Sleep is important for nearly all aspects of health in adolescence. Chronotype, a specific facet of sleep, refers to the “placement of sleep within the 24-hour day” (Buysse, Reference Buysse2014, p. 11) and reflects the body's internal clock, or the circadian rhythm. Chronotype is implicated in several domains of adolescent behavioral health, including internalizing and externalizing problems, inattention, substance use, and risk taking (Gau et al., Reference Gau, Shang, Merikangas, Chiu, Soong and Cheng2007; Haraden, Mullin, & Hankin, Reference Haraden, Mullin and Hankin2017; Haynie et al., Reference Haynie, Lewin, Luk, Lipsky, O'Brien, Iannotti and Simons-Morton2017; McGlinchey & Harvey, Reference McGlinchey and Harvey2015; Merikanto et al., Reference Merikanto, Pesonen, Kuula, Lahti, Heinonen, Kajantie and Räikkönen2017; Short, Gradisar, Lack, & Wright, Reference Short, Gradisar, Lack and Wright2013). The links between this transdiagnostic risk factor and adolescent health may be direct (Owens, Dearth-Wesley, Lewin, Gioia, & Whitaker, Reference Owens, Dearth-Wesley, Lewin, Gioia and Whitaker2016) and indirect via insufficient sleep, poorer sleep quality, and greater variation in sleep between weekdays and weekends (Roenneberg, Pilz, Zerbini, & Winnebeck, Reference Roenneberg, Pilz, Zerbini and Winnebeck2019; Russo, Bruni, Lucidi, Ferri, & Violani, Reference Russo, Bruni, Lucidi, Ferri and Violani2007; Tzischinsky & Shochat, Reference Tzischinsky and Shochat2011).
While often considered a stable trait that varies between individuals (Kuula et al., Reference Kuula, Pesonen, Merikanto, Gradisar, Lahti, Heinonen and Räikkönen2018; Roenneberg, Kumar, & Merrow, Reference Roenneberg, Kumar and Merrow2007), sleep chronotype is also shaped by time-varying factors such as development and environmental cues (Foley, Ram, Susman, & Weinraub, Reference Foley, Ram, Susman and Weinraub2018; Hoyt et al., Reference Hoyt, Deardorff, Marceau, Laurent, Windham, Greenspan and Hiatt2018; Roenneberg et al., Reference Roenneberg, Pilz, Zerbini and Winnebeck2019). Specifically, teenagers show relative delays in sleep chronotype, preferring to go to bed and wake later in comparison to children and adults (Hagenauer & Lee, Reference Hagenauer and Lee2012; LeBourgeois et al., Reference LeBourgeois, Hale, Chang, Akacem, Montgomery-Downs and Buxton2017). Pubertal development likely underlies the shift in sleep timing toward eveningness, especially among girls (Foley et al., Reference Foley, Ram, Susman and Weinraub2018; Hoyt et al., Reference Hoyt, Deardorff, Marceau, Laurent, Windham, Greenspan and Hiatt2018). However, sleep chronotype continues to delay past the midpoint of physical pubertal development, with peak eveningness reported to be around age 18 to 19 years (Fischer, Lombardi, Marucci-Wellman, & Roenneberg, Reference Fischer, Lombardi, Marucci-Wellman and Roenneberg2017). In addition to puberty, cognitive, behavioral, and social factors that change across adolescent development including greater levels of autonomy and the peer and family contexts may contribute to shifting sleep chronotype during adolescence. However, with an average school start time of 7:59 a.m., US youth often get fewer hours of sleep and experience greater misalignment between their biological and social clocks (Minges & Redeker, Reference Minges and Redeker2016; Roenneberg et al., Reference Roenneberg, Pilz, Zerbini and Winnebeck2019; Wheaton, Ferro, & Croft, Reference Wheaton, Ferro and Croft2015; Wittmann, Dinich, Merrow, & Roenneberg, Reference Wittmann, Dinich, Merrow and Roenneberg2006). Youth are likely to get fewer hours of sleep and incur more sleep debt during the week, and sleep more on weekends, leading to greater variability in sleep timing between scheduled and unscheduled days (Hansen, Janssen, Schiff, Zee, & Dubocovich, Reference Hansen, Janssen, Schiff, Zee and Dubocovich2005). While many adolescents report this shift toward eveningness, those who go to bed and wake even later than same-aged peers (i.e., relatively delayed sleep timing) especially suffer. They are at greater risk for behavioral health problems than morning-type peers (i.e., relatively advanced sleep timing; Gau et al., Reference Gau, Shang, Merikangas, Chiu, Soong and Cheng2007; Haynie et al., Reference Haynie, Lewin, Luk, Lipsky, O'Brien, Iannotti and Simons-Morton2017; McGlinchey & Harvey, Reference McGlinchey and Harvey2015; Merikanto et al., Reference Merikanto, Pesonen, Kuula, Lahti, Heinonen, Kajantie and Räikkönen2017; Short et al., Reference Short, Gradisar, Lack and Wright2013). Given its links to behavioral health, a better understanding of intraindividual and interindividual variability in sleep chronotype across development is needed.
Sleep chronotype is often approximated using one-time scales that assess subjective preferences to categorize individuals as morning or evening types (Adan & Almirall, Reference Adan and Almirall1991; Carskadon, Vieira, & Acebo, Reference Carskadon, Vieira and Acebo1993; Horne & Östberg, Reference Horne and Östberg1976; Werner, LeBourgeois, Geiger, & Jenni, Reference Werner, LeBourgeois, Geiger and Jenni2009). Preference scale scores are highly correlated with diary and actigraphy measures of sleep timing (Thun et al., Reference Thun, Bjorvatn, Osland, Martin Steen, Sivertsen, Johansen and Pallesen2012; Tonetti, Adan, Di Milia, Randler, & Natale, Reference Tonetti, Adan, Di Milia, Randler and Natale2015), but not with dim-light melatonin onset, the gold standard for measuring circadian rhythm (Dolsen, Wyatt, & Harvey, Reference Dolsen, Wyatt and Harvey2019). Crucially, self-report preference scales do not distinguish sleep timing from personal preference and self-perceptions of morning and nighttime alertness. As a result, the putative associations between sleep chronotype and adolescent behavioral health may be inflated by shared method variance and reporting bias when studies only rely on preference scales.
