The role of dietary flavonoids in the prevention of several chronic diseases is the subject of intense research interest and soya isoflavones have been the focus of particular attentionReference Cassidy, Albertazzi and Lise Nielsen1, Reference Usui2. To exert their action on health, micronutrients, such as soya isoflavones, have to be bioavailable. There have been major advances in the past few years regarding the absorption and the metabolism of soya isoflavonesReference Rowland, Faughnan, Hoey, Wahala, Williamson and Cassidy3 and it is apparent that soya isoflavones are sufficiently absorbed to exert biological effectsReference Cassidy, Bingham and Setchell4, reaching micromolar concentrations in bloodReference Rowland, Faughnan, Hoey, Wahala, Williamson and Cassidy3.
Isoflavones are mainly present as glucoside conjugates in most commercially available soya-containing products, except products resulting from soya fermentation, such as tempeh, tofu or several soya-based cheesesReference Choi and Rhee5. After ingestion, isoflavones are not absorbed in this form; their absorption requires hydrolysis of the sugar moiety of these compounds by gastric acidReference Xu, Harris, Wang, Murphy and Hendrich6, Reference Piskula, Yamakoshi and Iwai7 and intestinal and bacterial β-glucosidasesReference Day, DuPont, Ridley, Rhodes, Rhodes, Morgan and Williamson8, Reference Setchell, Brown, Zimmer-Nechemias, Brashear, Wolfe, Kirschner and Heubi9. A proportion of isoflavones are then absorbed and conjugated again mainly into glucuronic acid and, to a lesser extent, into sulphuric acid, increasing their solubility and consequently their ability to circulate in plasma. Another proportion of the isoflavones are metabolized by intestinal bacteria into other metabolites, such as equolReference Axelson, Sjovall, Gustafsson and Setchell10 or o-desmethylangolensinReference Adlercreutz, Musey, Fotsis, Bannwart, Wahala, Makela, Brunow and Hase11.
Isoflavones are abundant in soya and soya-derivative products such as dietary supplements. The range of different dietary soya-based supplements available through chemists, health-food shops and supermarkets has been growing many-fold over the last decade in Western countriesReference Nurmi, Mazur, Heinonen, Kokkonen and Adlercreutz12. In previous studies, the bioavailability of isoflavones was investigated using glycosilated pure compounds or aglycone pure compoundsReference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi13–Reference Bloedon, Jeffcoat and Lopaczynski15 or single soya-based foodsReference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord16, Reference Vergne, Titier and Bernard17. Interestingly, four recent articles aimed at studying factors affecting the bioavailability of isoflavones and especially compared different soya food matricesReference Anupongsanugool, Teekachunhatean, Rojanasthien, Pongsatha and Sangdee18–Reference Kano, Takayanagi, Harada, Sawada and Ishikawa21. Finally, it still remains essential to understand whether serum and urine levels are similar following consumption of a known dose of isoflavones, which are present in different food matricesReference de Pascual-Teresa, Hallund, Talbot, Schroot, Williams, Bugel and Cassidy19.
In the present study, the isoflavone content of sixty-nine soya-derivative products, available on the French and European commercial market, were analysed using an ELISA method. Isoflavones were measured after the hydrolysis of the sugar moiety, i.e. in their aglycone forms. The data on the concentrations of daidzein and genistein in foodstuffs reported in this article can be used in future studies to assess the dietary intake of these compounds and their impact on health in epidemiological studies. Moreover, the current study examined the apparent bioavailability of isoflavones comparing soya-based supplements with soya-based cheese. A group of twelve healthy Caucasian males were recruited to perform a crossover trial, in which each volunteer randomly consumed the two soya-derivative products, containing 35 mg soya isoflavones, equivalent aglycone.
Subjects and methods
Soya-based products
The commercial soya-derivative products were obtained from chemists, supermarkets or special organic food shops depending on the distribution circuits. For each trademark, a sample was used for the analysis and the measurements were performed in triplicate.
