Diabetes is a well-known public health issue with an increasing prevalence worldwide. The scientific community has estimated that 592 million people will be diagnosed with diabetes by 2030(Reference Shaw, Sicree and Zimmet1). In type 2 diabetes mellitus (T2DM), raised blood sugar levels known as hyperglycaemia(Reference Stettler, Allemann and Jüni2,Reference Niafar, Bahrami and Yagin3) can lead to various chronic complications, including CVD, kidney disease, retinopathy, neuropathy and amputation(Reference Stettler, Allemann and Jüni2–Reference Skyler4). Patients with diabetes are three times more likely to be hospitalised than healthy subjects. Higher risk of early death and shorter life expectancy have been observed in T2DM patients(Reference Björk5,Reference Jalili, Moradi and Babaei6) . One of the essential goals in the treatment of T2DM is the control of glycaemic parameters(Reference King7).
Mg is one of the most crucial micronutrient for humans, having a vital role in countless body reactions, including insulin secretion and activity, blood sugar regulation and in the energy and carbohydrates’ metabolism(Reference Singh, Verma and Agrawal8,Reference Asbaghi, Hosseini and Boozari9) . Mg deficiency has been reported in 25 % to 38 % of diabetic patients(Reference De Lima, Cruz and Pousada10). However, Kurstjens et al.(Reference Kurstjens11) reported that the prevalence of hypo-magnesemia in cohorts of diabetes patients was between 11 % and 65 % of patients. Low intracellular and extracellular Mg levels, known as hypo-magnesemia, can derive due to reduced Mg intake or elevated Mg losses in poorly controlled diabetes(Reference Resnick, Altura and Gupta12,Reference Garland13) . Hypo-magnesemia is linked to insulin resistance, decreased pancreatic insulin release, altered cellular glucose transport, impaired glucose tolerance and more rapid decline in kidney function(Reference Asbaghi, Moradi and Nezamoleslami14–Reference Gommers, Hoenderop and Bindels17). Hence, hypo-magnesemia in diabetes patients leading to faster progression of diabetes and risk of end-stage kidney disease, CVD, nephropathy, retinopathy and foot ulcers(Reference Asbaghi, Moradi and Nezamoleslami14–Reference Gommers, Hoenderop and Bindels17).
Previously, Song et al. 2006(Reference Rodriguez-Moran and Guerrero-Romero15,Reference Song, He and Levitan18) performed a systematic meta-analysis review on nine randomised clinical trials (RCT) to evaluate the efficacy of oral Mg supplementation on glycaemic control, lipids, blood pressure or Mg levels in patients with T2DM compared to the control group. The findings reported that Mg supplements were associated with a significant reduction in fasting blood sugar (FBS) but not HbA1c. However, several RCT(Reference Singh, Verma and Agrawal8,Reference Barragán-Rodríguez, Rodríguez-Morán and Guerrero-Romero19–Reference Sadeghian, Azadbakht and Khalili27) have been added to the literature, and the former results need to update.
Until now, the results of the studies on the efficacy of Mg supplementation on glycaemic control in T2DM patients are still inconsistent. Some studies have demonstrated that Mg supplementation is associated with improved glycaemic control and could prevent chronic complications of diabetes(Reference Guerrero-Romero and Rodriguez-Moran20,Reference Talari, Zakizade and Soleimani28) . Although, other studies have not demonstrated such results(Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero22). These clinical trials individually cannot provide a clear answer whether Mg affects the glycaemic control of T2DM patients, and any previous meta-analysis needs to update. For this reason, in this study, we performed a systematic review and meta-analysis of the RCT to assess the effect of oral Mg supplementation on glycaemic control in T2DM patients.
