Introduction
Cervical cancer is one of the most common malignancies in women [Reference Torre1], and its incidence in China in 2015 was 9.89% in 2015 [Reference Chen2]. Radical hysterectomy, chemotherapy, radiotherapy and concurrent chemo-radiotherapy are some of the treatment options available for cervical cancer [Reference Somashekhar and Ashwin3]. Despite the variety of treatment options, the tumour recurrence rate varies from 10% to 50%, and the 5-year survival rate is approximately 65% depending on the disease stage [Reference Carneiro4]. Cervical cancer screening to detect early-stage lesions has led to dramatic reductions in the incidence and mortality of cervical cancer [Reference Landy5].
Human papillomavirus (HPV) is established as an essential pathogenic factor in the development of cervical cancer [Reference Bouvard6, Reference de Sanjosé, Brotons and Pavón7]. Screening tests for HPV infection generally include 13 high-risk HPV (HR-HPV) genotypes (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) [Reference Poljak and Kocjan8–Reference Kaliamurthi12]. Among these genotypes, HPV-16, 18, 31, 33, 45, 52 and 58 are thought to be responsible for more than 90% of cases of cervical cancer [Reference Bosch13–Reference de Sanjosé16]. The latest US cervical cancer screening guideline recommends a screening scheme based on age, with no screening recommended for women aged <21 or >65 years, cytology alone every 3 years for women aged 21–29 years, and the combination of cytology with HR-HPV testing for women aged 30–65 years [Reference Saslow17]. However, the implementation of cervical cancer screening and recommended guidelines vary between different geographical regions due to differences in factors such as population and economic conditions [Reference Chrysostomou18–Reference Kaliamurthi20]. Furthermore, geographic variations are observed in the HPV genotype distribution [Reference Husain and Ramakrishnan21].
Data regarding HPV genotype distribution in women with HPV infection in the Sichuan area of China are limited. Therefore, the present retrospective analysis attempted to evaluate the HPV genotype distribution and association between the HPV genotype and cervical cytology findings in women in the Sichuan Province, China to provide new insights into the distribution of HPV genotypes in this region and clarify the relationship between HPV genotype and cervical cytology.
Methods
Study design and study participants
The present retrospective analysis was conducted in 1089 female outpatients with a positive HPV genotyping test result who underwent a cervical cytology test at the gynaecological clinic of the West China Second Hospital of Sichuan University (Chengdu, Sichuan, China) between January 2014 and December 2016. The inclusion criteria were: (1) female aged 15–74 years; (2) HPV genotyping and cervical cytology tests were performed at the same time; and (3) a positive result was obtained for the HPV genotyping test. The exclusion criteria were: (1) previous surgical treatment of cervical cancer; (2) immune system diseases; (3) chronic wasting diseases; and (4) previous organ transplantation.
Ethics
This study was approved by the Ethics Committee of West China Second Hospital of Sichuan University. All patients provided informed consent for cervical cytology and HPV testing. Consent for inclusion in the study was waived by our institution's ethics committee because the analysis was retrospective and anonymised.
Cervical cytology
All procedures were performed in accordance with the Cytology Room Work Regulations of our hospital in clinical laboratories with ISO15189 and the College of American Pathologists accreditation. Patients were asked not to rinse the vagina, administer vaginal drugs or have sexual intercourse for 3 days before the test. Samples of the exfoliated cervical cells were collected by trained and experienced specialists. The cervix was exposed with a disposable colposcope, the cervix mouth and surrounding secretion was wiped off, the cervical sample plate was used to rotate 360 degrees at the cervical orifice, and the exfoliated cells were immediately smeared on to a clean slide. The slide was then placed in a specimen bottle containing 95% ethanol. Papanicolaou smear cytology was performed using Papaniculaou stain, Gill's haematoxylin and EA-50 counterstain prepared in the cytology laboratory of our hospital using standardised procedures. The 2001 Bethesda System [Reference Nayar and Wilbur22] was used to report the cervical cytological diagnosis using the following terminology: (1) atypical squamous cells (ASC), including ASC of undetermined significance (ASC-US) and ASC-cannot exclude high-grade squamous intraepithelial lesion (ASC-H); (2) low-grade squamous intraepithelial lesion (LSIL); (3) high-grade squamous intraepithelial lesion (HSIL); (4) squamous cell carcinoma (SCC); (5) atypical glandular cells (AGC); (6) adenocarcinoma in situ (AIS); (7) endocervical adenocarcinoma (Endo-CA); (8) endometrial adenocarcinoma (Endo-MA); (9) extrauterine adenocarcinoma (Extr-AA); (10) unclassified cancer cells (UCC); or (11) negative for intraepithelial lesion or malignancy.