Two popular soya-based products were used to compare apparent bioavailability of isoflavones. A soya-based supplement in capsule form was provided by Arkopharma, Pharmaceutical Laboratories (Carros, France) and a soya-based cheese was provided by Le Sojami (Agen, France). Before the clinical trial, each formulation was assessed for daidzein and genistein content measured with an ELISA method and expressed in aglycone equivalents. The amount of each soya-based food consumed by the subjects was adjusted to ensure that the total intake of isoflavones, equivalent aglycone, was similar throughout the study.
The chemical composition of the isoflavones, contained in the two particular food products tested for isoflavone bioavailability comparison, was assessed using the extraction and HPLC methods described by Murphy et al. Reference Murphy, Song, Buseman and Barua22. Furthermore, the nutritional composition of these two soya-based items, based on the information supplied by the manufacturers, is given in Table 1.
Table 1 Food composition of the two soya-based products used for the bioavailability study†
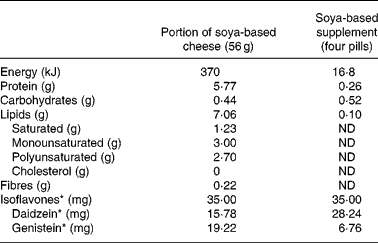
ND, Not determined.
* Expressed as aglycone equivalent.
† For details of procedures, see Subjects and methods.
Study subjects
Twelve healthy male Caucasian volunteers, aged 20 to 29 years with BMI between 20 to 25 kg/mReference Usui2, gave informed consent to enter the present study. Prior to the study, all subjects underwent a full clinical examination. None of the subjects had allergy or intolerance to soya. The subjects had to abstain from consuming any drugs, especially antibiotics, for at least 30 d prior to the beginning of the study and during the study. Soya foods and their derivatives were prohibited for 10 d prior to and during the study. The main foods containing polyphenols, such as red fruits, red wine, chocolates, tea or coffee, were prohibited for 3 d prior to the beginning of the study and during the study. The study was performed at the Clinical Investigation Center (Haut-Levêque Hospital, Pessac, France) and was approved by the local Medical Ethics Committee (Comité Consultatif pour la Protection des Personnes se prêtant à des Recherches Biomédicales, CCPPRB, Bordeaux A, France). During the kinetic analysis, meals were controlled by a dietitian and were strictly the same for every subject. No adverse effect in relation to the ingestion of the soya-based products was reported.
Design of the study
The design was a randomized, two-way crossover study, involving twelve young male volunteers, who were fed a soya-derivative product containing 35 mg isoflavones, as either supplements or cheese. On the basis of the 35 mg total aglycone isoflavones, each subject consumed four pills of Phytosoya® (Arkopharma), i.e. 28·24 mg daidzein and 6·76 mg genistein, and 56 g cheese Le Tartimi® (Le Sojami), i.e. 15·78 mg daidzein and 19·22 mg genistein. Volunteers were hospitalized at 12.00 hours for a 24 h period and randomly received a single dose of either Phytosoya® or soya-based cheese. After intake of the soya-based capsules or cheese, volunteers had lunch at 12.00 hours, dinner at 19.00 hours and breakfast at 07.00 hours the following morning. After a 2-week wash-out period, the study was repeated in the same conditions to complete the crossover design. Blood samples (10 ml) were drawn into Vacutainer® glass tubes (Becton Dickinson, Le Pont-De-Claix, France) containing heparin and lithium as anticoagulants, through an indwelling cannula, before (0) and 2, 4, 6, 8, 12, 18, 24 and 48 h after the intake of soya-based products. Plasma samples were prepared by centrifugation at 5000 g, 5 min, 4°C and stored frozen at − 20°C until further analysis. Urinary samples were collected before (0) and 6, 12, 18, 24 h after the ingestion of soya-derivative products. During the second day of the experiment, volunteers were instructed to collect all their urine in plastic bottles containing ascorbic acid (1 g/l). The volume of each micturition was measured and a 10 ml aliquot of each sample was removed and stored at − 20°C until analysis.