Methods
Literature search and selection
This systematic review and meta-analysis were conducted based on the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis(Reference Moher, Liberati and Tetzlaff29). A systematic literature search was performed by the PubMed, SCOPUS, Web of Science, Google Scholar and Embase databases up to 30 February 2020. The systematic search was carried out using the following medical subject heading terms, in abstracts and keywords without language or date limitations. This was conducted using the following search terms ((‘Type 2 diabetes’ OR T2DM OR diabetes) AND (Intervention OR ‘Intervention Study’ OR ‘Intervention Studies’ OR ‘controlled trial’ OR randomized OR randomized OR random OR randomly OR placebo OR ‘clinical trial’ OR Trial OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR RCT OR blinded OR ‘double blind’ OR ‘double blinded’ OR trial OR ‘clinical trial’ OR trials OR ‘Pragmatic Clinical Trial’ OR ‘Cross-Over Studies’ OR ‘Cross-Over’ OR ‘Cross-Over Study’ OR parallel OR ‘parallel study’ OR ‘parallel trial’)). Electronic database systematic searches were completed along with reference list and citation hand searches. The research process was conducted by two authors (OM and SM) separately and in duplicate. Any disagreement was resolved through discussion with a third researcher (MM).
Eligibility criteria
Two investigators selected eligible articles separately by reading titles, abstracts and, whenever required, the full text of the publications. All human RCT (either parallel or cross-over designs) reported the effects of Mg supplementation on glycaemic parameters, particularly FBS, fasting insulin, the homoeostatic model assessment of insulin resistance (HOMA-IR) and HbA1c were considered. Studies were excluded if they had one or more of the following characteristics: (i) non-RCT, (ii) RCT with treatment duration < 2 weeks, (iii) studies without a control group for oral Mg supplementation and (iv) insufficient data. To keep away from overlapping, we included studies with larger participants. Disagreements regarding the study selection process were resolved by face-to-face discussion.
Data extraction
The following data were extracted from the full text of the eligible studies using a pre-designed abstraction form: (i) first author’s name, (ii) year of the publication, (iii) location of the study, (iv) sample sizes of the Mg and control groups, (v) type and dose of the Mg supplementation and placebo, (vi) study duration and (vii) age, gender and BMI. In cases of lack of relevant data, we contacted the corresponding authors via e-mail to provide their help. The process of data extraction was undertaken independently by two investigators (OA and SM) to minimise potential errors. If there was a disagreement, it was resolved by consensus.
Study quality assessment
We used the Cochrane Collaboration’s tools for quality assessment of studies to perform a systematic assessment of bias(Reference Higgins, Altman and Gøtzsche30). This tool separates a judgement about the risk of bias from a description of the support for that judgement for a series of items covering different bias domains. Two researchers (OA and SM) independently evaluated the methods and the quality of the eligible studies using Cochrane Collaboration’s tools, covered the following domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting and (7) other possible sources of bias. For each item in the tool, the assessment of the risk of bias is in two parts. The support for judgement provides a succinct-free text description or summary of the relevant trial characteristic on which judgements of risk of bias are based and aims to ensure transparency in how judgements are reached(Reference Higgins, Altman and Gøtzsche30). The first part was a further classification: low risk (L), high risk (H) and unclear risk (U) of bias. Then, according to the guidelines, the general quality of each study was considered as good (low risk for more than two cases), fair (low risk for two cases) or weak (low risk for less than two cases)(Reference Higgins, Altman and Gøtzsche30).
Meta-analysis of data
To analyse the effect size for FBS, fasting insulin, HOMA-IR and HbA1c, the mean difference and its standard deviation for both intervention and control groups, as the comparison group, were extracted. We considered mg/dl, μIU/ml and percentage as units of FBS, fasting insulin and HbA1c, respectively. Also, for those studies that reported different units, we converted them with valid methods.
A one-stage robust error meta-regression model based on inverse variance weighted least squares regression and cluster robust error variances was used for the dose–response analysis between Mg supplementation and duration of intervention and glycaemic control factors(Reference Xu and Doi31). Statistical analysis was conducted using STATA, version 11.2 (Stata Corp). The statistical significant value was defined as P values < 0·05.
Results
Selection and identification of studies
Out of the initial 1986 published studies obtained by electronic and hand search, 713 were duplicates, 1273 were screened according to our inclusion criteria. Then, after excluded animal, review and unrelated studies, we assessed twenty-one RCT, of which three studies did not meet the desired criteria. Finally, eighteen eligible RCT (Fig. 1) were included in our final analysis(Reference Singh, Verma and Agrawal8,Reference De Lima, Cruz and Pousada10,Reference Rodriguez-Moran and Guerrero-Romero15,Reference Barragán-Rodríguez, Rodríguez-Morán and Guerrero-Romero19–Reference Talari, Zakizade and Soleimani28,Reference Paolisso, Sgambato and Pizza32–Reference De Valk, Verkaaik and Van Rijn36) .
