The associations between birth anthropometry and subsequent wheeze and atopy have been interpreted as evidence of in utero nutritional influences on fetal airway and immune development( Reference Godfrey, Barker and Osmond 1 – Reference Chatkin and Menezes 4 ). This concept is supported by the associations between reduced birth weight and reduced lung function in later life, suggesting that suboptimal fetal growth and nutrition increase the risk of airflow obstruction in later life( Reference Pei, Chen and Mi 5 – Reference Canoy, Pekkanen and Elliott 7 ). Studies on fetal ultrasound measurements provide more direct evidence of the importance of fetal growth in the development of wheeze, asthma and lung function( Reference Turner, Campbell and Smith 8 , Reference Turner, Prabhu and Danielian 9 ).
There are now many reports of the associations between childhood wheeze, asthma, and atopic disease and maternal nutritional status during pregnancy, particularly related to antioxidants, PUFA/lipids and vitamin D( Reference Nurmatov, Devereux and Sheikh 10 – Reference Devereux and Wagner 12 ). Although highlighted by animal models and a single human study, the potential role of maternal Fe status during pregnancy in the development of childhood wheeze, asthma and lung function remains relatively unexplored. In rodents, Fe deficiency during pregnancy induces hypertension in the offspring, coupled with changes in lipid metabolism and adult obesity( Reference Gambling, Dunford and Wallace 13 ). It has also been suggested that changes occur in lung structure, without any expansion of the alveoli (L Gambling and HJ McArdle, unpublished results).
The Avon Longitudinal Study of Parents and Children (ALSPAC) has reported associations between reduced umbilical cord Fe status and childhood wheeze; however, it was concluded that this could have been the consequence of multiple analyses( Reference Shaheen, Newson and Henderson 14 ). Moreover, the study had a relatively short follow-up duration and lacked lung function data. Given the relative ease of Fe supplementation during pregnancy, these novel findings justify further studies on the role of Fe in the development of lung function, wheeze, asthma and atopy.
The present study investigated the associations of maternal Fe status during pregnancy with fetal growth, birth anthropometry, childhood wheeze/atopy and lung function up to 10 years of age in a subgroup of children from the SEATON (Study of Eczema and Asthma To Observe the influence of Nutrition) birth cohort. This cohort was established to prospectively investigate the associations between maternal nutritional status during pregnancy and childhood asthma and atopic disease( Reference Martindale, McNeill and Devereux 15 ).
Experimental methods
Study design
Between 1997 and 1999, pregnant women were recruited to the SEATON. Maternal dietary intake and nutritional status were assessed, and the first- and second-trimester fetal measurement data were collected. Singleton children were followed up using a postal questionnaire( Reference Martindale, McNeill and Devereux 15 ) at 1, 2, 5 and 10 years of age. At 5 and 10 years of age, the children were invited to attend a clinical assessment. The present study was approved by the North of Scotland Research Ethics Committee (08/S0802/19), and written parental consent was obtained and children gave verbal and/or written assent.
Recruitment
Complete details of recruitment have been described previously( Reference Martindale, McNeill and Devereux 15 ). A total of 2000 healthy pregnant women attending an antenatal clinic for a routine dating ultrasound scan, at median 11 (interquartile range 8–12) weeks of gestation, were recruited. There was no selection for asthma, atopic disease, anaemia or Fe status, and the recruited women were mostly representative of the local obstetric population( Reference Martindale, McNeill and Devereux 15 ). At enrolment, an interviewer administered a questionnaire; atopic status was ascertained by skin-prick testing, and a non-fasting blood sample was collected. At 32 weeks of gestation, habitual dietary intake during the previous 3 months was assessed using a semi-quantitative FFQ( Reference Masson, McNeill and Tomany 16 ). In forty women of childbearing age, the rank correlation coefficient for Fe intake determined by this FFQ and 4 d weighed records was 0·60 (P< 0·001)( Reference Masson, McNeill and Tomany 16 ).