Serum and urinary isoflavone analysis
Daidzein, equol and genistein concentrations in masked blood samples were measured by the ELISA method previously describedReference Bennetau-Pelissero, Le Houerou, Lamothe, Le Menn, Babin and Bennetau23, Reference Bennetau-Pelissero, Arnal-Schnebelen, Lamothe, Sauvant, Sagne, Verbruggen, Mathey and Lavialle24. Briefly, samples were first digested for 48 h with β-glucuronidase aryl sulfatase from Helix pomatia (Roche Diagnostics, Meylan, France) in acetate buffer, pH 5. Aglycone isoflavones were then extracted three times with acidified ethyl actetate buffer (Fluka, Buchs, Switzerland). Finally, assays were performed following the classical indirect competitive ELISA using primary rabbit antibodies for daidzein, equol and genistein developed in the laboratoryReference Bennetau-Pelissero, Le Houerou, Lamothe, Le Menn, Babin and Bennetau23, Reference Le Houerou, Bennetau-Pelissero, Lamothe, Le Menn, Babin and Bennetau25. The secondary antibody was polyclonal swine anti-rabbit IgG, complexed with a peroxydase enzyme (Dako, Trappes, France) used with o-phenylenediamine as a substrate for revelation. Optical density (OD) was read at 490 nm (MRX II; Dynex Technologies, Issy-les-Moulineaux, France) and the concentration of isoflavones was determined from standard curves of each isoflavone (r 2 ≥ 0·99). Hydrolysis and extractions were checked against external standards. The inter-assay variation is 12·8 % for daidzein, 13·1 % for genistein and 13·6 % for equol measured comparing the same sample on ten different plates. The intra-assay variation 5 % for daidzein, 4·8 % for genistein and 5 % for equol measured on the same sample assayed twelve times on the same plate. The samples are run randomly on plates together with an assay control sample (same sample on each plate). The sensitivity of the assays given as the mid point of the standard curve is 15·6 ng/ml for daidzein and genistein and 10 ng/ml for equol. The detection limits of this specific analytical method are 3·9 ng/ml for daidzein and for genistein and 2·5 ng/ml for equol and have been improved from the previously reported studyReference Bennetau-Pelissero, Arnal-Schnebelen, Lamothe, Sauvant, Sagne, Verbruggen, Mathey and Lavialle24. The technique was validated against the HPLC method coupled to a UV detector for the supplements and foods and coupled to a cool array detector for biological fluid samplesReference Mathey, Lamothe, Coxam, Potier, Sauvant and Pelissero26.
Determination of the serum and urinary isoflavone pharmacokinetics
All pharmacokinetic parameters were performed by the pharmacokinetic software PK-FIT version 1.2 (RDPP, Montpellier, France). A non-compartmental pharmacokinetic analysis was used to analyse plasma isoflavone concentration-time data. The maximum observed concentration (C max) and time to reach peak concentration (T max) parameters were obtained directly from experimental observations without interpolation. The terminal slope (K e) of the concentration-time curve was determined by log-linear regression. Elimination half-life (T ½) of the terminal log-linear phase was calculated following the equation 0·693/K e. The area under the plasma concentration-time curve was extrapolated to infinity (AUC0 → ∞). It was determined by summing the areas from time 0 to the time of the last quantifiable concentration (t) (obtained by trapezoidal and log-trapezoidal methods: AUC0 → t) and the extrapolated area from t to infinity (AUCt → ∞). The extrapolated area was determined by dividing the last detectable concentration by the slope of the terminal log-linear phase. To ensure that statistical comparisons were valid, C max and AUC0 → ∞ values for daidzein and genistein were dose-adjusted to take into account the differences in the proportion of isoflavones within each soya food product.
Statistical analysis
All data are expressed as means and standard deviations. Comparison of the pharmacokinetic parameters of each isoflavone was based on a crossover analysis using intra-subject comparisons of the two soya-based products. It consisted of a two-step strategy as proposed by GrizzleReference Grizzle27. First, the interaction between the two soya-based products and the intake period was studied. If significant at a nominal significance level 0·10, the comparison between the two soya-based products only used the data from the first intake period. Otherwise, comparison between the two soya-based products was performed using the two intake periods. All comparisons used the Wilcoxon sign rank test. Except for the interaction analysis, all tests were considered statistically significant at a P value < 0·05.