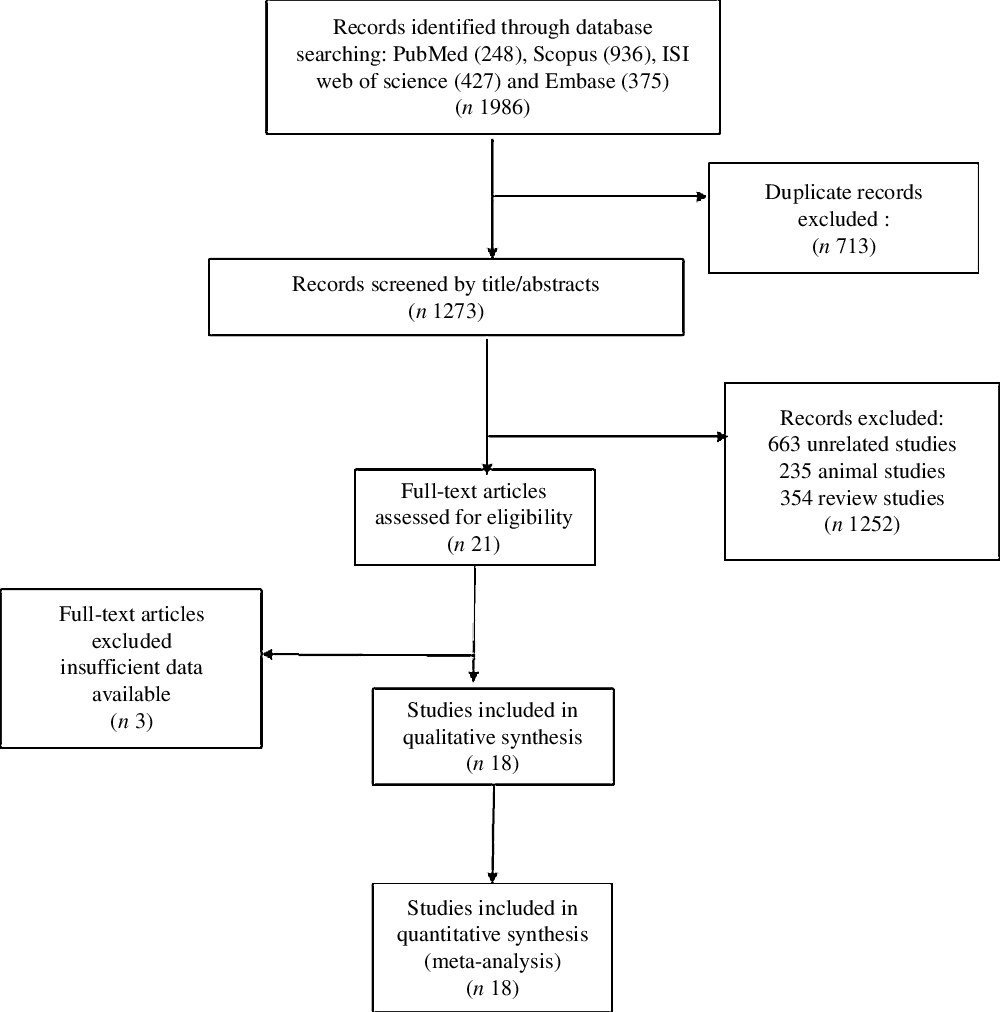
Fig. 1. Flowchart of study selection for inclusion trials in the systematic review.