A complementary approach to one-time questionnaires is to conduct repeated assessments of sleep timing using daily diaries that represent typical daily experiences (Fischer et al., Reference Fischer, Lombardi, Marucci-Wellman and Roenneberg2017). In this approach, sleep chronotype is operationalized as the midsleep point between sleep onset and offset on days without a fixed wake time (unscheduled days), adjusting for the accumulation of sleep debt on scheduled days (Roenneberg, Kuehnle, et al., Reference Roenneberg, Kumar and Merrow2007). By querying about sleep daily rather than asking youth to recall their average sleep habits over the past month, daily sleep diaries can minimize recall bias while maximizing external validity. Moreover, in contrast to preference scales, which are susceptible to influences from state mood, daily surveys obtain specific and more accurate data about bed and wake times. The current study examined midsleep point on free days as the focal continuous measure of sleep chronotype. For adolescents, free days were operationalized as weekend days or official school holidays. For parents, free days were operationalized as days free from parental work or youth school. Using this measure, we examined parent and youth chronotype across middle adolescence, and assessed its stability over 1 year.
Given the importance of chronotype for health, identifying family characteristics associated with adolescent sleep timing can help us determine whether the family social environment is a useful system to target in intervention efforts intended to improve youth sleep. Sleep chronotype has a strong genetic basis (Barclay, Eley, Buysse, Archer, & Gregory, Reference Barclay, Eley, Buysse, Archer and Gregory2010), and parent and adolescent chronotypes are likely correlated. Moreover, teen sleep habits are shaped in the home, against the backdrop of the family social environment. In addition to shared biological predispositions and the physical home environment, family members continue to influence adolescent sleep through parenting behaviors such as the reinforcement of bedtime rules (Buxton et al., Reference Buxton, Chang, Spilsbury, Bos, Emsellem and Knutson2015), which in turn may be shaped by parental attitudes, beliefs, and preferences about their family and health.
The current study examines adolescent and parent sleep timing in Mexican American families. Although Mexican American families make up a rapidly growing population in the United States (Vespa, Armstrong, & Medina, Reference Vespa, Armstrong and Medina2018), they are underrepresented in sleep research. Comparisons across race and ethnicity suggest that adolescents of Mexican descent sleep longer than adolescents of Chinese or European descent (Fuligni & Hardway, Reference Fuligni and Hardway2006). At the same time, there is significant within-group variability in nightly sleep, in part due to differences in the family social environment (McHale, Kim, Kan, & Updegraff, Reference McHale, Kim, Kan and Updegraff2011). Specifically, Mexican American adolescents of parents who placed a greater emphasis on the family or were more enculturated to the Mexican culture slept longer during the night (McHale et al., Reference McHale, Kim, Kan and Updegraff2011). Moreover, past work has shown that adolescents and parents of Mexican descent exhibit significant concordance in bedtime, wake time, and sleep duration (Fuligni, Tsai, Krull & Gozales, Reference Fuligni, Tsai, Krull and Gonzales2015). However, no research has examined sleep chronotype, a specific aspect of sleep linked to behavioral health concerns that are prevalent in Mexican American youth (Georgiades, Paksarian, Rudolph, & Merikangas, Reference Georgiades, Paksarian, Rudolph and Merikangas2018; Swendsen et al., Reference Swendsen, Burstein, Case, Conway, Dierker, He and Merikangas2012). To better understand adolescent sleep health in the family context, we examined the associations between parent and youth chronotype across middle adolescence. In addition, we compared the stability of sleep chronotype over 1 year in adolescents and parents.
In the current study, we used repeated daily measurements of sleep to assess chronotype over 2 consecutive years in 417 adolescents from Mexican American backgrounds. First, we estimated sleep chronotype using daily self-reports of bed and wake times across 14 days. We tested youth chronotype as a function of age, gender, and parent chronotype. We hypothesized that older youth would show more delayed chronotypes, consistent with past research on the effects of biological maturation on sleep timing (Fischer et al., Reference Fischer, Lombardi, Marucci-Wellman and Roenneberg2017). We predicted that youth and parent chronotypes would be concordant, extending our past finding that bed and wake times are correlated within dyads (Fuligni et al., Reference Fuligni, Tsai, Krull and Gonzales2015). Second, we examined stability in youth and parent sleep chronotype over 1 year, with the hypothesis that sleep chronotype will be highly correlated within individuals over time. Third, we evaluated whether contemporaneous associations between sleep chronotype and behavioral health problems observed in previous studies could be replicated in this Mexican American sample using daily diary-derived estimates (Haraden et al., Reference Haraden, Mullin and Hankin2017; Merikanto et al., Reference Merikanto, Pesonen, Kuula, Lahti, Heinonen, Kajantie and Räikkönen2017). We explored moderation by gender and age, and hypothesized that females with more advanced sleep chronotypes might be at highest risk of behavioral health problems, given past research suggesting that timing and rate of pubertal development differentially predicts sleep timing and behavioral health in males and females (Foley et al., Reference Foley, Ram, Susman and Weinraub2018; Hamlat, Snyder, Young, & Hankin, Reference Hamlat, Snyder, Young and Hankin2018; Mendle & Ferrero, Reference Mendle and Ferrero2012; Negriff & Susman, Reference Negriff and Susman2011).