Fetal measurements
Details regarding fetal measurements have been described elsewhere( Reference Turner, Prabhu and Danielian 9 ), and these measurements were recorded as part of routine antenatal care using an ATL (Ultramark 4A) or Toshiba (SSA-240A or SSA-340A) ultrasound scanner. The crown–rump length was measured during the first-trimester scan, and the femur length and biparietal diameter (inner–outer) were measured during the second-trimester scan. Fetal and neonatal measurements are expressed as z-scores( Reference Chitty, Altman and Henderson 17 , Reference Chitty, Altman and Henderson 18 ).
Outcome assessments
A questionnaire based on the International Study of Asthma and Allergy in Children format was posted to the parents of cohort children at 1, 2, 5 and 10 years of age. Doctor-confirmed asthma was defined as an affirmative response to the following two questions: ‘Has your child ever had asthma?’ and ‘Was this confirmed by a doctor?’. Similar questions were asked about ‘doctor-diagnosed eczema’ and ‘doctor-diagnosed hay fever’. At 5 and 10 years of age, the cohort children were invited to attend a detailed assessment. This included spirometry using a pneumotachograph (21/20; Vitalograph) with incentive software (Spirotrac IV version 4.22; Vitalograph) and application of standard quality control( Reference Miller, Hankinson and Brusasco 19 ). Spirometric variables are expressed as z-scores( Reference Stanojevic, Wade and Stocks 20 ). Atopic status was determined by skin-prick testing (dust mite, cat and dog allergens, grass pollen, egg and peanut; ALK Abello). Atopic sensitisation was defined as a mean wheal diameter ≥ 3 mm when compared with the negative control.
Determination of maternal iron status
Ferritin was used as a measure of Fe stores and serum soluble transferrin receptor (sTfR) was used as an indicator of Fe deficiency; both are more sensitive indicators of Fe status than dietary Fe intake or Hb concentration( Reference Rigas, Sørensen and Pedersen 21 , Reference Cogswell, Parvanta and Ickes 22 ). Maternal blood samples were collected at recruitment (11 weeks of gestation) and at delivery. As financial considerations only permitted analysis of a limited number of blood samples, the analysis was restricted to mother–child pairs with complete datasets during pregnancy (blood samples and fetal scans) and up to 10 years of age (questionnaire data at 1, 2, 5 and 10 years and spirometric measurement data at 5 and 10 years). Maternal serum ferritin and sTfR concentrations were quantified using ELISA (DE1872; Demeditec Diagnostics and Human sTfR Quantikine IVD, DTFR; R&D Systems, respectively). In both assays, 20 μl of serum were used and samples were run in duplicate; Western blotting confirmed negligible protein degradation during storage at − 80°C. All plates contained both intra- and inter-plate quality controls and were assayed according to the manufacturers’ instructions. The sTfR:log(ferritin) ratio (TfR-F index) was calculated as described by Cook et al. ( Reference Cook, Finch and Smith 23 ). Decreasing ferritin concentrations and increasing sTfR concentrations and TfR-F index are evidence of declining Fe status.