Characteristics of studies
The main characteristics of the included studies in this meta-analysis are described in Table 1. Overall, fifty effect sizes (seven effect sizes for HOMA-IR, eighteen effect sizes for FBS, nine effect sizes for fasting insulin, and sixteen effect sizes for HbA1c) were extracted from eighteen RCT, including 1097 participants, out of which 571 participants were in the Mg group, and 526 were the control group. Most RCT(Reference Singh, Verma and Agrawal8,Reference De Lima, Cruz and Pousada10,Reference Rodriguez-Moran and Guerrero-Romero15,Reference Barragán-Rodríguez, Rodríguez-Morán and Guerrero-Romero19–Reference Barbagallo, Dominguez and Galioto21,Reference Solati, Ouspid and Hosseini23–Reference Talari, Zakizade and Soleimani28,Reference Corica, Allegra and Di Benedetto33–Reference De Valk, Verkaaik and Van Rijn36) adopted a parallel design except for two studies that used a crossover setting(Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero22,Reference Paolisso, Sgambato and Pizza32) . The mean age of participants in these studies ranged from 25·5 ± 6·5 to 72·2 ± 2·0 years. These studies were published between the year 1989 and 2019. The RCT were conducted in Iran(Reference Solati, Ouspid and Hosseini23,Reference Razzaghi, Pidar and Momen-Heravi24,Reference Rashvand, Mobasseri and Tarighat-Esfanjani26–Reference Talari, Zakizade and Soleimani28) , Mexico(Reference Barragán-Rodríguez, Rodríguez-Morán and Guerrero-Romero19,Reference Guerrero-Romero and Rodriguez-Moran20) , Australia(Reference Rodriguez-Moran and Guerrero-Romero15,Reference Eibl, Kopp and Nowak35) , Italy(Reference Barbagallo, Dominguez and Galioto21,Reference Paolisso, Sgambato and Pizza32,Reference Corica, Allegra and Di Benedetto33) , Netherlands(Reference Barbagallo, Dominguez and Galioto21,Reference Paolisso, Sgambato and Pizza32,Reference Corica, Allegra and Di Benedetto33) , Norway(Reference Gullestad, Jacobsen and Dolva34), India(Reference Singh, Verma and Agrawal8) and Brazil(Reference De Lima, Cruz and Pousada10). The dose of the oral elemental Mg given in these studies ranged from 36·49 to 500 mg/d, and all of the included studies were used Mg as intervention. The duration of intervention also varied from 4 to 24 weeks among the studies. Based on Cochrane scores, five studies were classified as fair- or weak-quality studies(Reference Singh, Verma and Agrawal8,Reference Barbagallo, Dominguez and Galioto21,Reference ELDerawi, Naser and Taleb25,Reference Corica, Allegra and Di Benedetto33,Reference Eibl, Kopp and Nowak35) , and the rest were good-quality studies(Reference De Lima, Cruz and Pousada10,Reference Rodriguez-Moran and Guerrero-Romero15,Reference Barragán-Rodríguez, Rodríguez-Morán and Guerrero-Romero19,Reference Guerrero-Romero and Rodriguez-Moran20,Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero22–Reference Razzaghi, Pidar and Momen-Heravi24,Reference Rashvand, Mobasseri and Tarighat-Esfanjani26–Reference Talari, Zakizade and Soleimani28,Reference Paolisso, Sgambato and Pizza32,Reference Gullestad, Jacobsen and Dolva34,Reference De Valk, Verkaaik and Van Rijn36) . The result of the quality assessment is reported in Table 2.