Statistical analyses between the mean urinary excretions of isoflavones, according to the two soya-based products ingested, were performed by Student's t test. Differences were considered significant at P < 0·05.
Results
Isoflavone contents in commercial soya foods
Tables 2 and 3 present respectively the isoflavone content assessed by ELISA in forty-nine soya-based supplements and in twenty soya-based foods available on the French and European market. From these tables, it can be observed that content of isoflavones can vary greatly according to the brand. Indeed, consumers can ingest from 0·07 to 92·8 mg aglycone isoflavones per d, as specified by the manufacturers on the product packaging. Of the sixty-nine soya food products reported in this article, forty-six exhibit a major content of genistein in comparison with daidzein. Despite the lower concentrations of isoflavones in soya foods compared with soya-based capsules, isoflavone level intakes are of the same order of magnitude due to the size of the food portions ingested.
Table 2 Isoflavone content in diet supplements based on soya and freely available on the European market

* Values resulting from ELISA assessment are less than 50 % of the claimed values of isoflavones. The differences between claim doses and assessment doses may represent the weight of sugar conjugated with isoflavones.
† Claimed values are similar to the ELISA assessment values of isoflavones. Manufacturers have ever indicated the isoflavones in aglycone equivalent.
‡ Results already measured and publishedReference Bennetau-Pelissero, Arnal-Schnebelen, Lamothe, Sauvant, Sagne, Verbruggen, Mathey and Lavialle24 and were measured again for this study.
§ High values are due to the presence of another isoflavone, the biochanin A (data not shown).
Table 3 Isoflavone concentration of solid matrix (A) or liquid matrix (B) soya-based foodstuffs, available on the European market

Isoflavone contents in foods used for clinical study
Fig. 1(A),(B) represents the HPLC chromatograms corresponding to the soya-based products ingested during the clinical study. Isoflavones are mainly present as glycoside forms in the capsules: 97·4 % daidzein and 98·1 % genistein ingested were in glycoside form. For cheese, a larger proportion of aglycone isoflavones was measured. It was found that 57·8 % daidzein and 43·7 % genistein ingested were in glycoside form.

Fig. 1 HPLC chromatographic profiles of soya-based capsules (A) and soya-based cheese (B) ingested by the twelve volunteers. Peak identification: 1. daidzin; 2. genistin; 3. malonyl-daidzin; 4. acetyl-daidzin; 5. malonyl-genistin; 6. daidzein; 7. acetyl-genistin; 8. genistin. Tables represent the percentage of each isoflavone and their associated glycosides found in the two soya-based products. For details of subjects and procedures, see Subjects and methods.
Serum kinetics of isoflavones
The mean plasma concentration time-profiles of daidzein and genistein from 0 to 48 h after a single oral dose of both soya-based foodstuffs are represented in Fig. 2. At baseline, patients had no detectable concentrations of genistein and daidzein. The kinetics of both isoflavones present a similar pattern. Absorption is biphasic and occurs between 0 and 8 h. A first peak of daidzein concentration was reached at 2 h for both soya-based foodstuffs and the second one at 8 h following isoflavone intake, leading to a mean T max of 6·9 (sd 3·4) and 6·1 (sd 5·0) h for capsules and cheese respectively. In the case of genistein, T max values were 6·7 (sd 4·0) and 4·6 (sd 2·4) for capsules and cheese respectively. Pharmacokinetic analysis of the plasma concentration-time curves showed that the elimination T ½ of genistein was 15·3 (sd 7·4) and 11·9 (sd 4·4) h for capsules and cheese respectively. In the present study, no equol producers were found within the recruited population of volunteers.

Fig. 2 Time-course of plasma daidzein (A) and genistein (B) concentrations, adjusted to the intake of each isoflavone, in twelve volunteers following soya-based cheese (□) or soya-based capsules (♦) intake. Values are means with their standard errors of the mean. Graphics at the top represented time-course of plasma isoflavones without the dose intake adjustment. For details of subjects and procedures, see Subjects and methods.