Statistical analyses
Differences in enrolment characteristics between women with serum Fe measurement data and those without these data were analysed using the χ2 test. Student's t tests and ANOVA were used to relate maternal serum Fe status to maternal enrolment characteristics. The exposures of interest were unit increases in maternal serum ferritin concentrations, sTfR concentrations and the TfR-F index at 11 weeks of gestation and at delivery. Maternal dietary Fe and Fe supplement intake values were summated and energy was adjusted using the residual method( Reference Willett, Howe and Kushi 24 ) and divided into thirds. Linear regression was used to model the associations between maternal Fe intake, serum Fe status at recruitment and at delivery, and first- and second-trimester fetal measurements and birth anthropometry. The main outcomes were the longitudinal development of wheeze, ‘doctor-diagnosed asthma, atopic eczema, and hay fever’, atopic sensitisation and lung function (peak expiratory flow (PEF), forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC)) up to 10 years of age. The associations between maternal Fe intake and serum Fe status and the development of wheezing symptoms and lung function were investigated using generalised estimating equations with an exchangeable correlation structure. The associations between maternal Fe intake and serum Fe status and the development of asthma, atopic eczema, hay fever and atopic sensitisation were modelled using discrete-time hazard models. Unadjusted and adjusted results were computed for all analyses. The adjusted results included maternal smoking status, atopic status, age and socio-economic status (Scottish Index of Multiple Deprivation) and child sex and gestational age at birth. These potential confounding variables were chosen based on conceptual evidence and by statistical tests (i.e. if the variables achieved P< 0·25 in a univariate association with any of the endpoints)( Reference Nurmatov, Nwaru and Devereux 25 ). Interaction terms between serum Fe status and time (i.e. age when outcomes were ascertained) in relation to wheeze as well as lung function endpoints were included in the models to investigate whether the influence of maternal serum Fe status on the outcomes was time dependent. Bonferroni adjustment was used for the correction of multiple testing. The analyses were performed using Stata 11 (Stata Statistical Software: Release 11; StataCorp LP).
Results
Of the 1924 women with a singleton birth, serum Fe measurement data were available for 157 women at 11 weeks of gestation and at delivery. The mean concentrations of serum ferritin and sTfR at 11 weeks of gestation were 28·4 (sd 43·4) ng/ml and 12·0 (sd 3·0) nmol/l, respectively; the corresponding values at delivery were 10·2 (sd 19·2) ng/ml and 18·8 (sd 17·6) nmol/l, respectively. Women without serum Fe measurement data were more likely to smoke, were younger, and were of lower socio-economic status when compared with women with Fe measurement data (Table 1). Maternal smoking status and atopic status were not associated with serum ferritin concentrations, sTfR concentrations and TfR-F index at 11 weeks of gestation and at delivery (Table 1). There was no difference in dietary Fe intake (13·9 (sd 4·98) v. 13·6 (sd 3·77) mg/d) or Fe supplement use (37 v. 41 %, P= 0·33) in women with or without Fe measurement data. Maternal total Fe intake quantified at 32 weeks of gestation was not associated with serum Fe indices at 11 weeks of gestation, but was weakly associated with maternal serum Fe indices at delivery (Spearman's ρ for ferritin 0·27, P= 0·002; sTfR − 0·29, P< 0·001; TfR-F − 0·29, P= 0·001; and Hb 0·18, P= 0·023). At 32 weeks of gestation, fifty-eight (37 %) women were found to be taking Fe supplements. When compared with women not using Fe supplements, in women using Fe supplements, Fe supplement use was associated with reduced Fe status at recruitment (11 weeks of gestation), but with increased Fe status at delivery (Fig. 1).
Table 1 Maternal serum ferritin concentrations at recruitment (11 weeks of gestation) and at delivery by maternal and neonatal characteristics (Mean values and standard deviations; number of children and percentages)

SIMD, Scottish Index of Multiple Deprivation.
* P value for Pearson's χ2 test.
† The t test for covariates with two categories and ANOVA for covariates with more than two categories.

Fig. 1 Iron status (soluble transferrin receptor, Hb and ferritin) of women using (●) and not using (○) iron supplements. sTfR, soluble transferrin receptor.
Maternal serum Fe status (ferritin, sTfR and sTfR-F index) at 11 weeks of gestation was not significantly associated with any of the first- or second-trimester ultrasound measurements or with birth measurements (Table 2).
Table 2 Association between maternal serum iron status at 11 weeks of gestation and fetal ultrasound measurements at 11 and 20 weeks of gestation and birth measurements (Adjusted and unadjusted coefficients and 95 % confidence intervals)

F, ferritin; sTfR, soluble transferrin receptor; CRL, crown–rump length; BPD, biparietal diameter.
* CRL adjusted for gestational age at recruitment; BPD adjusted for gestational age at second visit; and child's measurements at birth adjusted for gestational age at birth.
