As heat waves become more frequent and longer in duration, heat-related morbidity and financial burden (approximately $900 million/year for dairy products and > $300 million/year in beef and swine in the USA alone) are also likely to increase(Reference St-Pierre, Cobanov and Schnitkey1). Environmentally induced heat stress (HS) negatively influences global animal agriculture and undermines genetic, nutritional and pharmaceutical advances in animal feed efficiency(Reference Pollman2). HS-induced devastating economic losses are a result of poor animal performance, reduced and inconsistent growth, decreased carcass quality and increased veterinary costs(Reference Renaudeau, Gourdine and St-Pierre3).
The intestinal epithelium plays important roles in nutrient digestion and absorption and immune functions(Reference Barszcz and Skomiał4). Studies have shown that HS jeopardises livestock health and productivity through reducing intestinal barrier integrity and increasing intestinal permeability(Reference Lambert5–Reference Tang, Jiang and Tang7). HS increases concentrations of gut-derived endotoxin and pathogenic bacteria in portal and systemic blood(Reference Hall, Buettner and Oberley8), causing gastrointestinal tract injury and heat exhaustion death(Reference Gathiram, Wells and Brock-Utne9 , Reference Gathiram, Wells and Brock-Utne10). The porcine jejunal cell line IPEC-J2, a non-transformed epithelial cell line(Reference Zakrzewski, Richter and Krug11), preserves most functions as primary intestinal epithelial cells. IPEC-J2 cells are well-established in vitro model to study intestinal epithelial functions(Reference Paszti-Gere, Matis and Farkas12–Reference Lei, Tang and Qiang15). Se is an essential micronutrient involved in various metabolic processes within the body, and it has been demonstrated to alleviate the negative impacts of HS on the animal’s gastrointestinal mucosal health and epithelial cell function(Reference Chadio, Kotsampasi and Menegatos16–Reference Niu, Liu and Yan18). HS is associated with the reduced performance and productivity in animals due to decreases in feed intake, nutrient utilisation, growth rate and feed efficiency(Reference Yang, Li and Xiang19). Dietary Se improves feed utilisation through the regulation of the metabolism of carbohydrates, lipids and proteins(Reference Stapleton20). Furthermore, HS has been found to promote the generation of free radicals(Reference Mujahid, Akiba and Toyomizu21,Reference Azad, Kikusato and Maekawa22) , cause inflammatory disorders(Reference Heneka, Sharp and Klockgether23), jeopardise immune responses(Reference Bernabucci, Lacetera and Baumgard24) and damage proteins, DNA and other biological molecules(Reference Yang, Tan and Fu25). Interestingly, Se is an integral component of at least twenty-five selenoproteins(Reference Kryukov, Castellano and Novoselov26), which contain at least one selenocysteine at their active site(Reference Naziroglu and Yurekli27). Se is often referred to as an antioxidant, mainly due to the role of certain selenoproteins in detoxifying hydrogen peroxidase or reversing the side effect of oxidised lipids or methionine residues. In addition, selenoproteins are crucial for the regulation of apoptosis, regeneration of damaged cells, preventing chronic inflammatory damage and immunomodulation(Reference Roman, Jitaru and Barbante28).
Our previous studies have shown that expression of selenoproteins in IPEC-J2 cells were affected by HS(Reference Lei, Tang and Qiang15), but the reciprocal metabolic impacts of Se sources on the prevention of intestinal epithelial cell damage and expression of selenoproteins during HS remain unclear. Therefore, the present study was conducted to investigate (1) the protective effect of different sources of Se (sodium selenite (SS) and selenomethionine (SeMet)) on alleviating IPEC-J2 cell damage induced by HS and (2) the possible link between the protective effects of Se sources on the expression profiles of selenoproteins.
Methods
Cell culture
The IPEC-J2 cells (porcine small intestinal epithelial) were a kind gift from Dr Per Torp Sangild (University of Copenhagen, Denmark) and were used between passages sixty-three and seventy-seven. The cells were grown in Dulbecco’s modified Eagle’s medium and Ham’s F-12 nutrient mixture (DMEM/F12) medium (Gibco) supplemented with 10 % (v/v) fetal bovine serum (Gibco) and 1 % penicillin-streptomycin (Gibco) at 37°C in 5 % CO2 humidified chamber (Forma 3111; Thermo Fisher Scientific). The media were changed every 2 d.
Cell viability assays
Cell viability was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) (Sigma) assay(Reference Mosmann29). IPEC-J2 cells were plated in ninety-six-well plates at 1 × 104 cells/well density and cultured at 37°C under 5 % CO2. At 80 % confluence, cells were washed with PBS and cultured with serum-free medium (DMEM/F12) at 37°C (control, CK), at 41·5°C (HS), at 41·5°C supplied with 0·42-µmol/l SS (provided by Chelota, China) and at 41·5°C supplied with 0·42-µmol/l SeMet (S3132; Sigma) for four different times (12, 24, 36 and 48 h) (n 6). After treatment, the cells were washed with PBS, 200 µl/well MTT (dissolved in serum-free DMEM/F12 at 0·5-mg MTT/ml) was added to each well (n 6) and incubated at 37°C for 4 h. Then the culture medium was removed and 150 μl/well of dimethyl sulfoxide was added to dissolve the crystal. The lysates were transferred to a new ninety-six well plates. And the absorbance was measured at 490 nm using a Microplate reader (Model 680; Bio-Rad).
Real-time quantitative PCR analyses
IPEC-J2 cells 1 × 105 cells/well were plated in twenty-four-well plates and cultured at 37°C under 5 % CO2. At 80 % confluence, the cells were cultured with serum-free medium (DMEM/F12) for another 24 h under four different conditions: at 37°C, at 41·5°C, at 41·5°C with 0·42-µmol/l SS supplementation, at 41·5°C with 0·42-µmol/l SeMet supplementation.