Comparison of isoflavone bioavailability between soya-based cheese and soya-based capsules
The mean pharmacokinetic parameters of isoflavones and the P values of the two soya-based products are shown in Table 4. Despite the equal amount of total isoflavone intake, the bioavailability varied according to the two soya-based products used. The adjusted Cmax of daidzein from soya-based cheese is significantly lower than that from soya-based capsules (P = 0·002). Similarly, the adjusted C max of genistein was significantly lower for cheese than that measured for capsules (P = 0·002). The adjusted AUC0 → ∞ of genistein was 171·3 (sd 47·2) and 331·7 (sd 155·5) ng/ml per h per mg ingested for cheese and capsules, respectively, and were significantly different (P = 0·002).
Table 4 Pharmacokinetic parameters for daidzein and genistein and relative bioavailability of isoflavones contained either in soya-based cheese or soya-based capsules‡
(Mean values and standard deviations)

NP, statistical analysis was not performed due to differences in oral administered doses between both soya-containing foods.
* Pharmacokinetic parameters were adjusted for oral administered intake of individual isoflavone.
† The interaction between period and food type was significant. As recommended by Grizzle, elimination T ½ data and P value effect reported are only those of the first period of the crossoverReference Grizzle27.
‡ For details of subjects and procedures, see Subjects and methods.
Moreover the adjusted AUC0 → ∞ for both the isoflavones in cheese was 1·27 μmol/l per h per mg isoflavones ingested (0·63 (sd 0·17) and 0·64 (sd 0·29) μmol/l per h per mg, for genistein and daidzein respectively). On the other hand, the globally adjusted AUC0 → ∞ for capsules is 1·92 μmol/l per h per mg ingested (1·23 (sd 0·58) and 0·69 (sd 0·22) μmol/l per h per mg for genistein and daidzein respectively), leading to an adjusted AUC0 → ∞ ratio of 1·50 between the two soya-based products.
Urinary excretion of isoflavones
From 0 to 24 h, the daidzein excretion profile was a bell-curve shaped with a maximal excretion peak between 6 and 12 h (Fig. 3(A)). For capsules and cheese respectively, 82·6 (sd 6·1) % and 65·4 (sd 7·2) % ingested daidzein was eliminated during the study period. The genistein excretion profile is similar to that observed for daidzein (Fig. 3(B)). For the capsules and cheese respectively, 46·3 (sd 7·4) and 26·9 (sd 4·6) % of the total ingested genistein was excreted in urine. Whatever the isoflavone ingested, the total urinary excretion was higher for the intake of capsules than that for the intake of cheese. Particular differences appeared in the urinary excretion of daidzein during the 6–12 h and 12–18 h periods and in the urinary excretion of genistein during the 6–12 h period.

Fig. 3 Urinary daidzein (A) and genistein (B) excretion profiles following the ingestion of capsules (■) or cheese (□), adjusted to the intake of each isoflavones. Results are expressed as means with their standard errors of the mean. Histograms at the top represented values without the dose intake adjustment. For details of subjects and procedures, see Subjects and methods.
Discussion
To assess fully the dietary impact of phyto-oestrogens on the health of the population, it is necessary to know their concentrations in the foods consumed and their circulating concentrations following intake.
Isoflavones are mainly present as glucoside conjugates in most commercially available soya-containing productsReference Hubert, Berger and Dayde28. However, isoflavones are hydrolysed as a free form before their intestinal absorption. Even if a conjugation with glucuronide or sulphate takes place during the metabolism of isoflavones, the isoflavone molecule still remains the active party due to its ability to bind to the oestrogen receptorsReference Muthyala, Ju, Sheng, Williams, Doerge, Katzenellenbogen, Helferich and Katzenellenbogen29 after entering the target cells. Provider claims often take into account the whole weight of the isoflavone molecules, including sugar moiety, which explains the most frequent discrepancies observed between the provider claims and the data measured in our laboratoryReference Bennetau-Pelissero, Arnal-Schnebelen, Lamothe, Sauvant, Sagne, Verbruggen, Mathey and Lavialle24. The weight of the glucose unit is approximately 40 % of the total glucoside weight and in the case of malonyl- and acetyl-forms the weight of the inactive sugar moiety increases up to 50 %. In addition, the relative proportions of each glycoside form are known to be influenced by the extraction steps taken before analysisReference Verbruggen and van Roojen30. Consequently, as already suggested by Nurmi et al., Erdman et al., the French Food Safety Agency and Messina et al., the recommended way to express isoflavone content in soya food products is to use the aglycone weightReference Nurmi, Mazur, Heinonen, Kokkonen and Adlercreutz12, Reference Erdman, Badger, Lampe, Setchell and Messina31–Reference Messina, Nagata and Wu33.