Method
Sample and procedures
Participants were 417 Mexican American adolescents (M age = 16.0) and their caregivers, who completed reports of sleep at Wave 1 (9th or 10th grade) or Wave 2 (10th or 11th grade). Of the 417 adolescents, 50.6% were female, and the majority of adolescents were born in the United States (87.3%). Caregivers, hereon referred to as parents, were 82% mothers, 13% fathers, and 4% other relative at Wave 1 and 87% mothers, 11% fathers, and 2% other relatives at Wave 2. Mean caregiver age at Wave 1 was 40.7 years (SD = 6.8, Range = 20–69). The majority of parents were foreign born (82.2%) and completed at most some high school: 65.9% less than a high school degree, 7.4% a high school degree only, 5.5% trade or vocational school, and 15.6% at least some college. Participants were recruited from two high schools in Los Angeles through letters and phone calls made to parents to determine youth eligibility. Of the parents who were reached by phone and deemed eligible by self-reporting a Mexican ethnic background, 63% enrolled. Families were contacted again, 1 year later, and asked to participate in Wave 2.
Of the 417 youth participants, 411 were enrolled at Wave 1 (M age = 15.5, Range = 13.9–18.9), and 323 returned for follow-up at Wave 2. Six additional participants enrolled in Wave 2 only, for a total Wave 2 sample of 329 adolescents (M age = 16.5, Range = 14.9–20.0). Ten participants were between 18 and 20 years old at Wave 1 or 2. Across the two waves, 403 caregivers provided sufficient sleep data to compute parent sleep chronotype: 82.9% mothers, 13.2% fathers, and 4.0% other. Of the 378 parents who completed sleep diaries at Wave 1, 277 returned for follow-up. Twenty-five additional parents completed sleep diaries at Wave 2 only. Participants provided written consent and assent as appropriate at each wave, and all procedures were approved by the institutional review board.
Research interviewers conducted home visits, during which parents reported demographic information on youth, and adolescents completed self-report questionnaires. After the completion of self-report questionnaires, adolescents and parents independently answered questions about their own nightly sleep on checklists daily for 14 consecutive days. Participants folded, sealed, and stamped the checklist with an electronic time stamper to indicate time of completion. Each daily checklist took about 5–10 min to complete. Interviewers called participants during the 2-week period to answer questions and encourage compliance, and collected the checklists at the end of the period. Most participants completed the daily diaries during the school year, and a small minority completed them over school holidays. To be included in the current study, participants had to provide sleep data on at least 1 free day, such as school holidays or weekends. Adolescents and parents were compensated $30 and $50, respectively, and they received an additional two free movie passes for correct checklist completion. On average, adolescents completed 96% and parents completed 95% of the checklists. Of these, 86% of adolescent diaries and 90% parent diaries were on time, completed before the next day at noon. All available diaries were used in our analyses.
Data from this sample were used in previous articles on daily concordance between sleep habits of parents and adolescents (Fuligni et al., Reference Fuligni, Tsai, Krull and Gonzales2015), optimum sleep for achievement and symptomatology (Fuligni, Arruda, Krull & Gonzales, Reference Fuligni, Arruda, Krull and Gonzales2018), and individual differences in effect of sleep on daily mood (Fuligni, Bai, Krull & Gonzales, Reference Fuligni, Bai, Krull and Gonzales2019). These reports did not examine chronotype, an important facet of sleep with unique implications for adolescent health.
Measures
Sleep
At each wave, adolescents and parents independently reported on bedtime (i.e., sleep onset), “What time did you go to bed last night?”; wake time (i.e., sleep offset), “What time did you wake up this morning?”; and sleep duration, “How much time did you sleep last night?” Self-reported sleep duration was checked against the interval between self-reported bed and wake times. Any report of duration that exceeded the interval between bed and wake times was recoded to be equal to the bed-to-wake time interval. If the reported sleep duration was shorter than the interval, reported sleep duration was kept as the most accurate estimate, and time of sleep onset was computed by subtracting reported sleep duration from wake time. Daily surveys are frequently used to assess sleep (Carney et al., Reference Carney, Buysse, Ancoli-Israel, Edinger, Krystal, Lichstein and Morin2012), and self-report data are moderately correlated with objective estimates obtained from actigraphy (Lockley, Skene, & Arendt, Reference Lockley, Skene and Arendt1999; Matthews, Hall, & Dahl, Reference Matthews, Hall and Dahl2014).
Scheduled and free days
For adolescents, school days were coded as scheduled days, whereas weekend days or official school holidays were coded as free days. At Wave 1, mean number of free days was 6.37 (SD = 3.41, Range = 2–14). At Wave 2, mean number of free days was 6.86 (SD = 3.58, Range = 4–14). Fifty-seven and 47 youth reported 100% free days during the diary period in Waves 1 and 2, respectively.
For parents, school days and/or self-reported workdays were coded as scheduled days and days when there was no school or work were coded as free days. Mean number of free days was 5.21 (SD = 3.18, Range = 1–14) at Wave 1 and 5.28 (SD = 3.25, Range = 1–14) at Wave 2. Twenty-seven and 21 caregivers reported only free days in Waves 1 and 2, respectively.
Youth behavioral health
Adolescents completed self-report questionnaires that assessed various aspects of their health at each wave (see Table 1).
Table 1. Mean and standard deviation of youth midsleep point (MSFc), sleep duration, behavioral health indicators, and parent MSFc by age range

Note: MSFc = midsleep point on free days, correcting for sleep debt; sleep duration in hours, averaged over all available daily diaries; CES-D = Center for Epidemiologic Studies Depression Scale, average of scores from 19 items, excluding Item 11, “my sleep was restless”; YSR = Youth self-report.