After 24-h treatment, cells were washed with cold PBS and harvested for total RNA extraction. Four wells of cells were pooled together for one sample and for each treatment six samples were collected (n 6). Total RNA was extracted using TRIzol (Life Technologies) following the manufacturer’s protocol. Complementary DNA was synthesised according to the PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa) protocol. Quantitative PCR was performed on ABI 7900HT system (Applied Biosystems) in a final volume of 10 μl using the SYBR Premix Ex Taq TM kit (TaKaRa). The PCR consisted of one cycle of 95°C for 30 s and forty cycles of 95°C for 5 s and 60°C for 30 s, followed by the dissociation step at 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. The relative mRNA abundance quantification has been described previously by our group using the ΔCt method(Reference Zhao, Li and Tang30–Reference Xu, Wang and Tang32). Primers for heat shock protein 70 (HSP70), three tight junction (TJ)-related genes, ten inflammation-related genes, twenty-five selenoprotein genes and two reference genes (β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) were designed with Primer Express 3.0 (Applied Biosystems) and are presented in Supplementary Table S1.
Reactive oxygen species detection
The total reactive oxygen species (ROS) level was determined using a commercial ROS assay kit (eBioscience), according to the manufacturer’s instructions. Four treatments were carried out as mentioned above except the cells for ROS detection were grown on twelve-well cell plates. After 24-h treatment, cells were washed with cold PBS and incubated in 500 µl/well 1 × ROS Assay Stain for 60 min in the dark. Then the cells were digested with 0·25 % trypsin and three wells of cells were pooled together for one sample (n 4) and centrifuged at 800 g for 2 min at 4°C. Then the cells were re-suspended with ROS assay buffer and analysed by a flow cytometry (FACSVerse). Data were analysed using BD FACSuite software.
Western blot
Cell treatment was carried out as mentioned for the ROS detection. After 24-h treatment, the cells were washed with cold PBS buffer, and the protocol for protein sample preparation was the same as described previously, that is, using the RIPA lysis buffer (50-mM Tris-HCl (pH 7·4), 150-mmol/l NaCl, 0·25 % sodium deoxycholate, 1 % NP-40, 1-mmol/l EDTA, 10-μl/ml protease inhibitor, 10-μl/ml phosphatase inhibitor 3, 10-μl/ml 100-μmol/l Na3VO4, 10-μl/ml 10-mg/ml phenylmethylsulfonyl fluoride)(Reference Lei, Tang and Qiang15). Four wells of cells were pooled together as one replicate and three replicates were collected for each treatment (n 3). Protein concentrations were determined using a commercial protein assay kit (Jiancheng Bioengineering); 30 µg/well proteins were separated on 10 % polyacrylamide gels and blotted onto polyvinylidene fluoride membrane (Millipore). The membrane was blocked in 5 % bovine serum albumin for 1 h and then incubated with specific antibody against target protein HSP70 (1:20 000; ab5439; Abcam), claudin-1 (CLDN-1, 1:1000; 13050-1-AP; Proteintech), occludin (OCLN; 1:1000; 13409-1-AP; Proteintech), zonula occludens-1 (ZO-1, 1:1000; 21773-1-AP; Proteintech), glutathione peroxidase 1 (GPX1; 1:1000; 616958; Zen BioScience), selenoprotein P (SELP) (1:1000; sc-22639; Santa Cruz) and β-actin (1:20 000; MAB1501; Millipore), respectively. Blots were then incubated with the appropriate secondary antibodies (horseradish peroxidase-linked IgG). Signal was detected by autoradiography and chemiluminescence with an enhanced chemiluminescence system (Millipore). Densitometric analyses of Western blot bands were performed using Image LabTM software system (Bio-Rad). The relative abundance of each target protein was expressed as the ratio of targeted protein to β-actin protein.
Statistical analysis
Statistical analysis was performed using SAS 8.2 software (SAS 8.2; SAS Institute). The statistical significance of difference between groups was evaluated using ANOVA followed by Duncan’s test for multiple comparisons. All data were expressed as mean values with their standard errors and significance level was set at P < 0·05.
Results
Cell viability
IPEC-J2 cells were exposed to 41·5°C for 12, 24, 36 and 48 h, respectively, and the effect of SS and SeMet supplementation on cell viability was evaluated (Fig. 1). The results showed that short-time (12 h) HS elevated (P < 0·05) the cell viability, but the cell viability was decreased (P < 0·05) dramatically with prolonged HS. Both sources of Se supplementation exhibited protective effect, which effectively prevented (P < 0·05) HS-induced reduction in cell viability at later stage.
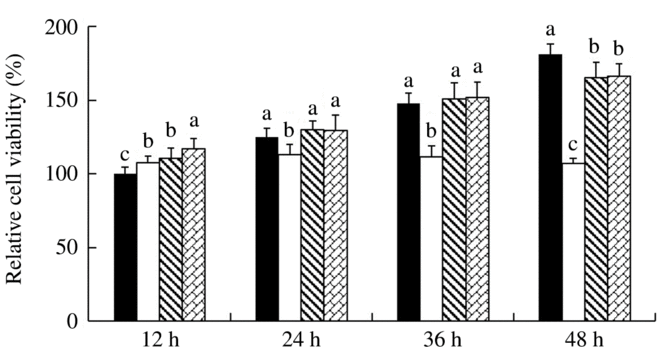
Fig. 1. Impact of sodium selenite (![]() ) and selenomethionine (
) and selenomethionine (![]() ) supplementation on the cell viability of IPEC-J2 cells subject to heat shock for 12, 24, 36 and 48 h. Data are presented as mean values with their standard errors, n 6. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) supplementation on the cell viability of IPEC-J2 cells subject to heat shock for 12, 24, 36 and 48 h. Data are presented as mean values with their standard errors, n 6. a,b,c Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control;
, Control; ![]() , heat shock only.