HPV detection
Multiplex polymerase chain reaction (PCR) with universal primers was used together with flow cytometric detection of in situ hybridisation to simultaneously detect 26 HPV types. Sample collection was performed using a dedicated cervical exfoliated cell collector (Shanghai Toujing Co. Ltd, Shanghai, China). The cervix was exposed with a disposable colposcope, and the sampling brush was inserted into the cervical mouth, rotated thrice in the same direction and stay for 10 s. Then, the cervical brush containing the secretion was removed from the cervix and then inserted into a cell preservation solution. The collected samples were then subjected to nucleic acid extraction, amplification and hybridisation using multiple HPV gene typing kits. The PCR hybridisation products were analysed using a bead-based multiplexed immunoassay system in a microplate format (Luminex xMAP technology with a Luminex Liquid Chip 200 flow cytometer; Luminex Corp., Austin, TX, USA). A total of 26 HPV subtypes were identified as being closely related to cervical cancer development, including 13 high-risk types (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), six intermediate-risk types (HPV-26, 53, 55, 66, 82 and 83) and seven low-risk types (HPV-6, 11, 40, 42, 44, 61 and 73) recognised by the World Health Organization International Agency for Research on Cancer. The HPV subtype corresponding to the probe was considered to be positive if the signal corresponding to the type-specific probe was >150.
Data collection
Medical records were used to extract relevant patient clinical information such as age, cervical cytology findings and HPV genotype.
Statistical analysis
A descriptive statistical method was adopted for categorical data. The correlations between the various cervical cytological diagnoses and HPV genotypes were analysed using the χ 2 test (sample size >40 and minimal theoretical frequency >5), χ 2 test with correction for continuity (sample size >40 and minimal theoretical frequency ⩾1 and <5) or Fisher's exact test (if the conditions for the χ 2 test were not satisfied). A P value of <0.05 was considered statistically significant. All data were analysed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
HPV genotype distribution