Substance use
Items were adapted from 2001 National Youth Risk Behavior Survey (Grunbaum et al., Reference Grunbaum, Kann, Kinchen, Williams, Ross, Lowry and Kolbe2002), which was designed for a nationally representative diverse sample including Hispanic Americans, and has substantial test–retest reliability across different ethnicities (Brener et al., Reference Brener, Kann, McManus, Kinchen, Sundberg and Ross2002). In the current study, adolescents responded yes/no to items assessing lifetime use of seven substances: cigarettes, alcohol, marijuana, cocaine, methamphetamines, other illegal drugs, and prescription drugs without a prescription. Items were summed such that scores represented the total count of substances ever used. The rate of substance use was relatively low; 46% and 39% reported that they had never tried any substances at Waves 1 and 2, respectively (skewness = 1.12, kurtosis = 3.73).
Depressive symptoms
Adolescents completed the widely used Center for Epidemiologic Studies Depression Scale (CES-D), a 20-item measure of past-week depressive symptoms, with high internal reliability, adequate test–retest reliability, and high construct validity (Radloff, Reference Radloff1991). Validation studies comparing CES-D scores between White and Mexican American youth show that the CES-D has high internal reliability and the same underlying factor structures in both samples (Crockett, Randall, Shen, Russell, & Driscoll, Reference Crockett, Randall, Shen, Russell and Driscoll2005), We excluded Item 11, “my sleep was restless,” and averaged scores from 19 items, rated on a 0 = rarely to 3 = mostly or all of the time scale, such that higher scores represented more problems. Internal consistency for the full scale was α = .73 at both waves.
Child Behavior Checklist: Youth Self-Report
Adolescents completed the Youth Self-Report (YSR) form of the Child Behavior Checklist (Achenbach, Reference Achenbach2009), a widely used measure of youth emotional and behavioral functioning. The YSR consists of 112 items scored on a 0 = not true of me to 2 = true or often true of me scale, and it demonstrates good psychometric properties, including high test–retest reliability and high external validity in ethnically diverse samples that include White, African American, and Latino youth (Achenbach & Rescorla, Reference Achenbach and Rescorla2003). The current study uses the internalizing broadband scale, consisting of 30 items (e.g., “I cry a lot ” and “I worry a lot,”) and the externalizing broadband scale, composed of 32 items (e.g., “I break rules at home, school or elsewhere” and “I get in many fights”). Item scores were summed for each broadband scale, such that higher scores represented more problems. Internal reliabilities ranged from .86 to .88 for the internalizing and externalizing scales at Waves 1 and 2.
Data analysis
The accelerated longitudinal design of the current study included data collected at the 9th and 10th grades for the 9th-grade cohort, and data collected at the 10th and 11th grades for the 10th-grade cohort. When pooled across the two waves and two cohorts, the 9th-grade cohort contributed to estimates for younger ages, the 10th-grade cohort contributed to estimates for older ages, and all participants contributed to estimates for overlapping ages. The full age range spanned from 13.9 to 20.0 years (M = 16.0; SD = 0.92) across the 2 years for both cohorts.
Using time of sleep onset and wake, chronotype was operationalized as the midsleep point on free days (MSF), while correcting for the difference between average sleep duration on free days and on all days (MSFc; Roenneberg, Kuehnle, et al., Reference Roenneberg, Kumar and Merrow2007). The correction adjusts for the tendency for individuals with greater sleep need to compensate for sleep shortage on scheduled days with longer sleep duration on free days (Roenneberg, Kuehnle, et al., Reference Roenneberg, Kumar and Merrow2007). Using all available reports of sleep, we computed MSFc for each participant at each wave, as shown in equations below.
We computed the midsleep point on free day, i, for individual j (MSF ij), and derived an average MSF for each individual at each wave (MSF .j). SleepFree .j refers to the average sleep duration over free days for individual j, and SleepAll .j refers to the average sleep duration over all diary days, at each wave. MSFc.j represents sleep chronotype for individual j at each wave adjusting for sleep debt. It is expressed as hours from midnight, with higher values representing more eveningness or more “delays” in chronotype.
We fitted two-level mixed effects regression models to examine MSFc as a function of age, gender, and parent chronotype. Mixed effects regression models account for within-person correlations in the accelerated longitudinal design in which waves are nested in individuals, and produce unbiased estimates when data are missing at random. We tested the linear and quadratic effects of age (grand mean centered, in years) and included gender (–1 = males; 1 = females) as a covariate. We further explored gender differences on the association between age and sleep chronotype. Both models included random intercept. Next, we examined the association between parent chronotype (grand mean centered, in hours) and youth chronotype. We controlled for youth age and gender, and excluded data points from primary caregivers who were “other relatives” to reduce heterogeneity in caregivers’ biological relationships to the teen. In addition, we tested for age and gender moderation on the association between parent and youth chronotype. Specifically, we first assessed the two-way interaction between youth age and parent chronotype in order to test whether the concordance of chronotypes waned in older youth. Second, we tested the two-way interaction between parent gender and parent chronotype to assess differences between mothers and fathers. Third, we explored differences by youth age and gender, by including the three-way interaction between age, youth gender, and parent chronotype and all associated lower order interaction terms. Models included random intercept and the random slope of parent chronotype.
To assess the stability of sleep chronotype over 1 year, we examined the Pearson's correlations of Wave 1 and Wave 2 MSFc for youth and parents. In addition, we compared the stability of youth MSFc to that of parents over the 1-year follow-up in a three-level regression model (waves, nested in family members, nested in families), with MSFc as the dependent variable, and family member (youth or parent), wave (1 or 2), and family member × wave as the independent variables. The model included a random intercept, controlled for each family member's gender, age, and age2, and excluded data points from “other relatives.” To further explore stability in youth MSFc specifically, we categorized youth as evening-types, morning-types, and intermediate-types, at each wave, using tertile cut points relative to others in the same age range: <15.0-year-olds, 15.0- to 15.9-year-olds, 16.0- to 16.9-year-olds, and >17.0-year-olds (Owens et al., Reference Owens, Dearth-Wesley, Lewin, Gioia and Whitaker2016). We described likelihood of individuals changing group membership from Wave 1 to 2, for each type.