The present study presents isoflavone content data in a selection of foodstuffs, which could be included in an existing phyto-oestrogen databaseReference Ritchie, Cummings, Morton, Michael Steel, Bolton-Smith and Riches34. These food products represent only a small proportion of the ‘Western’ soya-based products freely available on the market. In France, the isoflavone intake for a traditional meal was estimated to be of the order of 22·1 μg, from the database published by the French Food Safety Agency32. However, more isoflavones could be ingested by consuming several soya-based products, such as those presented in the current study. Regularly consumed, these products can induce non-negligible isoflavone concentrations in plasma and may lead to physiological effects.
Although, there has been interest in the potential risks and benefits of consuming diets enriched in soya isoflavones, to date, relatively limited research has been conducted comparing apparent bioavailability of isoflavones contained in soya-based supplements or in soya-based food and concentrations reached in blood and urine. The soya-based products selected for our clinical trial represented two different nutritional forms of isoflavone intake, since one is a food supplement, i.e. taken daily and several times throughout the day, and the other is a soya-based cheese, eaten occasionally during a meal. In all cases, to exert their biological effects, isoflavones have to be bioavailable.
The aim of the present clinical study was to investigate the concentrations of isoflavones reached in serum and urine in twelve healthy male volunteers given an oral dose of two different food products containing 35 mg isoflavones, equivalent aglycone. Since both soya-based products consist of different proportions of daidzein and genistein (approximately 3:4 for cheese and 4:1 for capsules), the total quantity of isoflavones was first considered. Daidzein and genistein contents in soya food can vary and depend on the raw material and processing conditions used to produce a particular food product. In each type of soya food, there are different forms of isoflavones in differing amounts. Based on the equivalent dose of isoflavones, the administration of different soya foods has shown no difference in particular isoflavone bioavailabilityReference Xu, Wang, Murphy and Hendrich35. Even though the metabolism is similar, no competition phenomenon of absorption, metabolism and elimination of these molecules has ever been reported. Furthermore, the total quantity of isoflavones is lower than those used to describe a small saturation effect in recent intervention studiesReference Setchell, Brown, Desai, Zimmer-Nechimias, Wolfe, Jakate, Creutzinger and Heubi36. Consequently, a normalization with the particular amount of each compound was performed to compare the pharmacokinetic parameters, as has classically been described by several authorsReference Anupongsanugool, Teekachunhatean, Rojanasthien, Pongsatha and Sangdee18, Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell20.
The food supplement was initially chosen due to its high daidzein content. It was expected to lead to rather high equol concentrations in plasma. Unfortunately, out of the twelve volunteers, no one produced equol. This is not linked to the ELISA used in the present study since our previous studies reported an equol producer percentage close to that mentioned by other authorsReference Vergne, Titier and Bernard17, Reference Bennetau-Pelissero, Arnal-Schnebelen, Lamothe, Sauvant, Sagne, Verbruggen, Mathey and Lavialle24, Reference Mathey, Lamothe, Coxam, Potier, Sauvant and Pelissero26. Furthermore, the design and the duration of the study cannot be responsible for this finding because a recent trial performed in the same conditions found four volunteers as equol producers out of a total of twelve volunteersReference Vergne, Titier and Bernard17. Finally, according to criteria used to define equol producers, a previous clinical study did not find any equol producers among the twelve volunteersReference de Pascual-Teresa, Hallund, Talbot, Schroot, Williams, Bugel and Cassidy19. Since equol is produced by only 30 to 50 % of the population, recruitment randomization alone may explain why no equol producers were involved in this trial.