, heat shock only.
Effect of heat stress and selenium supplementation on heat shock protein 70 expression
To determine the response of IPEC-J2 cells to HS and the protective effect of additional two-source Se addition, we investigated the expression of mRNA and HSP70 after 24-h treatment (Fig. 2). HS up-regulated (P < 0·05) mRNA expression of HSP70 in IPEC-J2 cells, which was reversed by either SeMet or SS supplementation (P < 0·05) (Fig. 2(A)). HSP70 protein abundance was also increased by HS in IPEC-J2 cells (P < 0·05). Unlike mRNA expression, SeMet supplementation further increased (P < 0·05) HSP70 abundance compared with HS groups (Fig. 2(B)).
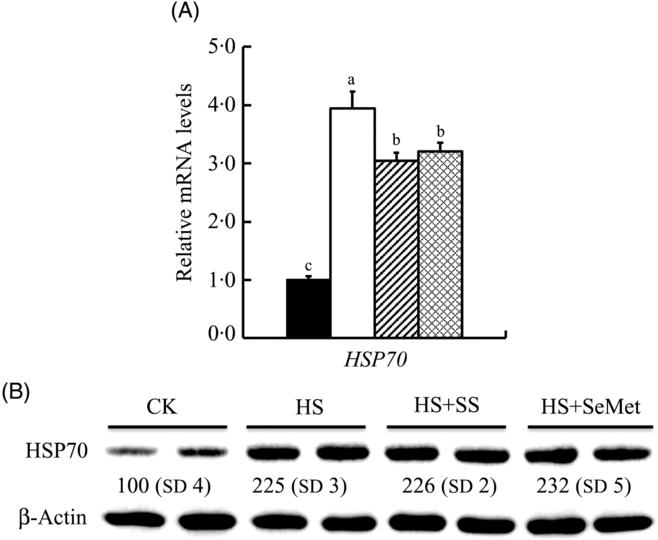
Fig. 2. Impact of sodium selenite (SS; ![]() ) and selenomethionine (SeMet;
) and selenomethionine (SeMet; ![]() ) supplementation on the mRNA abundance (A) and protein level (B) of heat shock protein 70 (HSP70) in IPEC-J2 cells subject to heat stress (HS) for 24 h. Data are presented as mean values with their standard errors, n 6 (gene) or 3 (protein). a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) supplementation on the mRNA abundance (A) and protein level (B) of heat shock protein 70 (HSP70) in IPEC-J2 cells subject to heat stress (HS) for 24 h. Data are presented as mean values with their standard errors, n 6 (gene) or 3 (protein). a,b,c Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control (CK);
, Control (CK); ![]() , HS only.
, HS only.
Effect of heat stress and selenium supplementation on tight junction-related protein abundance
We also investigated effect of HS and Se supplementation on expression of three TJ-related proteins in IPEC-J2 cells (Fig. 3). HS down-regulated (P < 0·05) mRNA levels of CLDN-1 and ZO-1, but up-regulated (P < 0·05) mRNA expression of OCLN. Interesting, SeMet and SS supplementation reversed (P < 0·05) the effect of HS on the expression of these genes, showing increased (P < 0·05) expression of CLDN-1 and ZO-1 and decreased (P < 0·05) expression of OCLN in IPEC-J2 cells compared with the HS group (Fig. 3(A)). Consistent with the mRNA levels, HS also decreased (P < 0·05) the protein levels of CLDN-1 and ZO-1 but had no effect on the expression of OCLN. Both SS and SeMet supplementation recovered (P < 0·05) the expression of CLDN-1 and ZO-1 in IPEC-J2 cells exposed to HS, and the SeMet group even exhibited abundant proteins CLDN-1 and ZO-1 compared with that of the control group (Fig. 3(B)).
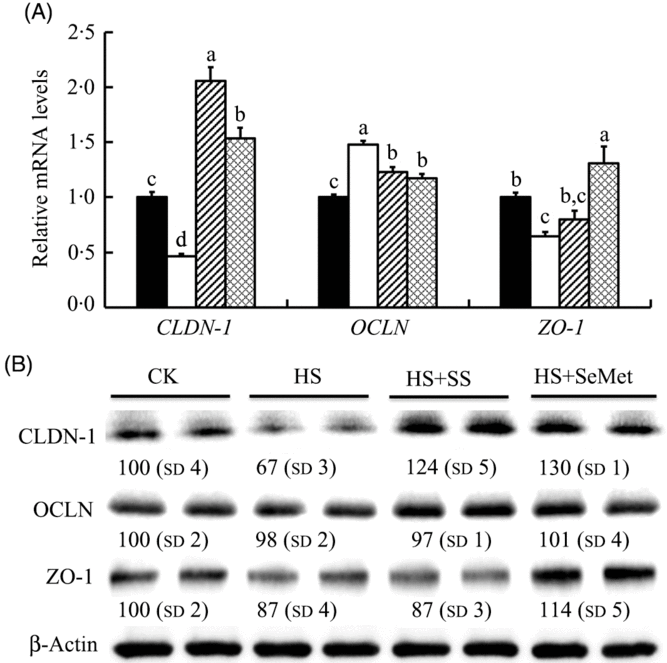
Fig. 3. Impact of sodium selenite (SS; ![]() ) and selenomethionine (SeMet;
) and selenomethionine (SeMet; ![]() ) supplementation on the mRNA abundance (A) and protein levels (B) of three tight junction-related proteins in IPEC-J2 cells subject to heat stress (HS) for 24 h. Data are presented as mean values with their standard errors, n 6 (gene) or 3 (protein). a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
) supplementation on the mRNA abundance (A) and protein levels (B) of three tight junction-related proteins in IPEC-J2 cells subject to heat stress (HS) for 24 h. Data are presented as mean values with their standard errors, n 6 (gene) or 3 (protein). a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control (CK);
, Control (CK); ![]() , HS only; CLDN-1, claudin-1; OCLN, occludin; ZO-1, zonula occludens-1.