Finally, we evaluated whether contemporaneous associations between sleep chronotype and behavioral health problems could be replicated in this Mexican American sample using daily diary-derived MSFc estimates. We conducted two-level regression models examining youth substance use, depressive symptoms, and YSR internalizing and externalizing problems as dependent variables in separate models. Lifetime count of substances tried was estimated in Poisson models, depressive symptoms in linear and quadratic models, and YSR internalizing and externalizing problems in linear models. We included the random intercept, the random slope of MSFc, and covariates, age, gender and sleep duration, and tested age, gender, and Age × Gender as moderators. In addition, we included age2 as a control when estimating YSR internalizing and externalizing problems (Bongers, Koot, van der Ende, & Verhulst, Reference Bongers, Koot, van der Ende and Verhulst2003; van der Ende & Verhulst, Reference van der Ende and Verhulst2005). We conducted additional sensitivity analyses by including as a covariate social jetlag, operationalized as the difference between midsleep point on free days and on scheduled days, given its link to sleep chronotype (Roenneberg et al., Reference Roenneberg, Pilz, Zerbini and Winnebeck2019). Finally, we explored the cross-sectional associations between Wave 2 chronotype and Wave 2 behavioral health outcomes, over and above the control of Wave 1 levels of symptoms in prospective models. All analyses were conducted on Stata 15.0.
Results
Attrition analysis
Of the 411 youth who completed daily checklists at Wave 1, 323 (78.6%) also completed them at Wave 2. Adolescents who completed checklists at both waves did not differ from those who completed them only at Wave 1 with respect to age, gender, sleep chronotype, substance use, depressive symptoms, and internalizing and externalizing problems.
Youth chronotype according to age, gender, and parent chronotype
Table 1 describes means and standard deviations of youth and parent MSFc and youth behavioral health problems, by youth age group. Group means for youth MSFc ranged from 3:38 a.m. to 4:05 a.m., with a pooled mean of 3:57 a.m. Parents showed earlier chronotypes with mean MSFc ranging from 2:56 a.m. to 3:13 a.m.; pooled mean was 3:08 a.m.
Next, we conducted mixed effects regression analyses to examine the linear and quadratic effects of age on youth chronotype. MSFc showed a significant curvilinear association with youth age (Table 2; Figure 1), such that peak MSFc was observed at age 16.9 years. Gender and the Age × Gender interaction were not significantly associated with MSFc. We excluded caregivers who self-identified as “other relative,” and found that later parent chronotype was associated with later youth chronotype (B = 0.27, SE = 0.05, p < .001, 95% confidence interval [CI] [0.18, 0.36]) over and above youth age and gender. Age, parent gender, and youth gender did not moderate this linear relationship.

Figure 1. Youth MSFc as a quadratic function of age. Line represents predicted values of MSFc, based on the significant quadratic effect of age; scatter points represent residuals.
Table 2. Youth- and parent-related predictors of MSF
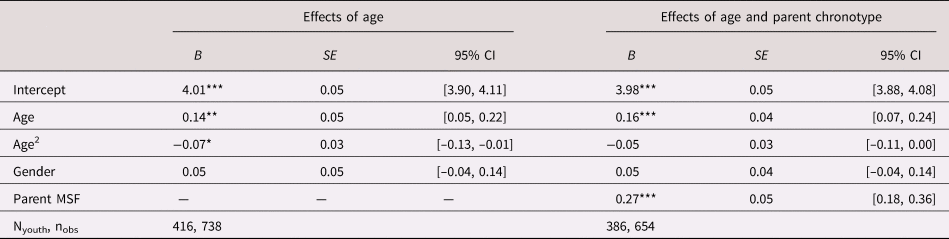
Note: MSFc is coded in hours and grand mean centered; age is coded in years and grand mean centered. Models include random intercept and random slope of parent MSFc when applicable. Adolescent gender is effect coded –1 = male, 1 = female. *p = .05. **p = .01. ***p = .001.
Stability in youth and parent chronotype
Youth MSFc at Wave 1 and 2 were moderately correlated, r (321) = .45, p < .001. We found comparable correlations for parent MSFc, r (275) = .45, p < .001. Next, our three-level regression showed that MSFc differed between parents and teens, and from Wave 1 to 2. The interaction term between family member (i.e., parent vs. youth) and wave was significant in the estimation of MSFc (B = 0.35, SE = 0.09, p < .001, 95% CI [0.17. 0.53]), over and above the control of individuals’ age and gender. Teens had more delayed chronotypes than parents at Wave 1 (B = 0.71, SE = 0.08, p < .001, 95% CI [0.55, 0.86]), and this difference was greater in magnitude at Wave 2 (B = 1.05, SE = 0.09, p < .001, 95% CI [0.88, 1.22]). Parents’ MSFc became more advanced from Wave 1 to 2 (B = –0.14, SE = 0.07, p = .041, 95% CI [–0.27, –0.01]), while teens' became more delayed (B = 0.21, SE = 0.06, p = .001, 95% CI [0.08, 0.33]).
