONTR piglets, LPS challenge increased mRNA abundance of TLR4, MyD88, NOD2 and RIPK2 (P<0·05), and tended to increase mRNA abundance of NF-κB p65 (P=0·052). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of TLR4 (linear, P=0·01; quadratic, P<0·01), MyD88 (linear, P<0·05; quadratic, P<0·05), NOD1 (linear, P<0·05; quadratic, P<0·05) and NOD2 (linear, P<0·05), and tended to decrease mRNA abundance of TRAF6 (linear, P=0·070) and NF-κB p65 (linear, P=0·082).
Table 4 Effects of asparagine (Asn) supplementation on muscle mRNA expression of toll-like receptor 4 (TLR4) and nucleotide-binding oligomerisation domain proteins (NOD) and their downstream signals in weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors; n 6 (one piglet per pen))

CONTR, non-challenged control; GM, gastrocnemius muscle; LDM, longissimus dorsi muscle; MyD88, myeloid differentiation factor 88; IRAK1, IL-1 receptor-associated kinase 1; RIPK2, receptor-interacting serine/threonine-protein kinase 2; TRAF6, TNF receptor-associated factor 6.
* P values were obtained using treatment as the main effect and by analysing data from the GM and LDM as repeated measures.
† The LPS pigs were compared with CONTR pigs to determine the effect of LPS. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs.
There was a trend for treatment×segment interaction observed for the mRNA abundance of TNF-α (P=0·094). Compared with CONTR piglets, LPS challenge increased mRNA abundance of TNF-α in gastrocnemius and LD muscles (P<0·01). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of TNF-α in gastrocnemius and LD muscles (linear and quadratic, P<0·05).
Muscle mRNA abundance of negative regulators of toll-like receptor 4 and nucleotide-binding oligomerisation domain proteins signalling pathways
The mRNA abundance of radioprotective 105 (RP105) in gastrocnemius muscle was lower than that in LD muscle (P<0·001; Table 5), and the mRNA abundance of toll-interacting protein (Tollip) in gastrocnemius muscle tended to be higher than that in LD muscle (P=0·094). Significant treatment×segment interactions were observed for the mRNA abundance of RP105 and suppressor of cytokine signalling 1 (SOCS1) (P<0·01). Compared with CONTR piglets, LPS challenge increased mRNA abundance of RP105 in gastrocnemius muscle, and SOCS1 in gastrocnemius and LD muscles (P<0·05). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of RP105 (linear and quadratic, P<0·05) and SOCS1 (linear, P<0·05; quadratic, P=0·081) in LD muscle.
Table 5 Effects of asparagine (Asn) supplementation on muscle mRNA expression of negative regulators of toll-like receptor 4 (TLR4) and nucleotide-binding oligomerisation domain proteins (NOD) signalling pathways in weaning piglets at 4 h after the administration of Escherichia coli lipopolysaccharide (LPS) challenge (Mean values with their pooled standard errors; n 6 (one piglet per pen))

CONTR, non-challenged control; RP105, radioprotective 105; GM, gastrocnemius muscle; LDM, longissimus dorsi muscle; SOCS1, suppressor of cytokine signalling 1; Tollip, toll-interacting protein; SIGIRR, single Ig IL-1 R-related molecule; ERBB2IP, Erbb2-interacting protein; CENTB1, centaurin β1.
* P values were obtained using treatment as the main effect and by analysing data from the GM and LDM as repeated measures.
† The LPS pigs were compared with CONTR pigs to determine the effect of LPS. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged pigs.
No significant treatment×segment interaction was observed for the mRNA abundance of Tollip, single Ig IL-1 R-related molecule (SIGIRR), Erbb2-interacting protein (ERBB2IP) and centaurin β1 (CENTB1). Overall, compared with CONTR pigs, LPS challenge decreased mRNA abundance of Tollip (P=0·05), and tended to increase mRNA abundance of CENTB1 (P=0·074). Among the LPS-challenged piglets, Asn supplementation decreased mRNA abundance of CENTB1 (linear and quadratic, P<0·01), and tended to increased mRNA abundance of Tollip (quadratic, P=0·064).