Table 1. Characteristic of included studies in meta-analysis of included studies in meta-analysis
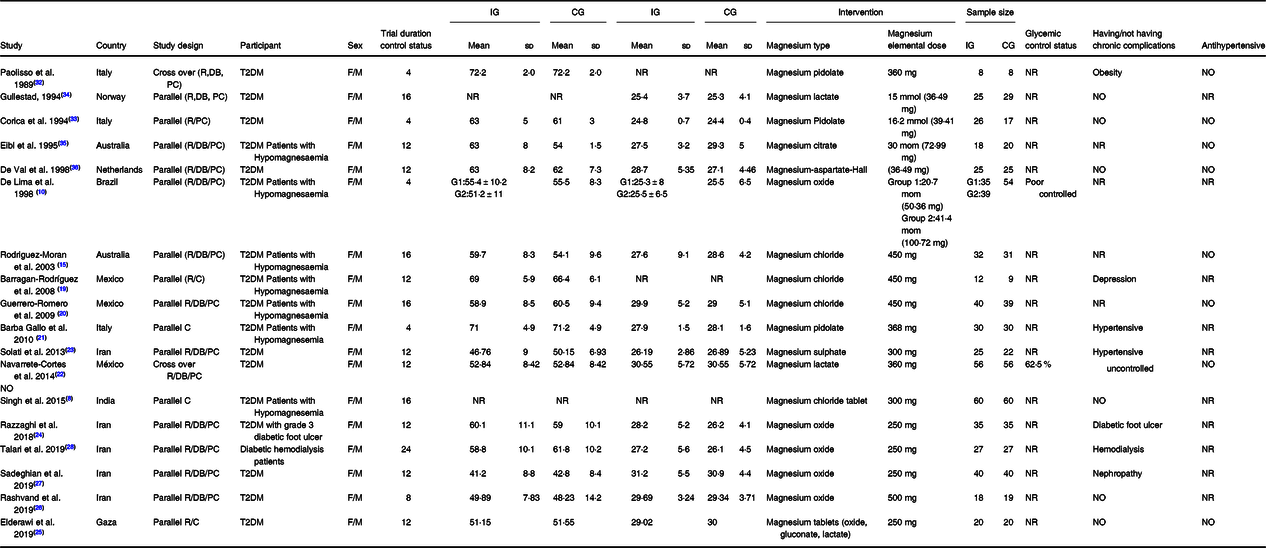
R, randomised; C, controlled; PC, placebo-controlled; DB, double blind; T2DM, type 2 diabetes mellitus; F, female; M, male; IG, intervention group; CG, control group; NR, not reported.
Table 2. Quality assessment

L, low-risk of bias; H, high-risk of bias; U, unclear-risk of bias.
Non-linear dose–response meta-analysis
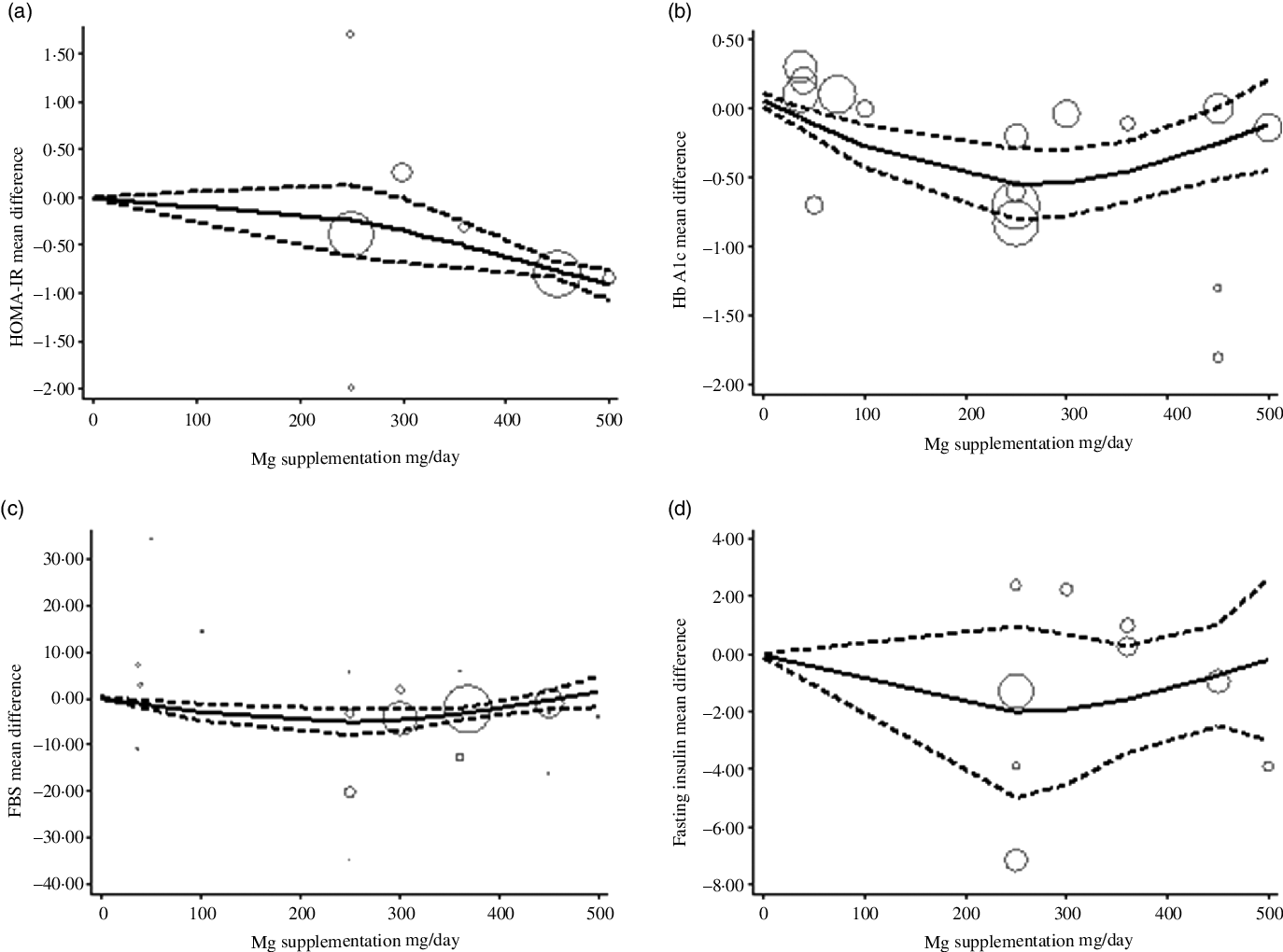
The estimated mean difference in HbA1c at 500 mg/d was −0·73 % (95 % CI: −1·25, −0·22) suggesting modest improvement in HbA1c with strong evidence (P value: 0·004). And in FBS at 360 mg/d was −7·11 mg/dl (95 % CI: −14·03, −0·19) suggesting minimal amelioration in FBS with weak evidence (P value: 0·092) against the model hypothesis at