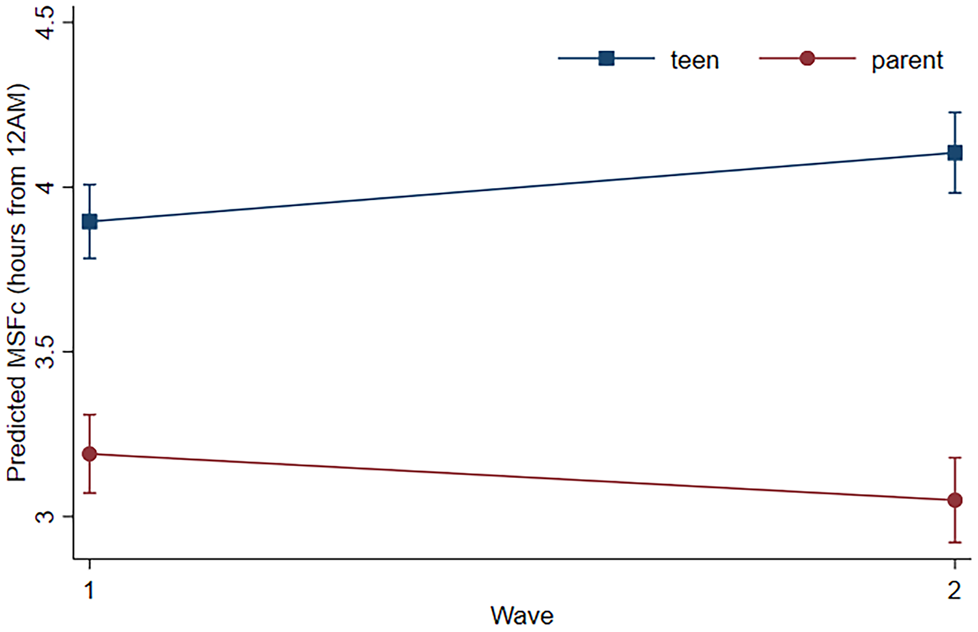
Figure 2. Predicted MSFc for parent and youth at Waves 1 and 2, controlling for individual age, age2, and gender. All simple effects were significant at p < .05; error bars represent 95% confidence intervals.
For each age group at each wave, youth with MSFc in the top tertile were coded as evening types, the lowest tertile as morning types, and the middle tertile as intermediate types. We examined shifts in chronotype tertiles, using the terms “advanced,” to signify that MSFc shifted toward morningness, and “delayed,” to describe that MSFc shifted toward eveningness, from Wave 1 to 2. Of the 115 morning types at Wave 1, 46.1% (N = 53) remained as such at Wave 2, 39.1% (N = 45) became intermediate, and 14.8% (N = 17) delayed to evening types. Of the 102 intermediates at Wave 1, 30.4% (N = 31) advanced to morning types at Wave 2, and 34.3% (N = 35) delayed to evening types. Of the 105 evening types at Wave 1, 58.1% (N = 61) remained as such, 25.7% (N = 27) became intermediate types, and 16.2% (N = 17) advanced to morning types. Morning types were the least likely to show stability in chronotype from Wave 1 to 2, χ2(2) = 10.8, p = .004, and gender and age were not associated with 1-year stability. Results suggest that there is moderate stability in individual differences from Wave 1 to Wave 2, and that youth who were morning types in Wave 1 are perhaps more likely to “catch up” to their evening-type peers over 1 year.
Chronotype and behavioral health problems
Next, we examined the contemporaneous associations between MSFc and youth substance use count, depressive symptoms, and internalizing and externalizing problems, over and above age and gender. We tested whether each association was moderated by age, gender, and Age × Gender. Findings were consistent with or without the control of sleep duration, and Table 3 presents models that include the covariate.
Table 3. Contemporaneous associations between youth MSFc and substance use, depressive symptoms, internalizing problems, and externalizing problems
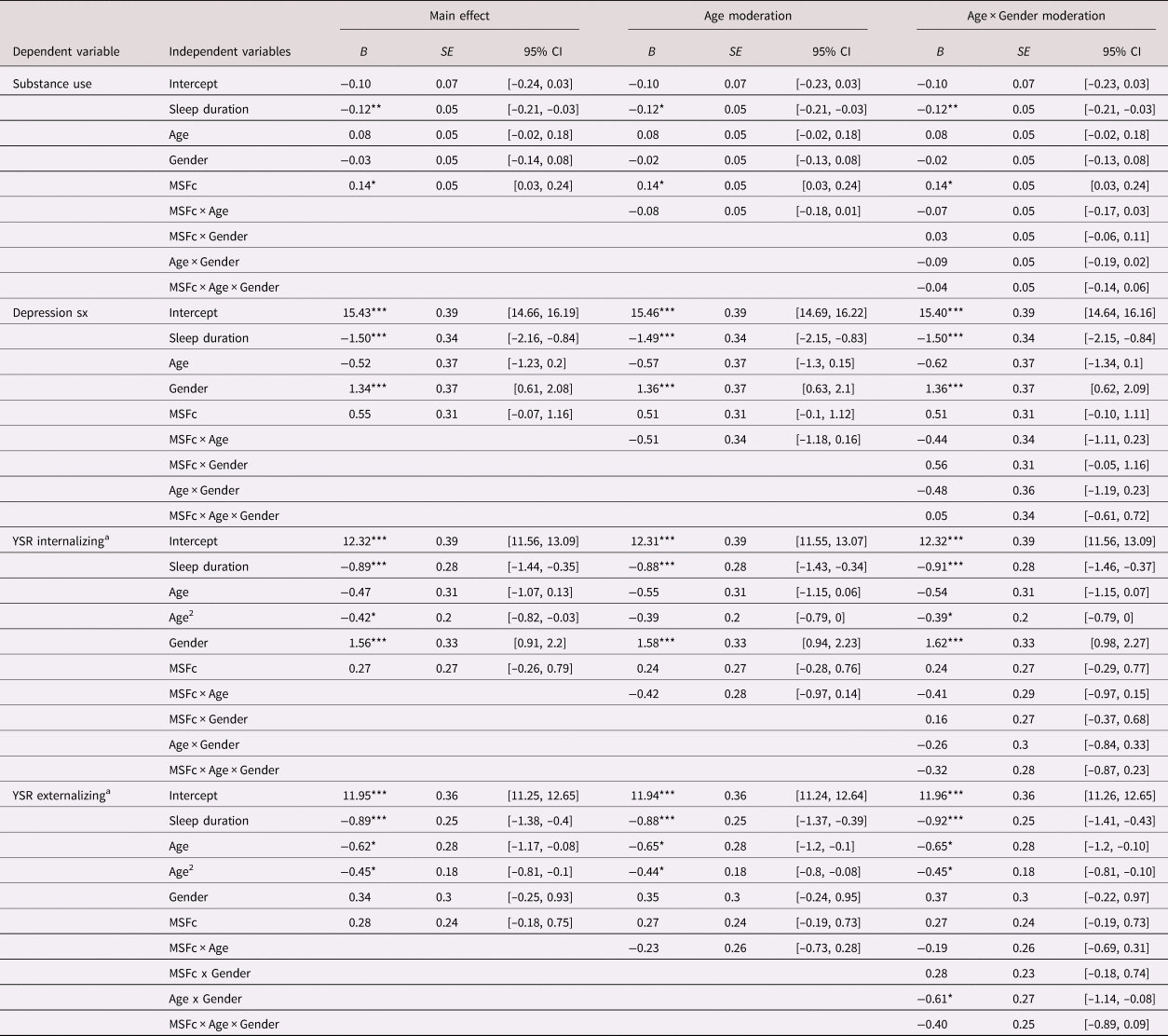
Note: MSFc is coded in hours and grand mean centered; age is coded in years and grand mean centered. All models included random intercept and slope of MSFc. Adolescent gender is effect coded –1 = male, 1 = female; B = log of expected counts from Poisson regressions estimating substance use count or unstandardized coefficients from linear regressions estimating depressive symptoms, and internalizing and externalizing problems. aModels excluded one outlier at Wave 1; age2 did not significantly interact with MSFc or gender in the estimation of internalizing or externalizing problems, and thus is not included in the final models. *p < .05. **p < .01. ***p < .001.