Discussion
Protein or amino acid supplementation exerts a regulatory effect on both protein synthesis and protein degradation( Reference Vary, Jefferson and Kimball 32 , Reference Hulmi, Lockwood and Stout 33 ). As stated in a recent review, protein or amino acids are more effective than carbohydrate in increasing lean or fat-free body mass and whole-muscle cross-sectional area( Reference Hulmi, Lockwood and Stout 33 ). Amino acid supplementation also stimulates the synthesis of both myofibrillar and sarcoplasmic proteins( Reference Vary, Jefferson and Kimball 32 ). However, the molecular mechanisms by which protein or amino acids affect muscle protein metabolism are presently unknown. Our present study offered a new basis to explain the beneficial effects of amino acid in the skeletal muscle. We found that Asn supplementation had beneficial effects on muscle atrophy, as indicated by the increase of muscle protein and RNA contents, and the down-regulation of ubiquitin ligases MAFbx and MuRF1 mRNA transcription. These changes were accompanied by increased phosphorylation (activation) of Akt1 protein, and reduced phosphorylation (inhibition) of AMPK and FOXO1 in muscles. Also, these changes were concurrent with decreased circulating level of TNF-α and decreased mRNA transcription of muscle TLR4 and NOD and their downstream signals. Overall, the present data suggest that Asn exerts a positive effect on LPS-induced muscle atrophy, which is associated with regulating Akt, AMPKα, TLR4 and NOD signalling.
Muscle atrophy occurs when protein degradation exceeds protein synthesis, leading to a net loss of muscle protein( Reference Fanzani, Conraads and Penna 1 , Reference Sacheck, Ohtsuka and McLary 34 ). The muscle protein, RNA and DNA are common metrics for assessing the protein synthetic capacity, translational efficiency and cell size, and they are negatively related to protein degradation( Reference Smith, Atherton and Reeds 35 ). MAFbx and MuRF1, induced early in the atrophy process( Reference Shiota, Abe and Kawai 36 ), are thought to be the accurate markers of the atrophy process( Reference Jamart, Gomes and Dewey 6 ). The increased expression of the MAFbx and MuRF1 precedes the loss of muscle weight( Reference Shiota, Abe and Kawai 36 ), and have been described in several models of muscle atrophy( Reference Jaitovich, Angulo and Lecuona 37 ). In this study, LPS administration resulted in decreased DNA content and increased mRNA expression of MAFbx and MuRF1 in muscles, indicating LPS-induced muscle atrophy. Our results are in agreement with previous observations of up-regulation of MAFbx and MuRF1 in endotoxaemia( Reference Dehoux, van Beneden and Fernández-Celemín 38 ), and their central role in the initiation and regulation of muscle protein degradation via the ubiquitin-proteasome pathway during atrophy( Reference Bodine, Latres and Baumhueter 39 ). Asn supplementation to the LPS-challenged piglets increased protein and RNA content in muscles, and decreased muscle mRNA expression of MAFbx and MuRF1, indicating that Asn is effective in attenuating LPS-induced muscle atrophy. However, in this study, neither LPS nor Asn treatment affected the protein abundance of MuRF1 and MAFbx. Many reports on mRNA and protein abundances find that mRNA and protein are differentially expressed, and the discrepancy may be attributed to different levels of regulation between transcript and protein product( Reference Maier, Güell and Serrano 40 , Reference Koussounadis, Langdon and Um 41 ). Previous studies have shown that, within 8–24 h post injection, LPS increased the protein abundance of MuRF1 and MAFbx( Reference Orellana, Suryawan and Wilson 2 , Reference Crossland, Constantin-Teodosiu and Gardiner 15 ). We speculated that gene up-regulation may occur at an earlier stage than protein production.
Akt and AMPK are considered to regulate protein degradation in muscle through FOXO and FOXO target genes (i.e. MAFbx and MuRF1)( Reference Fanzani, Conraads and Penna 1 , Reference Nakashima and Yakabe 18 ). In our present experiment, LPS challenge increased phosphorylation of AMPKα, and decreased phosphorylation of Akt, which is consistent with the findings of Orellana et al.( Reference Orellana, Suryawan and Wilson 2 ) and Frost and Lang( Reference Frost and Lang 4 ). These data indicate that injection of LPS enhanced AMPK activity but inhibited Akt activity in skeletal muscle. In the present study, consistent with decreased mRNA expression of MAFbx and MuRF1 in muscle, Asn supplementation to the LPS-challenged pigs decreased the phosphorylation of AMPKα and increased the phosphorylation of Akt and FOXO1. AMPK, in an active (phosphorylated) state, can enhance the activity of FOXO transcription factor family members, leading to muscle wasting( Reference Nakashima and Yakabe 18 ). On the contrary, the phosphorylation of Akt inhibits muscle protein degradation by phosphorylating and inactivating FOXO transcription factors( Reference Orellana, Suryawan and Wilson 2 ). Thus, we speculated that Asn’s ability to attenuate muscle atrophy may be related to preventing LPS-induced inhibition of Akt and activation of AMPKα and FOXO1.