† All outcomes adjusted for maternal smoking status during pregnancy, birth order, maternal age, maternal Scottish Index of Multiple Deprivation, maternal atopic status, gestational age at birth, and sex of child. CRL also adjusted for gestational age at dating scan; BPD also adjusted for gestational age at second scan; outcomes at birth also adjusted for gestational age at birth, mode of delivery and sex of child.
‡ Analysed using linear regression models and based on the standardised values of the child's measurements.
The prevalence of wheeze, asthma, eczema, hay fever and atopic sensitisation at 10 years of age is summarised in Table 3.
Table 3 Prevalence of asthma and atopic outcomes in the 157 study children at 10 years of age (Number of children and percentages)
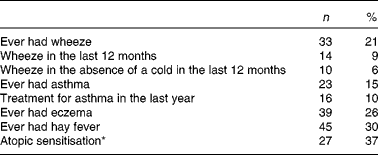
* Skin-prick testing was done in seventy-three children.
Maternal dietary Fe intake (including and excluding Fe supplement intake) and Fe supplement use at 32 weeks of gestation were not associated with any fetal or birth measurement or with asthma, wheeze, lung function or atopic outcomes up to 10 years of age (data not shown).
Longitudinal associations with maternal serum iron status at 11 weeks of gestation
In unadjusted and adjusted models, maternal serum ferritin concentrations at 11 weeks of gestation were found to be not associated with wheeze, hay fever, atopy, atopic eczema and asthma endpoints (Table 4). A unit increase in maternal serum ferritin concentrations at 11 weeks of gestation was associated with increased standardised lung function measurements (PEF, FEV1 and FVC) up to 10 years of age. In models that included interaction terms, a significant positive interaction was found between maternal serum ferritin concentrations at 11 weeks of gestation and time (age when children's lung function was measured) in relation to lung function measurements (Table 4). In adjusted models, a unit increase in maternal sTfR concentrations (i.e. reduced Fe status) at 11 weeks of gestation was found to be associated with an increased OR of ‘wheeze in the past year’ and ‘wheeze without cold in the past year’, but no association was found with ‘doctor-diagnosed asthma, hay fever, and eczema’, atopic sensitisation or lung function. In adjusted models, increasing sTfR-F index (i.e. reduced Fe status) was found to be associated with an increased risk of ‘wheeze in the past year’ and ‘wheeze without cold in the last year’, but no association was found with any other outcome (Table 4). After Bonferroni adjustment for multiple testing, the association between maternal first-trimester serum ferritin concentrations and childhood FEV1 remained significant. Dichotomisation of women into those with and without sufficient serum ferritin concentrations ( ≥ 15 and < 15 μg/ml) and Hb concentrations ( ≥ 105 and < 105 g/l; ≥ 10·5 and < 10·5 g/dl) at 11 weeks of gestation was performed and their associations with childhood outcomes were examined (online supplementary Tables S1 and S2). There were no significant associations between dichotomised Fe status and childhood outcomes; moreover, we lacked sufficient power to detect statistically meaningful effect estimates or undertake any confounder adjustments.
Table 4 Association between maternal serum iron status at 11 weeks of gestation and longitudinal development of asthma and atopic outcomes up to 10 years of age (Odds ratios and 95 % confidence intervals)

F, ferritin; sTfR, soluble transferrin receptor; PEF, peak expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
* Adjusted for maternal smoking status during pregnancy, birth order, maternal age, maternal Scottish Index of Multiple Deprivation, maternal atopic status, gestational age at birth and sex of child.
† Analysed using generalised estimating equations with an exchangeable correlation structure.
‡ Analysed using discrete-time hazard models.
§ Hence, no interaction with time evaluated.
∥ Standardised z-score values of lung function measurements used in the analyses.