Controlling for average sleep duration, later MSFc was associated with higher count of tried substances (B = 0.14, SE = 0.05, p = .011, 95% CI [0.03, 0.24]; IRR = 1.15) but not depressive symptoms, internalizing problems, or externalizing problems. Age and gender did not moderate this association.
We conducted sensitivity analyses with the inclusion social jetlag. The contemporaneous associations between youth chronotype and substance use remained significant over and above sleep duration and social jetlag (B = 0.15, SE = 0.07, p = .019, 95% CI [0.02, 0.28]; IRR = 1.17). Wave 2 MSFc was not reliably associated with any of the five behavioral health outcomes at Wave 2 over and above the control of Wave 1 levels of the symptoms.
Discussion
This study tested a daily diary approach to assessing sleep chronotype in Mexican American adolescents and parents. We operationalized sleep chronotype as the midsleep point between bed and wake times on non-school and non-work days, correcting for sleep debt accumulated on scheduled days. From 14 to 17 years, older age was associated with more eveningness; chronotype advanced toward the direction of morningness during late adolescence. Adolescent and parent chronotypes were contemporaneously correlated. However, youth chronotype was more delayed than parent chronotype at Wave 1 and this difference widened over the 1-year follow-up; chronotype for the average youth in the sample became more delayed, while parent chronotype became more advanced. Later chronotype was contemporaneously associated with greater lifetime history of substance use in all youth. Future studies should consider within- and between-person differences in chronotype across the life span, to better understand its implications on health.
Our youth appeared to have earlier MSFc overall and reach peak eveningness at younger ages than shown in previous samples (Fischer et al., Reference Fischer, Lombardi, Marucci-Wellman and Roenneberg2017; Maslowsky & Ozer, Reference Maslowsky and Ozer2014; Roenneberg, Kuehnle, et al., Reference Roenneberg, Kuehnle, Juda, Kantermann, Allebrandt, Gordijn and Merrow2007; Wittmann et al., Reference Wittmann, Dinich, Merrow and Roenneberg2006). In contrast to prior studies (Fischer et al., Reference Fischer, Lombardi, Marucci-Wellman and Roenneberg2017), we detected no differences by gender. These discrepancies must be interpreted in the context of two methodological differences. The daily diary approach used in the current research is distinct from the more commonly used one-time questionnaires on sleep preference or timing. Preference scales rely on subjective reports of preferred bed and wake times (Carskadon et al., Reference Carskadon, Vieira and Acebo1993). Sleep timing scales can provide information about actual behaviors and habits (Roenneberg, Kuehnle, et al., Reference Roenneberg, Kuehnle, Juda, Kantermann, Allebrandt, Gordijn and Merrow2007), and the daily diary approach improves upon one-time sleep timing questionnaires by providing an in-depth assessment of timing while minimizing recall bias and increasing external validity. Geographic and demographic differences between samples must also be considered (Randler & Rahafar, Reference Randler and Rahafar2017). The current study focused on a relatively smaller sample of 9th to 11th graders between ages 14 to 20 years in the southwest United States; we had relatively fewer observations after the age of 18 years. Thus, our estimate of sleep chronotype may be less accurate for older youth.
The relatively homogenous sample of Mexican American families is a unique strength that distinguishes the current study from past investigations of sleep chronotype. Families of Mexican descent make up a rapidly growing population in the United States (Vespa et al., Reference Vespa, Armstrong and Medina2018). However, Mexican American youth have been underrepresented in sleep research. In our sample, we found that sleep chronotype was only moderately stable from Wave 1 to 2 for both adolescents and parents. While adolescent and parent chronotypes were contemporaneously correlated, their 1-year trajectories diverged, with youth showing more delay and parents showing more advancement. Over half the youth who were morning types relative to their same-aged peers at Wave 1 became intermediate or evening types at Wave 2. Findings likely reflect a confluence of biological and environmental factors that influence sleep health in adolescence and parenthood.