, HS only; CLDN-1, claudin-1; OCLN, occludin; ZO-1, zonula occludens-1.
Effect of heat stress and selenium supplementation on reactive oxygen species content
ROS content in IPEC-J2 cells were measured by a flow cytometry after 24-h exposure to HS (Fig. 4). The HS group exhibited numerically higher ROS content compared with the CK group in IPEC-J2 cells, though the response was not significant (P > 0·05). SS supplementation increased (P > 0·05) cellular ROS, while SeMet supplementation reduced (P > 0·05) cellular ROS content in value compared with the HS group. However, these responses were also not significant.
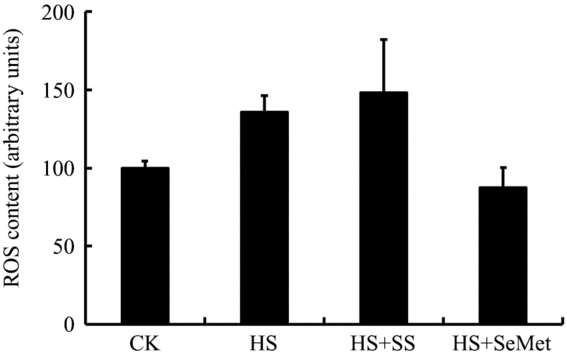
Fig. 4. Impact of sodium selenite (SS) and selenomethionine (SeMet) supplementation on reactive oxygen species (ROS) content in IPEC-J2 cells subject to heat stress (HS) for 24 h. Data are presented as mean values with their standard errors, n 4. CK, control.
Effect of heat stress and selenium supplementation on mRNA abundance of inflammation-related genes
We further investigated the response of mRNA levels in ten inflammation-related genes to SeMet and SS in IPEC-J2 cells under HS. The results showed that HS up-regulated (P < 0·05) the expression of six inflammation-related genes (IL-6, IL-8, intercellular adhesion molecule-1 (ICAM-1), interferon β (IFN-β), inducible nitric oxide synthase 2 (INOS-2) and monocyte chemotactic protein 1 (MCP-1)) and down-regulated (P < 0·05) the expression of transforming growth factor-β (TGF-β) and TNF-α in IPEC-J2 cells (Fig. 5). Both SS and SeMet supplementation effectively prevented (P < 0·05) the up-regulation of IL-6, IL-8, ICAM-1, IFN-β, INOS-2 and MCP-1 by HS, and SeMet exhibited a better protective effect (Fig. 5(A)). SS and SeMet supplementation showed no effect on the expression of TGF-β, TNF-α (Fig. 5(B)) and IL-1β (Fig. 5(C)). Of all the genes assayed, IL-10 result was too close to background to be interpreted.
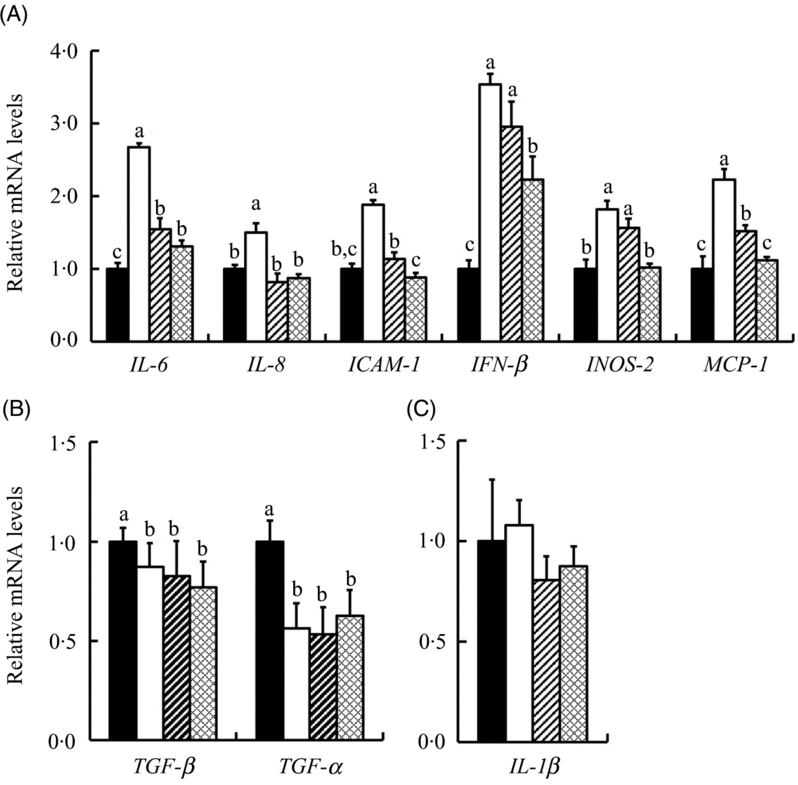
Fig. 5. Impact of sodium selenite (SS; ![]() ) and selenomethionine (SeMet;
) and selenomethionine (SeMet; ![]() ) supplementation on the mRNA profiles of ten inflammation-related genes in IPEC-J2 cells subject to heat stress for 24 h. Data are presented as mean values with their standard errors, n 6. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
) supplementation on the mRNA profiles of ten inflammation-related genes in IPEC-J2 cells subject to heat stress for 24 h. Data are presented as mean values with their standard errors, n 6. a,b,c Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control;
, Control; ![]() , heat stress only; ICAM-1, intercellular adhesion molecule-1; IFN-β, interferon β; INOS-2, inducible nitric oxide synthase 2; MCP-1, monocyte chemotactic protein-1; TGF-β, transforming growth factor β.