Longitudinal associations with maternal serum iron status at delivery
Maternal serum ferritin and sTfR concentrations at delivery were not associated with any of the wheeze, asthma, atopic or lung function parameters measured up to 10 years of age (Table 5). Increasing sTfR-F index at delivery was associated with an increased risk of atopic sensitisation (P= 0·037); an increased risk of ‘doctor-diagnosed eczema’ was of borderline significance (P= 0·068) after adjustment for confounders. Further analyses with dichotomised maternal serum ferritin and Hb concentrations revealed no significant associations with childhood outcomes (online supplementary Tables S1 and S2).
Table 5 Association between serum iron status at delivery and longitudinal development of asthma and atopic outcomes up to 10 years of age (Odds ratios and 95 % confidence intervals)

F, ferritin; sTfR, soluble transferrin receptor; PEF, peak expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
* Adjusted for maternal smoking status during pregnancy, birth order, maternal age, maternal Scottish Index of Multiple Deprivation, maternal atopic status, gestational age at birth and sex of child.
† Analysed using generalised estimating equations with an exchangeable correlation structure.
‡ Analysed using discrete-time hazard models.
§ Hence no interaction with time was performed.
∥ Standardised z-score values of lung function measurements used in the analyses.
Discussion
In the present exploratory study, no association was found between first-trimester maternal Fe status and fetal ultrasound and birth measurements. However, we report for the first time that decreasing first-trimester maternal serum ferritin concentrations (i.e. reduced Fe stores) are associated with reduced FEV1, FVC and PEF in children up to 10 years of age and that increasing maternal serum sTfR concentrations and TfR-F index (i.e. Fe deficiency) are associated with an increased risk of childhood wheeze up to 10 years of age. A significant positive interaction was found between first-trimester maternal ferritin status and children's age, suggesting that the magnitude of the adverse effect of low maternal Fe status during pregnancy increases as children grow. In addition, an adverse association was observed between reduced maternal Fe status at delivery (increasing sTfR-F index) and an increased risk of atopic sensitisation in children.
The associations found in the present study are consistent with the notion that maternal Fe status during pregnancy influences fetal lung development such that children born to mothers with lower Fe status are established on a suboptimal lung developmental trajectory characterised by reduced lung function during the first decade of life. The associations found in the present study between suboptimal maternal Fe status and lung function and wheeze are consistent with the results of longitudinal studies that have tracked neonatal lung function and shown that children established on a suboptimal lung growth trajectory are more likely to wheeze( Reference Stern, Morgan and Wright 26 ). The lack of an association with maternal Fe intake and Hb concentrations is not surprising, as it is well established that neither is a good indicator of Fe status.
Fetal lung development occurs in a relatively hypoxic environment, and in animal models, Fe has been found to be involved in airway development. Chelation of Fe by desferrioxamine in ex vivo lung buds from embryonic day mice has been found to reduce the vascular network surrounding the developing lung buds and to reduce epithelial branching( Reference Groenman, Rutter and Wang 27 ). FeCl3 reverses the inhibitory effects of chelation. This animal study has suggested that Fe influences airway development through regulatory effects on vascular endothelial growth factor signalling and up-regulation of hypoxia-inducible factor-1α. It also supports findings from the present study that suboptimal Fe status adversely affects fetal airway development. Women given Fe supplements during pregnancy have a lower risk of preterm birth and give birth to larger babies( Reference Cogswell, Parvanta and Ickes 22 ). In addition, first-trimester Fe intake is also positively associated with birth outcome( Reference Scholl and Reilly 28 ). Intake later in pregnancy does not exhibit this association( Reference Alwan, Greenwood and Simpson 29 ). These results are consistent with the importance of Fe in the regulation of differentiation rather than in proliferation. Although associations between maternal Fe status and childhood wheeze, lung function and atopic outcomes were found in the present study, associations with fetal measurements or birth anthropometry could not be demonstrated; this may be a consequence of a small study sample size.