HPV genotyping was performed in 4517 outpatients, of which 1089 (24.1%) were positive for HPV infection. A total of 1368 HPV genotypes were detected (Fig. 1), of which 1145 (83.70%) were high-risk type. The genotypes detected most frequently were HPV-52 (18.64%), HPV-16 (16.59%), HPV-58 (13.23%), HPV-18 (6.80%), HPV-56 (5.56%) and HPV-59 (5.56%), all of which are considered high-risk subtypes (Fig. 1). HPV-53 (1.75%) and HPV-61 (3.44%) were the most common intermediate-risk and low-risk genotypes, respectively (Fig. 1). Table 1 presented the frequency of each HPV genotype according to age. The difference in the HPV genotype distribution between age groups was statistically non-significant.
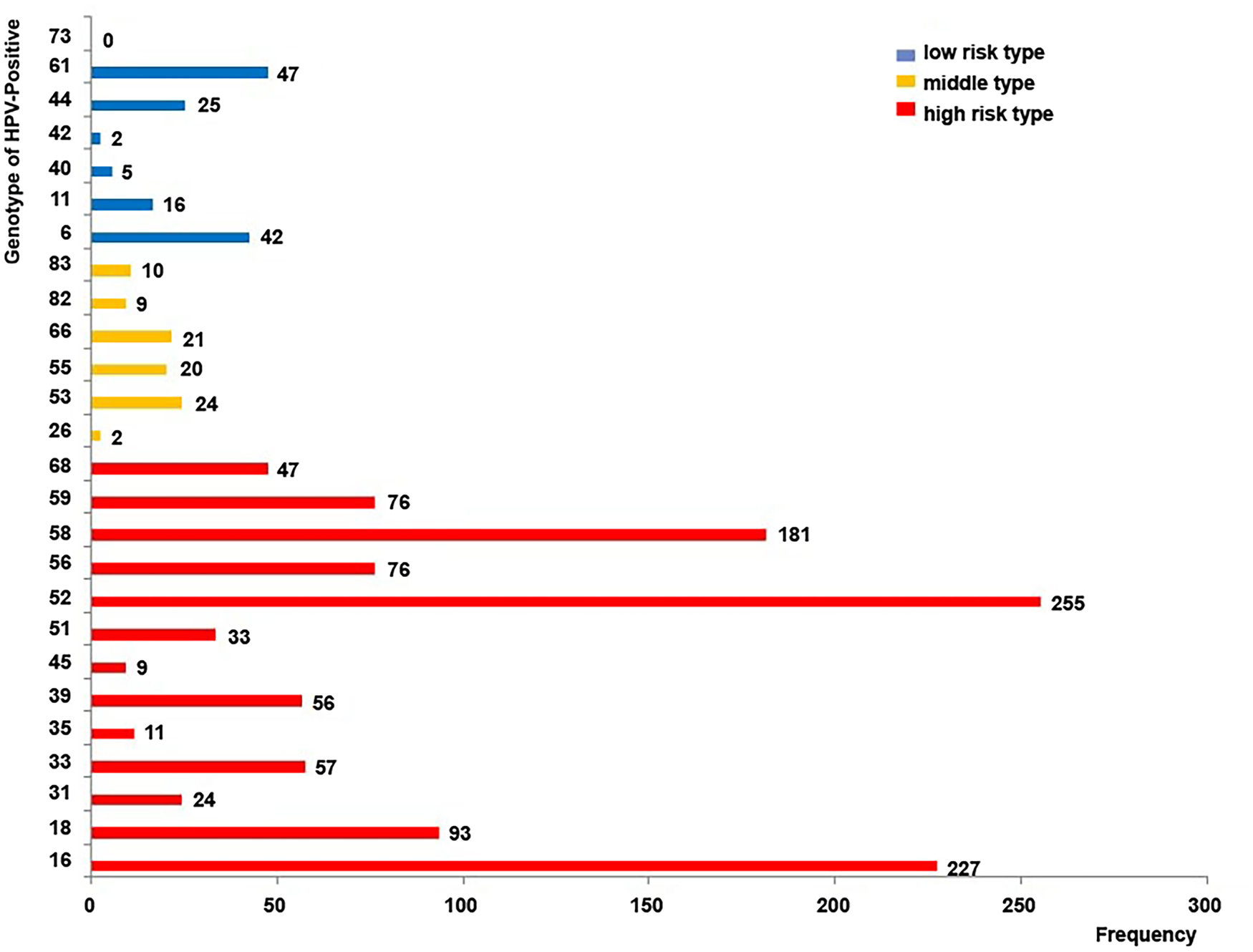
Fig. 1. Human papillomavirus (HPV) genotype distribution in 1089 women who tested positive for infection. The numerical value next to each bar illustrates the number of cases in which the genotype was detected. High-risk types: HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68; intermediate-risk types: HPV-26, 53, 55, 66, 82 and 83; low-risk types: HPV-6, 11, 40, 42, 44, 61 and 73.
Table 1. Human papillomavirus (HPV) genotype frequency stratified by age

Data are presented as the number of patients in that age group positive for the HPV genotype (% of patients in that age group positive for the HPV genotype). Note that the percentage values for each row total >100% since some of the patients were positive for more than one genotype.
Of the 1089 patients who tested positive for HPV infection, 869 (79.80%) were infected by a single HPV type (Fig. 2). High-risk genotypes accounted for 87.46% of the HPV infections in these 869 women, with the most common genotypes being HPV-52 (20.83%), HPV-16 (20.71%), HPV-58 (14.61%), HPV-18 (5.98%), HPV-59 (5.41%) and HPV-56 (4.03%) (Fig. 2). Of the 220 patients who tested positive for multiple (2–5) genotypes (Fig. 3), 172 (78.2%) were infected with two types, 39 (17.7%) were infected with three types, and nine (4.1%) were infected with four or five types of HPV. Therefore, the average infection rate was 2.27 genotypes/patient. High-risk genotypes accounted for 77.15% of the HPV types in patients with multiple infections, and the most common genotypes were HPV-52 (14.83%), HPV-58 (10.82%), HPV-16 (9.42%), HPV-18 (8.22%), HPV-56 (8.22%) and HPV-59 (5.81%) (Fig. 3).

Fig. 2. Human papillomavirus (HPV) genotype distribution in 869 women who tested positive for a single HPV genotype. The numerical value next to each bar illustrates the number of cases in which the genotype was detected. High-risk types: HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68; intermediate-risk types: HPV-26, 53, 55, 66, 82 and 83; low-risk types: HPV-6, 11, 40, 42, 44, 61 and 73.