, heat stress only; ICAM-1, intercellular adhesion molecule-1; IFN-β, interferon β; INOS-2, inducible nitric oxide synthase 2; MCP-1, monocyte chemotactic protein-1; TGF-β, transforming growth factor β.
Effect of heat stress and selenium supplementation on mRNA abundance of selenoprotein encoding genes
We explored mRNA abundance of twenty-five selenoprotein encoding genes in the IPEC-J2 cells (Fig. 6). HS increased (P < 0·05) mRNA expression of fourteen selenoprotein genes (GPX2, GPX3, GPX4, GPX6, iodothyronine deiodinase 1 (DIO1), DIO2, thioredoxin reductase 1 (TXNRD1), selenoprotein K (SELENOK), SELENOS, SELENOT, SELENOW, SELENOF, methionine sulfoxide reductase B1 (MSRB1) and selenophosphate synthetase 2 (SEPHS2)) (Fig. 6(A) and (B)), decreased (P < 0·05) mRNA expressions of five selenoprotein genes (GPX1, TXNRD2, SELENOH, SELENOI and SELENOM) (Fig. 6(C)) and exhibited no effect on the expression of SELENON and SELENOP (Fig. 6(D)) in the IPEC-J2 cells. SS and SeMet supplementation exhibited impact on the expression of selenoprotein encoding genes in cells under HS, which decreased the expression of eleven selenoprotein encoding genes (GPX2, GPX3, GPX6, DIO2, TXNRD1, SELENOK, SELENOS, SELENOT, SELENOF, MSRB1 and SEPHS2) (Fig. 6(A)) and increased the mRNA abundance of three selenoprotein genes (GPX4, DIO1 and SELENOW) (Fig. 6(B)). Although HS suppressed the expression of five selenoprotein encoding genes, SS and SeMet supplementation modestly recovered mRNA levels of those genes (Fig. 6(C)). Although there was no effect of HS on mRNA expression of SELENON and SELENOP, SS and SeMet supplementation up-regulated (P < 0·05) the expression of these two genes (Fig. 6(D)). There was no difference in gene expression profiles when the IPEC-J2 cells were subject to HS, SS and SeMet supplementation, which included four selenoprotein genes (Supplementary Table S2). Taken together, SS and SeMet supplementation alleviated the impact of HS on the expression of selenoprotein encoding genes, SeMet groups exhibited better recovery effect than SS based on the expression of these selenoprotein encoding genes.
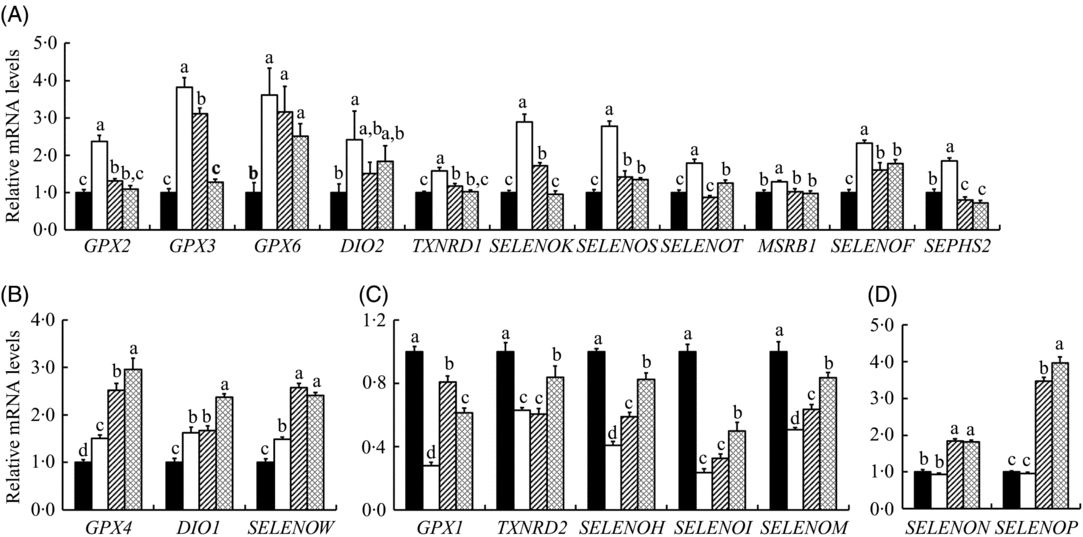
Fig. 6. Impact of sodium selenite (SS; ![]() ) and selenomethionine (SeMet;
) and selenomethionine (SeMet; ![]() ) supplementation on the mRNA profiles of selenoprotein encoding genes in IPEC-J2 cells subject to heat stress for 24 h. Data are presented as mean values with their standard errors, n 6. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05).
) supplementation on the mRNA profiles of selenoprotein encoding genes in IPEC-J2 cells subject to heat stress for 24 h. Data are presented as mean values with their standard errors, n 6. a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). ![]() , Control;
, Control; ![]() , heat stress only; GPX, glutathione peroxidase; DIO, iodothyronine deiodinase; TXNRD, thioredoxin reductase; SELENO, selenoprotein; MSRB1, methionine sulfoxide reductase B1; SEPHS2, selenophosphate synthetase 2.
, heat stress only; GPX, glutathione peroxidase; DIO, iodothyronine deiodinase; TXNRD, thioredoxin reductase; SELENO, selenoprotein; MSRB1, methionine sulfoxide reductase B1; SEPHS2, selenophosphate synthetase 2.