We are aware of one study that has investigated fetal Fe status in relation to childhood wheeze, asthma and atopic outcomes. In the ALSPAC, umbilical cord Fe concentration was found to be inversely associated with childhood wheeze up to 42 months of age( Reference Shaheen, Newson and Henderson 14 ). Although the study concluded that fetal exposure to Fe might possibly influence the risk of wheezing in early childhood, caution was advised because of the multiple analyses conducted. The associations between maternal Fe status and childhood wheeze observed in the present study are consistent with those reported by the ALSPAC( Reference Shaheen, Newson and Henderson 14 ), and the association between maternal first-trimester serum ferritin concentrations and FEV1 remained significant after Bonferroni adjustment. In the present study pregnant women who were Fe deficient appeared to have been more likely to use Fe supplements, leading to improved Fe status at delivery; however, we were unable to find any association between Fe supplementation and childhood outcomes, probably because only fifty-eight of the 157 women took a variety of Fe supplements during pregnancy.
A further finding of the ALSPAC was an inverse association between umbilical cord Fe status and childhood eczema up to 30 months of age, but was qualified because of the multiple analyses performed. In the present study, a borderline significant association was observed between low maternal Fe status at delivery (sTfR-F index) and ‘doctor-diagnosed eczema’ in the first decade of life. Although the differences in significance between the present study and the ALSPAC may reflect differences in sample size, in the present study, maternal Fe status at delivery (sTfR-F index) was inversely associated with childhood atopic sensitisation. Although the associations between maternal serum Fe status and atopic outcomes observed in the present study are consistent with those reported in the ALSPAC, adjustment for multiple testing resulted in the associations becoming non-significant in the ALSPAC.
The results of the present study suggest differential associations between maternal serum Fe status at pregnancy and childhood outcomes; while first-trimester Fe status was associated with childhood lung function and wheeze, maternal serum Fe status at delivery was associated with childhood atopic outcomes. This suggests that maternal Fe status at delivery possibly influences the first critical interactions between the infant's immune system and allergens that certainly commence soon after birth (if not earlier). In animal models, Fe has been found to promote T-helper-cell differentiation away from the Th2 phenotype. In murine models of airway eosinophilia, parenteral Fe supplementation has been found to be associated with reduced airway eosinophilia, airway hyper-responsiveness and reduced lung tissue IL-4, IL-5 and IL-13 concentrations( Reference Brion, Leary and Smith 30 , Reference Maazi, Shirinbak and Bloksma 31 ). The timing of any putative Fe supplementation is likely to be critically important in correcting deficiency-induced effects. Data showing that Fe deficiency-induced changes in the brain can be reversed by early, but not later, supplementation have been obtained from human subjects and monkeys( Reference Hale, Potts Kant and Greer 32 – Reference Golub, Hogrefe and Unger 36 ).
Several disparities are evident in the present study. A possible explanation for the absence of an association between maternal Fe intake and childhood outcomes is that the data on serum parameters most notably associated with outcomes were obtained at 11 weeks of gestation, whereas maternal dietary intake was quantified once at 32 weeks of gestation, and although women were asked to report their dietary intake in the previous 3 months, it is not possible to assume that Fe intake at 32 weeks of gestation is the same as that at the first trimester. Indeed, although maternal Fe intake at 32 weeks of gestation was correlated with maternal serum ferritin and sTfR concentrations at delivery, there were no correlations with serum Fe parameters at 11 weeks of gestation. In the UK population, the prevalence of Fe-deficiency anaemia is estimated to be about 0–6 %( 37 ), although our own data from a cohort in Aberdeen suggest that the prevalence Fe deficiency may be much higher( Reference Fosset, McGaw and Abramovich 38 ). The observed association of sTfR concentrations with wheeze, but not with asthma, is likely to be a result of insufficient power to detect an association with asthma as substantially more children wheezed than had asthma. The differential associations between the parameters of Fe status (ferritin and sTfR) and childhood outcomes (lung function and wheeze/atopy) may be a consequence of Fe potentially influencing both fetal lung growth and immune responses. The consequence of low fetal tissue Fe stores (ferritin) by adversely affecting fetal lung growth is likely to be impaired lung function, whereas Fe deficiency (sTfR) by influencing immune differentiation and airway inflammation is likely to manifest as wheezing and atopic sensitisation.