Fig. 3. Human papillomavirus (HPV) genotype distribution in 220 women who tested positive for multiple HPV genotypes. The numerical value next to each bar illustrates the number of cases in which the genotype was detected. High-risk types: HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68; intermediate-risk types: HPV-26, 53, 55, 66, 82 and 83; low-risk types: HPV-6, 11, 40, 42, 44, 61 and 73.
Cervical cytology findings
Of the 1089 patients with HPV infection, cervical cytology revealed abnormal cells in 430 (39.49%) patients. Of the 430 patients, 384 (89.30%) were diagnosed with one type of abnormality, 45 (10.47%) were diagnosed with two types of abnormality, and one (0.23%) was diagnosed with three types of abnormality. The abnormalities detected in the 430 patients with positive cytology findings were ASC-US (236 cases, 54.88%), LSIL (151 cases, 35.12%), HSIL (63 cases, 14.65%), AGC (21 cases, 4.88%), ASC-H (10 cases, 2.33%) and UCC (four cases, 0.93%). Therefore, HSIL was observed in 5.79% of all patients with HPV infection. SCC, AIS, Endo-CA, Endo-MA and Extr-AA were not observed in any patients. Table 2 presented the cervical cytology diagnoses stratified by age.
Table 2. Cervical cytological diagnoses stratified by age

Data are presented as the number of patients in that age group positive for the cervical cytology diagnosis (% of patients in that age group positive for the cervical cytology diagnosis). Note that the percentage values for each row total to >100% as some of the patients exhibit more than one type of cervical cytological abnormality. AGC, atypical glandular cells; ASC-H, atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; UCC, unclassified cancer cells.
Associations between cervical cytology diagnoses and HPV subtypes
The associations between cervical cytology diagnoses and HPV genotypes are presented in Table 3. HPV-66 (continuity-corrected χ 2 = 4.35, P = 0.037) was significantly associated with ASC. HPV-52 (χ 2 = 6.85, P = 0.009) and HPV-56 (χ 2 = 4.95, P = 0.026) were significantly associated with LSIL. HPV-16 (χ 2 = 36.35, P < 0.001), HPV-33 (continuity-corrected χ 2 = 6.00, P = 0.014) and HPV-58 (χ 2 = 8.84, P = 0.003) were significantly associated with HSIL, and when each of these genotypes was detected, the rate of HSIL diagnosis was 14.09%, 14.04% and 10.50%, respectively. HPV-16 (continuity-corrected χ 2 = 7.72, P = 0.005) was also significantly associated with AGC (Table 4).
Table 3. HPV type prevalence with abnormal cervical cytology

HPV genotype attributable fraction in the total of 340 HPV and cytology both positive patients.
HPV, human papillomavirus; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot rule out HSIL; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; AGC, atypical glandular cell. HR-HPVs include HPV16, 18, 31, 33, 35, 39, 45, 51,52, 56, 58, 59, 66 and 6 putative high-risk types (26, 53, 55, 68, 82 and 83), LR-HPVs include HPV 6, 11, 40, 42, 44, 61 and 73.
Seven cases of AGC complicated with ASCUS, three cases of AGC complicated with ASC, and 11 cases of AGC complicated with HSIL.
Table 4. Associations between cervical cytology diagnoses and human papillomavirus (HPV) genotypes

AGC, atypical glandular cells; ASC, atypical squamous cells; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Discussion
Cervical cancer is the third most common cancer in women worldwide [Reference Arbyn23]. Studies have reported a 9.6/100 000 morbidity rate and a 4.3/100 000 mortality rate of cervical cancer in China. The mortality has increased in lower socioeconomic areas [Reference Ferlay24, Reference Yang25].
HR-HPV infection is recognised as a significant factor for cervical cancer pathogenesis. Over 150 HPV subtypes have been identified currently, with 40 possessing the ability to cause a reproductive tract infection [Reference de Villiers26, Reference Bernard27]. The present study identified HPV-52, HPV-16, HPV-58, HPV-18, HPV-56 and HPV-59 as the six most common HPV subtypes in 1089 women with HPV infection in the Sichuan Province. Studies have identified HPV-52, HPV-16, HPV-58 and HPV-18 among the seven HPV genotypes responsible for the vast majority of cervical cancer cases worldwide [Reference Bosch13–Reference de Sanjosé16]. This finding is mirrored in the present study, too. However, the present study identified HPV-56 and HPV-59 as the fifth and sixth most common subtypes. This finding differed from other studies, suggesting that the distribution of HPV genotypes in patients differed from those reported for other regions of China or other countries. This finding is concurrent with other studies demonstrating geographic variation in the distribution of HPV genotypes associated with cervical cancer [Reference Husain and Ramakrishnan21].