this sample size (Fig. 2(a)–(d)). The estimated mean difference in FBS and HbA1c at 24 weeks was −15·58 mg/dl (95 % CI: −24·67, −6·49) and −0·48 (95 % CI: −0·77, −0·19), respectively, suggesting modest improvement in FBS (P value: 0·034) and HbA1c (P value: 0·001) with strong evidence against the model hypothesis at this sample size (Fig. 3(a)–(d)) (Table 3).
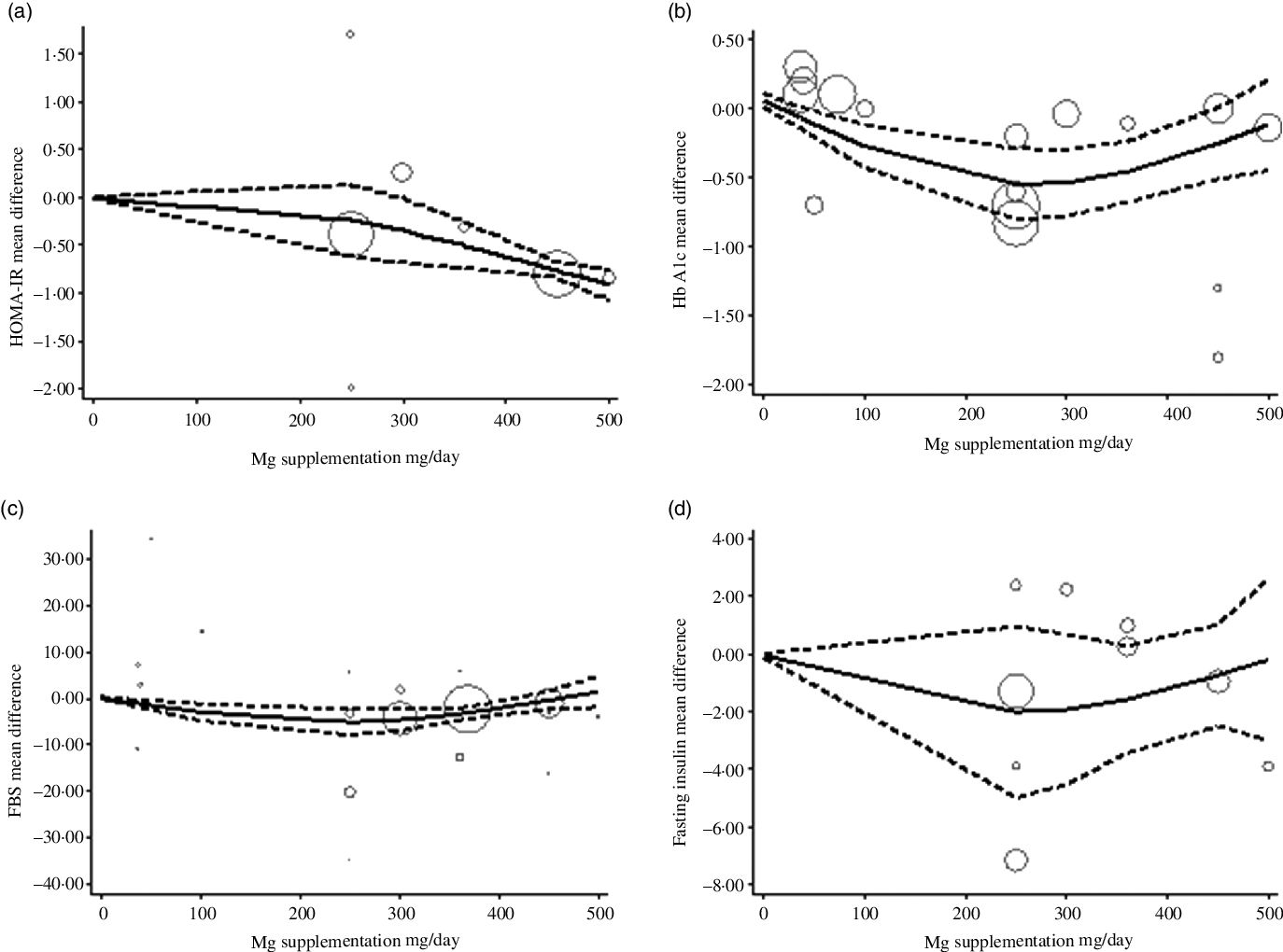
Fig. 2. The solid lines represent the estimate non-linear dose–response for magnesium supplementation on; (a) homoeostatic model assessment of insulin resistance (HOMA-IR); (b) HbA1c; (c) fasting blood sugar (FBS); and (d) Fasting insulin. The dashed lines represent 95 % CI.
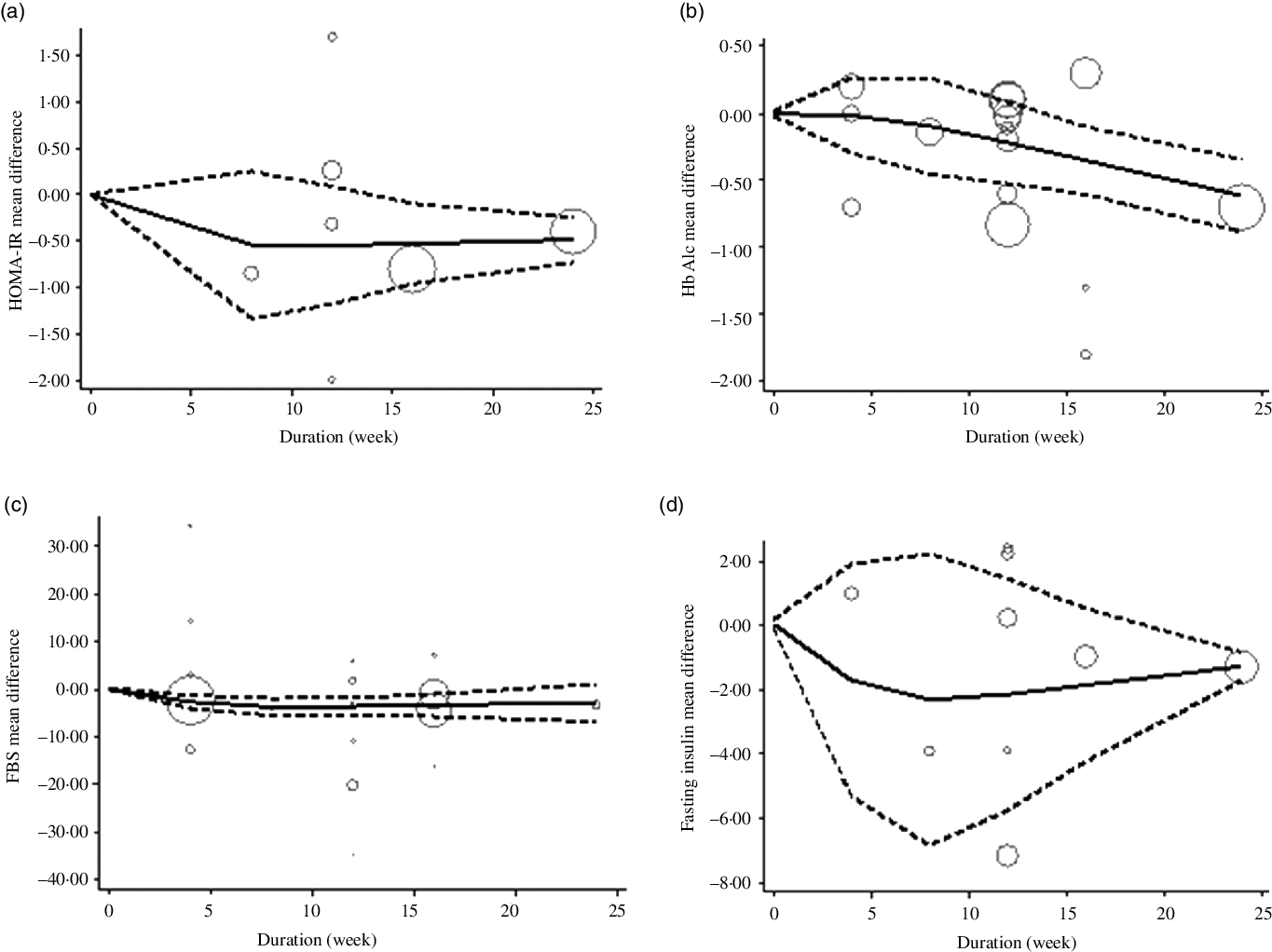
Fig. 3. The solid lines represent the estimate non-linear dose–response for duration of intervention on (a) HOMA-IR; (b) HbA1c; (c) fasting blood sugar (FBS) and (d) fasting insulin. The dashed lines represent 95 % CI.