Effect of heat stress and selenium supplementation on selenoproteins
Effect of HS and the Se supplementation on protein levels of GPX1 and SELP in IPEC-J2 cells were also investigated (Fig. 7). HS decreased (P < 0·05) the protein levels of GPX1, while both SS and SeMet supplementation inhibited (P < 0·05) this HS-induced reduction in the IPEC-J2 cells. Although HS exhibited no impact on protein level of SELP, SS and SeMet supplementation increased (P < 0·05) its level in heat-stressed IPEC-J2 cells, and SeMet group exhibited a higher (P < 0·05) abundance compared with the SS group.
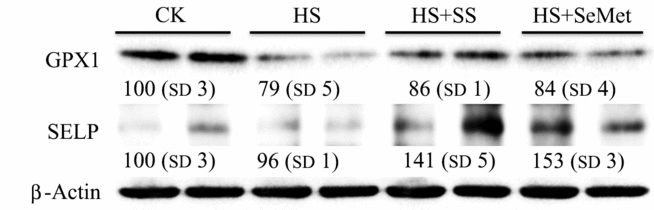
Fig. 7. Impact of sodium selenite (SS) and selenomethionine (SeMet) supplementation on the selenoprotein levels in IPEC-J2 cells subject to heat stress (HS) for 24 h. CK, control; GPX, glutathione peroxidase; SELP, selenoprotein P.
Discussion
Our previous study indicates HS-induced cell injury is associated with abnormal expression of selenogenome in porcine IPEC-J2 cells(Reference Lei, Tang and Qiang15). In the present study, we further investigate whether Se supplementation (either organic or inorganic Se) has protective effect on HS-induced IPEC-J2 cell injury. Studies have shown that HS results in oxidative damage to proteins and DNA, induces many abnormalities of cell functions, including changes in protein synthesis and function, changes in metabolism and membrane fluidity and even induces cell death(Reference Collier, Collier and Rhoads33). In the present study, the cell viability was decreased significantly with the prolongation of HS (more than 24 h) (Fig. 1). Both SS and SeMet supplementation effectively alleviated the decreased cell viability by HS, especially at later stages (Fig. 1), indicating Se supplementation provided a protective effect against the cell apoptosis induced by HS.
HSP are a family of proteins that maintain cell survival during hyperthermia and usually categorised according to their molecular weight(Reference Martin and Hartl34). Among the HSP, HSP70 is a well-known member frequently used as a biomarker of cellular HS(Reference Pei, Wu and Qin35). HSP70 expresses at low level under normal conditions, but its level increases greatly during cellular stress. After 24-h treatment, both the mRNA and protein abundance of HSP70 were increased by HS in the IPEC-J2 cells (Fig. 2), which is consistent with our previous study(Reference Lei, Tang and Qiang15). Western blot result revealed that SeMet supplementation increased the expression of HSP70 compared with HS groups in the IPEC-J2 cells (Fig. 2(B)). Under HS condition, up-regulation expression of the HSP70 can bind to unfolded or misfolded proteins and help restore their native conformation(Reference Bouchama and Knochel36). It is reported that HS significantly elevates the expression of HSP70 in the rat intestinal epithelial IEC-18 cells, which may play a role in protecting intestinal epithelial cells from oxidant and thermal injury(Reference Musch, Ciancio and Sarge37). Studies have revealed that HSP70 rescues cells from apoptosis through inhibition of anti-apoptotic protein caspase-3(Reference Jäättelä, Wissing and Kokholm38), and it can activate protein kinase B (Akt) to promote cell survival(Reference Kayama, Nakazawa and Thanos39). In rodent cardiomyocytes, HSP70 is capable of protecting cells against endotoxemia, hypoxia and metabolic stress(Reference Iwaki, Chi and Dillmann40). Our results showed that supplementation of SeMet increased the HSP70 protein level, and, apparently, it has beneficial effect for IPEC-J2 cells under HS. The addition of SS and SeMet decreased the mRNA profiles of HSP70 in stressed cells (Fig. 2(A)), which may indicate that Se alleviates the HS and the cells not need synthesise more HSP70 transcript to copy with thermal injury.
HS compromises the intestinal barrier function resulting in increased permeability and leading to increased concentrations of lipopolysaccharide in portal and systemic blood(Reference Hall, Buettner and Oberley8). Intestinal epithelium cells are tightly bound together by intercellular TJ proteins, which regulate the paracellular permeability and are crucial for integration of the epithelial barrier. TJ are complex structures composed of over fifty proteins, we compared the expression of three TJ-related proteins (CLDN-1, OCLN and ZO-1). The results showed that SeMet supplementation alleviated the down-regulation of CLDN-1 and ZO-1, SS supplementation alleviated the down-regulation of CLDN-1 by HS (Fig. 3). CLDN-1 is the structural backbone of TJ and seals the space between two adjacent epithelium cells(Reference Furuse, Sasaki and Fujimoto41). ZO-1 is one of the plaque proteins that bind to other proteins and form a scaffold or interact with specific transmembrane proteins to anchor them to the cytoplasm(Reference Fanning and Anderson42). Interestingly, the first PDZ domain of ZO-1 interacts with the CLDN proteins(Reference Itoh, Furuse and Morita43). The decreased gene and protein expression of these two proteins indicates increased permeability of the epithelial barrier. Both SS and SeMet supplementation effectively prevented the loss of TJ, and SeMet even improved the expression of those two TJ in the IPEC-J2 cells exposed to HS. SeMet exhibited a better preventive effect. OCLN regulates intermembrane diffusion and paracellular diffusion of small molecules(Reference Balda, Whitney and Flores44). Our results showed that HS increased mRNA abundance but had no impact on the protein expression of OCLN; SS and SeMet supplementation decreased mRNA profiles but exhibited no effect on protein abundance of OCLN in IPEC-J2 cells subjected to HS (Fig. 3).