The present study has a number of strengths and limitations. As this is an observational study, we cannot demonstrate causality. Even though adjustment for many potentially confounding factors was done, we cannot exclude the possibility of residual confounding by other unmeasured factors, although further analyses adjusting for alternative metrics of socio-economic status, e.g. parameters of maternal education, father's occupation-based social class, and maternal dietary intakes of vitamin E, vitamin D, Zn and Se, did not materially alter the reported associations. In the present study, maternal smoking status was found to be not significantly associated with Fe status; however, adjustment for maternal smoking status was done because there is some evidence that maternal smoking status is associated with increased cord blood Hb concentrations, sTfR concentrations, and sTfR-F index and decreased ferritin concentrations( Reference Sweet, Savage and Tubman 39 , Reference Chelchowska and Laskowska-Klita 40 ). One of the strengths of the present study is that maternal Fe status was quantified at 11 weeks of gestation and at delivery using several parameters. Traditionally, maternal Fe status is inferred from Hb concentration and haematocrit measurement. These are not good indicators of Fe status, and more recently, serum ferritin concentration has been used as an indicator, as it reflects liver Fe stores( Reference Cook, Finch and Smith 23 , Reference Sweet, Savage and Tubman 39 ). However, ferritin concentrations can increase as a result of inflammation and hence can give rise to falsely high values. Serum transferrin receptor concentrations increase during Fe deficiency, and the ratio of these two parameters gives the best estimate of Fe status( Reference Cook, Finch and Smith 23 , Reference Sweet, Savage and Tubman 39 ). Another strength of the present study is that the original cohort study was established to prospectively investigate the associations between maternal diet during pregnancy and childhood asthma and atopic disease and comprised healthy women recruited during pregnancy irrespective of asthma/atopy and Fe/Hb status. However, the present study sample is a subpopulation of the original cohort because financial considerations resulted in the number of mother–child pairs studied being small and the analysis being limited to those mother–child pairs with complete datasets during pregnancy and in the first 10 years of life. Women with Fe measurement data differed from those without these data, with regard to smoking status, age and socio-economic status, but not with regard to Fe supplement use, and at 10 years of age, children with Fe measurement data were found to less likely wheeze when compared with those without these data (9·0 v. 12·5 %), although these differences were non-significant. These differences suggest that women without Fe measurement data were at a greater risk of developing Fe deficiency and their children more likely to wheeze. Such findings would bias any association between Fe and wheeze towards the null and the reported associations may lead to the underestimation of the associations between maternal Fe status and childhood outcomes.
In summary, this small nested cohort study is the first to demonstrate inverse associations between first-trimester serum Fe status and childhood wheeze and lung function. Inverse associations were found between maternal serum Fe status at delivery and childhood atopic outcomes consistent with those reported previously by the only study on maternal Fe status during pregnancy and childhood asthma and atopic outcomes. Because Fe replacement during pregnancy is relatively straightforward, the associations between maternal Fe status during pregnancy and childhood asthma and atopic disease warrant further investigation. In the first instance, the associations found in the present study and the ALSPAC require further replication in a larger prospective cohort study. It may be possible to follow up children born to women recruited to intervention studies of Fe replacement during pregnancy. Ultimately, a double-blind randomised controlled trial of Fe replacement during pregnancy with long-term follow-up of children will be required.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514003122
Acknowledgements
The present study was funded by Asthma UK (00/011, 02/017), the Medical Research Council (80219), and the University of Aberdeen's Development Trust.
The authors’ contributions are as follows: K. A., N. P., H. H. and L. G. were involved in data acquisition and B. I. N. and G. D. performed the data analysis. All authors contributed to either the original or the present study hypothesis, the conception and design of the birth cohort, and/or the design and conduct of the cohort follow-up as well as to the writing and revision of this article.
None of the authors has any conflicts of interest to declare.