Several facets of sleep are highly heritable, including sleep timing. A study of 430 adult individuals in the United States found that genes accounted for about 21% to 41% of variability, with sleep time and morning alertness being particularly heritable (de Castro, Reference de Castro2002). Environmental factors, some of which may be shared by parents and adolescents, also significantly influence sleep chronotype. Cues in the physical environment, such as light, nutrients, and temperature influence circadian rhythms, the physiological basis of sleep chronotype (Randler, Reference Randler2008; Roenneberg et al., Reference Roenneberg, Pilz, Zerbini and Winnebeck2019). In addition, factors in the family social environment, such as family rules and expectations around bedtimes and screen use, shape adolescent sleep (Buxton et al., Reference Buxton, Chang, Spilsbury, Bos, Emsellem and Knutson2015; Randler, Bilger, & Díaz-Morales, Reference Randler, Bilger and Díaz-Morales2009). Such rules and expectations are likely determined by parents’ own sleep habits (Fuligni et al., Reference Fuligni, Tsai, Krull and Gonzales2015), which may contribute to the linkage between parent and youth chronotype. Furthermore, cultural factors, including family values, may help to improve adolescent sleep habits. Adolescents of Mexican descent may benefit from the emphasis on family interdependence and strong family support observed in Latino families (Campos & Kim, Reference Campos and Kim2017). These values may help to create a cohesive family environment, where adolescents can learn and practice healthy sleep habits. Future research can further examine adolescent sleep in the broader family context, and identify short-term family processes that promote healthy sleep behaviors.
In addition to genetic and environmental influences, biological and behavioral changes across adolescence may significantly contribute to sleep chronotype. For younger females specifically, the timing and tempo of pubertal development may underlie the shift in sleep timing toward eveningness, as well as the risk for behavioral health problems (Crockett, Carlo, Wolff, & Hope, Reference Crockett, Carlo, Wolff and Hope2013; Foley et al., Reference Foley, Ram, Susman and Weinraub2018; Hoyt et al., Reference Hoyt, Deardorff, Marceau, Laurent, Windham, Greenspan and Hiatt2018). Despite the linkage between pubertal development and sleep chronotype in the literature, we did not detect statistically significant moderation by gender or age on the association between chronotype and behavioral health problems in this sample. This may be because our 9th to 11th graders were likely past the initiation and midpoint of pubertal development (i.e., Tanner Stage 3; Foley et al., Reference Foley, Ram, Susman and Weinraub2018; Hoyt et al., Reference Hoyt, Deardorff, Marceau, Laurent, Windham, Greenspan and Hiatt2018), which could hold true for our sample of Mexican American females who may experience pubertal maturation at an earlier age than White peers (Hoyt et al., Reference Hoyt, Deardorff, Marceau, Laurent, Windham, Greenspan and Hiatt2018).
Nonetheless, we detected only moderate stability in sleep chronotype in both youth and parents, consistent with past research indicating age-related changes in sleep chronotype across the life span. This suggests that cognitive, behavioral, and social development, including self-regulation capabilities and family, peer, and school/work demands, likely contribute to sleep timing during adolescence and in parenthood. We found that youth who had more delayed chronotypes were likely to have tried more substances. However, this may be bidirectional. Early initiation of substance use and other risky behaviors may contribute to an exaggerated delay in chronotype, whereas delayed chronotype may create more opportunity for substance use and behavioral problems (Haynie et al., Reference Haynie, Lewin, Luk, Lipsky, O'Brien, Iannotti and Simons-Morton2017).
Findings have significant implications for behavioral health interventions that aim to prevent substance use. The consistent links between chronotype and substance use, over and above sleep duration, indicate that delayed sleep timing may be a risk factor for or a consequence of substance use. Chronotype should be an essential component of sleep health assessments, and youth with more delayed sleep timing may benefit from additional screenings for substance use. Prior work has shown that treating sleep alongside substance use can lead to improvements in both domains of behavioral health (Britton et al., Reference Britton, Bootzin, Cousins, Hasler, Peck and Shapiro2010; Conroy & Arnedt, Reference Conroy and Arnedt2014). However, past interventions have focused on sleep duration or sleep disturbance. The current study suggests that chronotype may be a malleable target of intervention (Harvey et al., Reference Harvey, Hein, Dolsen, Dong, Rabe-Hesketh, Gumport and Silk2018). Specifically, given the linkages between youth and parent chronotypes, youth may benefit from a family-focused intervention that addresses sleep as a family issue.
Study findings should be interpreted in the context of its limitations. We assumed non-school days to be free days. However, participants may have had to wake up at specific times to attend to other activities. Self-reports and objective measures may provide different estimates of sleep timing. Future studies can use both methods to evaluate concordance across methods. In addition, we assumed that reports on the daily surveys represent typical daily experiences. However, unique and unforeseen daily activities may limit the generalizability of our measure of sleep chronotype. Moreover, additional studies that assess the psychometric properties of the current approach to measuring sleep chronotype may help to identify developmentally sensitive cut points for morning, intermediate, and evening types across adolescence. The age of our sample only spanned from 13 to 20 years, and no measures of pubertal development were available. Extending the lower and upper age limit may yield a different estimate for age of peak eveningness. The study was conducted on youth with Mexican backgrounds in the southwest United States, limiting the generalizability of our findings to other ethnic groups in other geographic regions.
The current study advances the field by prospectively examining sleep chronotype—an important facet of sleep—during a particularly dynamic period of maturation. We leveraged daily self-reports to assess sleep timing in everyday life among a large sample of Mexican American youth. Whereas past studies have looked to the extremes (e.g., “night owls”) to infer risk, the current study suggested that incremental delays in sleep chronotype in the order of minutes to hours may provide clinically useful information about correlated indicators of behavioral health. Moreover, the study indicates that sleep timing is only moderately stable in youth and parents. Targeting sleep health in the family as a whole may help to improve that of individual family members.
Acknowledgment
We thank the participating families and Thomas Weisner for his contributions to the larger project.
Financial support
The research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01-HD057164), the National Institute of Mental Health Grant T32MH073517, and the UCLA California Center for Population Research, which is supported by the National Institute of Child Health and Human Development Grant P2C-HD041022.
Conflict of interest
Authors do not have any conflicts to disclose.