ROS are generated endogenously by living organisms during oxidative metabolism. Under normal conditions, ROS is an important secondary messenger that affects intracellular signaling and redox regulation(Reference Imai and Nakagawa45), and a balance exists between the generation of ROS and the antioxidant defences. However, under HS, the antioxidant defences of cells are out of balance, and surplus ROS can trigger severe damage to biological molecules (lipids, proteins and nucleic acids)(Reference Martindale and Holbrook46), with cell membrane fluidity disruption and apoptosis(Reference Green and Reed47). In our study, although there was no statistical difference, the ROS content in HS group showed higher value in IPEC-J2 cells compared with CK cells, and SeMet supplementation reduced ROS content (Fig. 4). It is indicated that HS broke the redox balance and invoked oxidative stress in the IPEC-J2 cells. The stability of ROS is dependent on the levels and activity of antioxidant enzymes responsible for their neutralisation. The results suggested that supplementation of Se in organic form can reduce the levels of ROS.
HS has been found to cause inflammatory disorders and jeopardise immune responses(Reference Lei, Tang and Qiang15). Se is known to improve immune responses by altering the production of certain cytokines in immune cells and by enhancing the resistance of the immune cells to oxidative stress(Reference Habibian, Sadeghi and Ghazi48). In the present study, both SS and SeMet supplementation prevented the HS-induced up-regulation of six inflammation-related genes (IL-6, IL-8, ICAM-1, IFN-β, INOS-2 and MCP-1) in the IPEC-J2 cells (Fig. 5(A)). IL-6 is an important mediator for fever and acute phase response, it can change the body’s temperature through initiating the synthesis of PG E2 in the hypothalamus(Reference Banks, Kastin and Gutierrez49). Moreover, IL-6 stimulates the inflammatory and immune processes in many diseases such as diabetes and atherosclerosis, and it is a diagnosis indicator of inflammation and infectious diseases( Reference Kristiansen and Mandrup-Poulsen50). ICAM-1 has a low basal level in epithelial cells but is up-regulated in response to a variety of inflammatory mediators(Reference Hubbard and Rothlein51). Thus, SS and SeMet supplementation help to prevent the up-regulation of proinflammatory cytokines in IPEC-J2 cells under HS, thereby alleviating HS-induced immune dysfunction. The SeMet groups exhibited a lower expression of proinflammatory genes compared with SS (Fig. 5(A)), implying a better protective effect. It was reported that probiotics promote the gut health through the stimulation of epithelial innate immune responses (e.g. TNF-α), rather than by suppression of inflammation(Reference Pagnini, Saeed and Bamias52). Se supplementation also had an inhibitory effect on TNF-α in heat-stressed broiler chicks(Reference Habibian, Sadeghi and Ghazi48). Interestingly, in our study, SS and SeMet supplementation did not prevent the HS-induced down-regulated expression of TNF-α in IPEC-J2 cells (Fig. 5(B)), which might imply that SS and SeMet resisted HS-induced inflammatory injury not through further inhibiting the expression of TNF-α.
To reveal the reciprocal metabolic impact of SS and SeMet on the prevention of HS-induced IPEC-J2 cell damage, the gene profiles of twenty-five selenoproteins in IPEC-J2 cells were compared. The twenty-five selenoprotein genes were shown as three expression patterns when the IPEC-J2 cells were subject to HS (Fig. 6). First, fourteen selenoprotein encoding genes were up-regulated by HS, and eleven of them (GPX2, GXP3, GPX6, DIO2, MSRB1, SELENOK, SELENOS, SELENOT, SELENOF, SEPHS2 and TXNRD1) were down-regulated by supplementation SS or SeMet (Fig. 6(A)), while GPX4, DIO1 and SELENOW were up-regulated by supplementation of SS or SeMet (Fig. 6(B)). HS globally up-regulated most selenoprotein encoding genes in the differentiated of C2C12 cells(Reference Tang, He and Yan53). Of the selenoproteins GPX2, GPX3, GPX4 and GPX6 belong to the GPX family that can catalyse the reduction of hydrogen peroxide (H2O2) and lipid hydroperoxides using GSH(Reference Gromer, Eubel and Lee54). DIO1 is plasma membrane proteins, and DIO2 is localised in the endoplasmic reticulum (ER) membrane(Reference Munira, Diego and Balazs55). DIO2 are oxidoreductases and participate in thyroid hormone metabolism by catalysing the activation of tetraiodothyroxine to triiodothyronine(Reference Baqui, Gereben and Harney56). Selenoprotein S (SELS) and selenoprotein K (SELK) have a similar structural characteristic, and they are involved in the ER-associated degradation of unfolded and misfolded proteins(Reference Shchedrina, Everley and Zhang57). Selenoprotein T (SELT), known as a glycosylated transmembrane protein, may have a potential function related to selenoprotein W (SELW), study shows that SELT knockdown in mouse fibroblasts is compensated by increasing the expression of SELW(Reference Sengupta, Carlson and Labunskyy58). SEP15 contains a Cys-rich domain in the N-terminal part of the protein, and it may play a role in catalysing the isomerisation or reducing disulphide bonds(Reference Labunskyy, Hatfield and Gladyshev59). The protein encoded by SEPHS2 (selenophosphate synthetase 2, SPS2) is involved in the selenoprotein biosynthesis, and it can catalyse the synthesis of mono selenophosphate and as a key donor of Se(Reference Huang, Rose and Hoffmann60). In general, enzymes encoded by GPX2, GXP3, GPX6, DIO2, MSRB1, SELENOK, SELENOS, SELENOT, SELENOF, SEPHS2 and TXNRD1 are involved principally in the antioxidant activities, reduction of oxidised proteins and membranes, regulation of apoptosis and degradation of misfolded proteins in the ER. Therefore, up-regulation of these selenoenzymes may exert a mediate protective response to HS-induced damage in the IPEC-J2 cells and may be necessary for the maintenance of cell survival. Interestedly, SS or SeMet recovered the expression level of eleven genes in cells (Fig. 6(A)), which indicated that supplementation Se might alleviate the cells’ damage induced by HS and enhance the cells’ resistibility to HS.