Pro-inflammatory cytokines can lead to muscle wasting directly or via alterations of Akt/FOXO/ubiquitin-proteasome pathway( Reference Crossland, Constantin-Teodosiu and Gardiner 15 , Reference Mann and Reid 42 ). In addition, skeletal muscle metabolism is under hormonal control( Reference Izquierdo, Häkkinen and Antón 43 ), and many of the hormonal responses to sepsis and endotoxaemia are mediated by enhanced synthesis and secretion of pro-inflammatory cytokines( Reference Frost, Nystrom and Jefferson 44 ). In our study, LPS challenge increased the concentrations of plasma TNF-α, cortisol and glucagon, and decreased plasma glucose concentration, and increased TNF-α mRNA expression in muscles. Cytokines have been shown to increase catabolic hormones such as cortisol( Reference Burton, Nicholson and Hall 45 ) and glucagon( Reference Grunfeld, Zhao and Fuller 46 ). The metabolic effects of cortisol are enhanced with skeletal muscle protein breakdown to provide gluconeogenic substrate and amino acids for liver protein synthesis( Reference Burton, Nicholson and Hall 45 ). Blood glucose level, which is regulated by the balance between anabolic and catabolic (glucagon and cortisol) hormones, is related to muscle fibre composition and could partially indicate ultimate pork quality( Reference Tappy 47 , Reference Choe, Choi and Lee 48 ). In the present study, Asn supplementation to the LPS-challenged pigs decreased the concentrations of TNF-α, cortisol and glucagon in plasma, and the mRNA expression of TNF-α in muscles. The data support the notion that dietary Asn supplementation may attenuate muscle atrophy partially by reducing pro-inflammatory cytokines.
Activation of TLR4 and NOD signalling pathways can induce over-production of pro-inflammatory cytokines, and elicit collateral host-tissue injury. To avoid excessive and harmful inflammatory responses, TLR4 and NOD signalling are subjected to extensive negative regulation through extracellular and intracellular mechanisms( Reference Kondo, Kawai and Akira 49 , Reference Coll and O’Neill 50 ). Of them, negative regulators of TLR4 (such as RP105, SOCS1, Tollip and SIGIRR) and NOD (such as ERBB2IP and CENTB1) play a central role in this process( Reference Kondo, Kawai and Akira 49 , Reference Coll and O’Neill 50 ). To explore the molecular mechanism(s) by which Asn reduces muscle pro-inflammatory cytokines, we examined the roles of these intracellular signalling pathways. In the present experiment, consistent with the decreased plasma and muscle TNF-α concentrations, Asn supplementation to the LPS-challenged pigs decreased mRNA abundance of TLR4 and NOD signalling-related genes (TLR4, MyD88, TRAF6, NOD1, NOD2 and NF-κB p65). In addition, we found that LPS challenge increased mRNA abundance of RP105, SOCS1 and CENTB1, and tended to decrease mRNA abundance of Tollip. Asn attenuated the alteration of mRNA levels of these negative regulators induced by LPS. Therefore, it is possible that the beneficial roles of Asn on muscle atrophy are closely related to reducing the expression of muscle pro-inflammatory cytokines through inhibiting the TLR4 and NOD signalling pathways via modulation of their negative regulators. We speculate that the effect of Asn on TLR4 and NOD pathways might be due to the following mechanisms. Asn can be converted to arginine and glutamine through complex metabolism( Reference Wu, Bazer and Davis 7 ). Chen et al.( Reference Chen, Chen and Tian 51 ) reported that arginine supplementation inhibited the excessive activation of the TLR4–MyD88 signalling pathway. In addition, Zhou et al.( Reference Zhou, Li and Zheng 52 ) found that glutamine protected the intestinal tract in preterm neonatal rats with necrotising enterocolitis via reducing TLR2 and TLR4 expression. In this way, it is possible that Asn may be converted to many other amino acids to regulate the TLR4 and NOD signalling pathways.
In summary, Asn supplementation has beneficial effects on muscle atrophy because of inhibition of muscle proteolysis via Akt activation and AMPKα and FOXO1 inhibition, and also decreasing the inflammatory processes via inhibition of TLR4 and NOD signalling pathways.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 31422053 and 31372318), and the Project of the Hubei Provincial Department of Education (grant no. T201508).
The authors’ contributions are as follows: Y. L. designed the research; Y. L., X. W., S. W., D. P., W. L., H. Z., J. Z., H. S. and S. L. conducted the research; Y. L., X. W. and D. P. analysed the data; Y. L. and X. W. wrote the article; Y. L., X. L. and J. O. edited and revised the manuscript; Y. L. had primary responsibility for final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S000711451600297X