In the present study, absorption and bioavailability parameters were in agreement with those classically described in literature. Moreover elimination T ½ was found to be longer than T ½ previously described in the literatureReference Rowland, Faughnan, Hoey, Wahala, Williamson and Cassidy3. With the present results, we confirmed longer elimination T ½, which had already been demonstrated in our previous study, in which the same soya-based capsules of Phytosoya® were ingested by healthy volunteersReference Vergne, Titier and Bernard17. Moreover, Richelle et al. reported a similar elimination half-life of 17·8 (sd 2·7) h for ingested glycoside genisteinReference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord16. With long elimination T ½, both genistein and daidzein are potentially able to accumulate in plasma, achieving a steady state level. Shorter T ½ do not allow this kind of kinetic pattern, except in the case of daily repeated ingestion. Such a practice is common to Asian people consuming soya foods as a natural component of the traditional dietReference Yamamoto, Sobue, Kobayashi, Sasaki and Tsugane37.
In any study dealing with bioavailability, the accuracy of pharmacokinetic parameters is dependent on obtaining sufficient plasma samples during the elimination phase. The 48 h point samples showed values near zero ng/ml, giving significance to this work. When pharmacokinetic parameter adjustment is performed with the real amounts ingested, the genistein concentrations are consistently higher in plasma than daidzein concentrations. The daidzein parameters of volume of distribution and a clearance rate were classically admitted to be higher and faster than those of genisteinReference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi13 and can explain this observation. Furthermore, whatever the soya-based products ingested, the higher urinary excretion of daidzein compared with that of genistein can be observed, in accordance with literatureReference Vergne, Titier and Bernard17, Reference Watanabe, Yamaguchi, Sobue, Takahashi, Miura, Arai, Mazur, Wahala and Adlercreutz38. The total urinary excretion of added isoflavones was higher for capsule intake than that of cheese intake, which is in accordance with the higher intestinal absorption of isoflavones contained in capsules.
The same total quantities of isoflavones, contained in two solid forms, were given to the subjects. Isoflavones from soya-based capsules were found to be more bioavailable than those from soya-based cheese, in spite of the differences in isoflavone composition between the two soya-based products. This result could be due to the high complexity of the cheese matrix, with its high content of lipids and proteins, which may limit intestinal absorption. Indeed, in such a matrix, isoflavones are expected to bind to other molecules by hydrogen or Van Der Waals bonds. Concomitantly, capsules are filled with a soya extract containing 10 % isoflavones. Soya extracts result from a complex manufacturing processReference Choi and Rhee5, which may weaken or break the links between isoflavones and other molecular structures present in the food supplements. This could explain why the isoflavones contained in capsules were absorbed faster and in greater amounts than those contained in soya-based cheese, even though the two soya-based products do not present a similar conjugation pattern of isoflavones. Setchell et al. showed greater bioavailability of glucosides, as measured from the area under the curve, using single purified forms of isoflavoneReference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi13. Izumi et al. found greater bioavailability of aglycones, on the basis of C maxReference Izumi, Piskula, Osawa, Obata, Tobe, Saito, Kataoka, Kubota and Kikuchi39, but they did not measure isoflavone concentrations between 6 and 24 h, whereas Setchell et al. reported that the mean time to reach C max was prolonged to 9 h after glycoside ingestionReference Setchell, Brown, Desai, Zimmer-Nechemias, Wolfe, Brashear, Kirschner, Cassidy and Heubi13. Two other clinical studies found no significant differences in the absorption efficiency for aglycones and glycosidesReference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord16, Reference Zubik and Meydani40. No clear statement exists on the bioavailability of aglycone isoflavones and their glycosides forms. Recently, no difference was found in the bioavailability of isoflavones following a single ingestion of tempeh and textured vegetable protein in twenty-one young healthy males (16·29 (sd 4·65) and 19·79 (sd 7·87) μmol/h per litre per mg, respectively, for the area under the curve of daidzein normalized to mg ingested and 26·91 (sd 13·50) and 22·98 (sd 14·12) μmol/h per litre per mg, respectively, for the area under the curve of genistein normalized to mg ingested)Reference Cassidy, Brown, Hawdon, Faughnan, King, Millward, Zimmer-Nechemias, Wolfe and Setchell20. Tempeh is a fermented food that contains approximately 50 % aglycone isoflavones, whereas textured vegetable protein contains less than 15 % aglycone isoflavones. The conjugation patterns of these two products are similar to those of the soya-based cheese and the soya-based supplement used in the present clinical study. Consequently, the difference in the conjugation patterns of these two soya-based products may not induce differences in the bioavailability of isoflavones and thus lead us to interpret our results accordingly.