We noticed SS or SeMet up-regulated GPX4, DIO1 and SELENOW (Fig. 6(B)). The increased expression of GPX4 may contribute to detoxification of ROS such as phospholipid hydroperoxide and H2O2 induced by HS(Reference Koyama, Omura and Ejima61). SELW also has an antioxidant function. A recent study has shown that overexpression of SELW significantly reduces the sensitivity of hamster ovary and lung cancer cells to H2O2 cytotoxicity(Reference Jeong, Kim and Chung62). Therefore, the increase in the expression of those genes suggested protection of IPEC-J2 cells from HS.
The second pattern was that HS down-regulated five selenoprotein genes (GPX1, TXNRD2, SELENOH, SELENOI and SELENOM) in IPEC-J2 cells, SS or SeMet supplementation alleviated the down-regulation of these selenoprotein genes (Fig. 6(C)). GPX1 is one of selenoproteins that is highly sensitive to changes in both Se status and oxidative stress conditions(Reference Papp, Lu and Holmgren63). GPX1 utilises GSH as a substrate for the reduction in H2O2, which involves the cellular processes modulated by hydroperoxides including cytokine signalling and apoptosis(Reference Gromer, Eubel and Lee54), and its deficiency is correlated with increased susceptibility to oxidative stress(Reference Handy, Lubos and Yang64). Therefore, it is not strange that both SS and SeMet supplementation recovered the expression of mRNA (Fig. 6(C)) and protein (Fig. 7) levels of GPX1 in IPEC-J2 cells. TXNRD2 is reported to protect cells from mitochondrial-mediated oxidative stress and apoptosis during embryogenesis(Reference Conrad, Jakupoglu and Moreno65). SELH is involved in up-regulating the levels of GSH and the activity of GPX and the total antioxidant capacity in response to the redox state, with protective effects against superoxide and cell damage(Reference Panee, Stoytcheva and Liu66). SELI may be connected to membrane phospholipids and ER protein degradation mechanisms that participate in the regulation of more general gene expression(Reference Shchedrina, Zhang and Labunskyy67). SELM is a thiol-disulfide oxidoreductase, it can encode an N-terminal peptide that is cleaved after translocation in the ER(Reference Huang, Rose and Hoffmann60). Seemingly, down-regulation of these five genes may reflect an oxidative injury induced by HS in the IPEC-J2 cells, as most of these genes encode redox-associated selenoproteins. SS and SeMet supplementation alleviated the down-regulation of these five selenoprotein genes induced by HS, which indicated a protective effect by the addition of Se.
The third pattern of regulation included a lack of effect by HS on SELENON, SELENOP, SELENOO and SELENOV, both SS and SeMet supplementation further up-regulated the mRNA expression of SELENON and SELENOP (Fig. 6(D)) while had no effect on the expression of SELENOO and SELENOV (Supplementary Table S2). Moreover, Western blot result also revealed that SS and SeMet supplementation up-regulated the protein expression of SELP, and SeMet group exhibited a higher expression compared with the SS group (Fig. 7). SELP plays an important role in transporting Se from plasma to certain tissues. SELENOP knockout mice presents very low Se concentrations in brain, testis and fetus, with severe pathophysiological consequences in each tissue(Reference Kasaikina, Hatfield and Gladyshev68). SELN is a ubiquitous glycoprotein highly expressed in fetal tissues, muscle, brain and lung. Its specific biological function remains unclear, but there is an indication that some forms of congenital muscular dystrophy are due to SELENON gene mutations(Reference Moghadaszadeh, Petit and Jaillard69). Their lack of responses to the HS may imply a physiologic necessity for a constant production of corresponding selenoproteins to cope with hyperthermia-related metabolic stress. SS and SeMet elevated mRNA and protein levels of SELP, the elevated expression of SELP may provide sufficient Se for corresponding functional selenoprotein bio-synthesis, thus helping to alleviate the HS-induced injury in IPEC-J2 cells.
In conclusion, HS decreased the cell viability, up-regulated gene and protein expression of HSP70, down-regulated gene and protein expression of TJ-related genes and induced inflammatory response by up-regulation of inflammation-related genes in porcine IPEC-J2 cells. The HS-induced cellular injury was accompanied by abnormal expression of selenoprotein encoding genes and selenoprotein. Se supplementation alleviated HS-induced damage in the intestinal epithelial cells mainly through regulation expression of selenoproteins. Se recovered most profiles of mRNA and proteins of selenoproteins in hyperthermia stressed cells, and the profiles were much similar to that of CK cells. In general, SeMet exhibited a better protective effect compared with SS. Our results revealed the relation of HS to gut-mediated immune response and the breaching of the TJ in oxidative stress with inflammatory effects, and the mitigating role of Se in various forms, thus providing a new target for further exploration of selenoproteins against the impairment induced by hyperthermia in the intestinal tissues of animals and for further research on climate change scenarios.
Acknowledgements
This work was supported partly by the National Natural Science Foundation of China (31772643 and 31272468), the Foundation of Educational Department of Sichuan (17ZA0318) and by the Special Research Funding for Discipline Construction in Sichuan Agricultural University (03570126).
H. Z. and J. T. designed the research; J. T. and L. C. conducted the experiments; G. J., G. L., X. C., G. T., J. C., H. S. and H. Z. collected sample and analysed the data; H. Z. and J. T. wrote the paper; and H. Z. had primary responsibility for the final content. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519001910