The present study demonstrated a high rate of infection with HPV-58, which is consistent with other reports from other areas in China [Reference Cai, Ding and Chen28–Reference Chao31]. However, regional disparities in HPV subtype distribution exist [Reference Cai, Ding and Chen28–Reference Chao31]. The infection rates with HPV-31 and HPV-33 in the present study were lower for our cohort than for those in European and American countries [Reference Crow32]. The present study identified HPV-45 as the least common of all the HR-HPV subtypes. This finding is in contrast with the study by de Sanjosé et al., who reported HPV-45 as the third most common subtype detected in association with cervical cancer, accounting for 12% of HPV subtypes in women with cervical adenocarcinoma [Reference de Sanjosé16].
Studies conducted outside of China have suggested that HPV-18 is the second most carcinogenic genotype among the HPV subtypes and that infection with HPV-18 is observed in approximately 10–15% of cervical cancer cases [Reference de Sanjosé16, Reference Walboomers33, Reference Muñoz34]. Furthermore, HPV-18 tends to be associated more with SCC than with adenocarcinoma of the cervix. This finding is in contrast with the findings of the present study, which indicated that HPV-18 was not associated with any particular type of cervical cytological abnormality. Li et al. observed that the HPV-16 and HPV-58 infection rates among females in western China were higher than those in other areas of the world [Reference Li35, Reference Li36]. Moreover, a meta-analysis based on the global HPV subtype distribution observed East Asian countries such as China, Korea and Japan exhibited higher HPV-52 and HPV-58 infection rates in association with HSIL and ICC than African countries [Reference Chan30, Reference Ding37, Reference Asato38]. The HPV-52 and HPV-58 subtypes are highly distributed in many regions, identifying them as significant causes of local cervical lesions. Simultaneously, we also observed that 220 HPV-positive patients were positive for multiple genotypes, of which 211 (95.9%) were co-infected with types 2–3, and the average infection rate was 2.27 genotypes per patient. High-risk genotypes accounted for 77.15% of HPV types in patients with multiple infections, and HPV-52 and HPV-58 were the most commonly associated. Therefore, future research on HPV infection in local populations must consider these two subtypes.
The present study also observed that HPV-16 was associated with HSIL and AGC, which are considered high-grade lesions. Notably, AGC was only associated with HPV-16 infection, revealing that HPV-16 may be a particularly carcinogenic subtype among the various HPV genotypes. Since HPV infection could potentially be detected before the development of any cervical cytological abnormalities, testing for HPV infection must be performed and the genotype involved must be identified to prevent the development of cervical cancer. In particular, testing and immunisation strategies that target HR-HPV genotypes such as HPV-16 could effectively prevent the development of cervical cancer or allow early curative treatment.
The present study has some limitations. The single-centre design of the study prevents the generalisation of the findings to the entire region of Sichuan or Southwest China. Nevertheless, given that the study site is the largest maternity and children's hospital in the southwestern region of China, the patient population was from various regions of Sichuan Province, and the sample size was relatively large. Additionally, the retrospective design of the study exposes the results to information or selection bias.
In conclusion, the present study illustrates that HPV infection in the Sichuan region of China has specific characteristics that differ from those in other geographic regions. Furthermore, HPV-16, HPV-58 and HPV-52 were the major subtypes associated with cervical lesions identified by cytological tests. The findings of this study extend our knowledge of the epidemiology of HPV infection in Sichuan Province and hence provide a basis for the formulation of health policies aimed at controlling HPV infection locally. Additionally, the present study can serve as a reference in the planning and implementation of future large-scale, multicentre, prospective studies on the distribution of HPV genotypes.
Data
Data availability is not applicable to this article as no new data were created or analysed in this study.
Acknowledgements
The authors would like to thank all the study participants.
Financial support
Key R & D Projects of Science and Technology Department of Sichuan Province in 2020, 2020YFS0106.
Conflict of interest
None.