Interestingly, our observation of biphasic pharmacokinetic profiles of isoflavones occurring at 2 h (peak 1) and 4–8 h (peak 2) after the soya-based product intake confirms previous studiesReference Setchell, Brown, Zimmer-Nechemias, Brashear, Wolfe, Kirschner and Heubi9, Reference Zubik and Meydani40. The presence of two distinct peaks may indicate entero-hepatic recirculation as has already been suggested by several authorsReference Rowland, Faughnan, Hoey, Wahala, Williamson and Cassidy3, Reference Richelle, Pridmore-Merten, Bodenstab, Enslen and Offord16, Reference Watanabe, Yamaguchi, Sobue, Takahashi, Miura, Arai, Mazur, Wahala and Adlercreutz38. Furthermore, the presence of distinct peaks 1 and 2 may also define separate locations of preferred isoflavone absorption during digestion. Franke et al. suggested that the times at which peaks 1 and 2 occurred indicated that the location of uptake is respectively the small and the large intestineReference Franke, Custer and Hundahl41. In the case of soya-based beverage intake, the peaks occurred at 1 h and 4–6 h. In the present study, the delay observed may be due to the time frame for solid matrices to reach the large intestine.
If we considered the AUC0 → ∞ of both isoflavones, isoflavones contained in capsules were more bioavailable by 50·4 % than those contained in soya-based cheese. A comparison with Asian populations could be attempted, using as a reference the study from Arai et al. Reference Arai, Watanabe, Kimira, Shimoi, Mochizuki and Kinae42. The isoflavone intake of 115 Japanese women was found to be 47·2 mg per d, with an inter-individual variation reported from 12·0 to 118·9 mg. If soya-based cheese is assumed to be equivalent in value to other categories of soya-based Japanese food, 75·52 g soya-based cheese is necessary to mimic the mean Japanese isoflavone intake. Concerning soya-based supplements, this would be achieved with 33·04 mg isoflavone, i.e. 3·8 capsules of Phytosoya®. Moreover, the great variation in isoflavone intake, reported by Arai et al. Reference Arai, Watanabe, Kimira, Shimoi, Mochizuki and Kinae42, may be equivalent to a range of 8 to 83 mg ingested by soya-based supplements. However, it must be kept in mind that this extrapolation is based on various hypotheses, which are far from being generally accepted. This parallel is merely theoretical and should be taken with caution.
In conclusion, assuming that isoflavone bioavailability is not influenced by the isoflavone conjugation pattern, the present work shows that isoflavones contained in the soya-based capsule are more bioavailable than those contained in soya-based cheese, after a single ingestion in twelve young male volunteers. Nevertheless, there is still little evidence to conclude that soya-based supplements are biologically active to dateReference Faure, Chantre and Mares43 and further studies are needed to establish the effectiveness of soya isoflavones on health. The bioavailability of an active compound must be known before investigating the potential physiological effects. As a consequence, it seems crucial to understand the mechanism by which all these compounds are absorbed and to increase the knowledge on factors influencing their bioavailability.
Acknowledgements
The authors thank the volunteers who participated in the study, Danièle Lamazière, the dietitian who checked food composition for the study period, Jean-James Garreau for providing the cheese, Patricia Baile and Philippe Abbe for their kind assistance with the HPLC method and Christelle Rosier-Sala for her kind help in reviewing the manuscript. Special thanks to Russell Wallace for his kind help with the English language. This study was supported by Research Ministry of France, RARE Program N°03P221 and by the Région Aquitaine. Sébastien Vergne is the recipient of a fellowship (CIFRE N°856/2003) from Arkopharma, Pharmaceutical Laboratories and National Association of Technical Research, Research Ministry of France.