Table 3. Non-linear dose–response meta-analysis

FBS, fasting blood sugar; HOMA-IR, Homoeostatic Model Assessment of Insulin Resistance.
Discussion
Diabetes is associated with an elevated risk for CVD(Reference Silva, Wickramatilake and Lekamwasam37,Reference Miraghajani, Esmaillzadeh and Najafabadi38) . Multiple laboratory tests are recommended to diagnose, manage, monitor and follow-up during the treatment of diabetic patients. These include plasma glucose, HbA1c, insulin(Reference Milosevic and Panin39,Reference Sacks, Arnold and Bakris40) and HOMA-IR(Reference Katsuki, Sumida and Gabazza41).
This condition comes with great costs for both the individual and society. Therefore, therapeutic strategies, including a range of dietary, supplements have been developed to improve the glycaemic control(Reference Niafar, Bahrami and Yagin3,Reference Nasri, Behradmanesh and Maghsoudi42,Reference Yalameha43) . Among dietary supplements, Mg has aroused curiosity among the scientific community. It might be because it is a crucial enzymatic cofactor in the various biological functions such as glucose metabolism and insulin signalling(Reference Barbagallo and Dominguez44).
Existing evidence diverges on the possible effects of oral Mg supplementation in the clinical management of glycaemic parameters. Therefore, the purpose of this review is to critically assess the scientific evidence regarding the efficacy of oral Mg supplementation on the glycaemic control in T2DM patients.
The present meta-analysis of eighteen RCT indicated that the increment of Mg intakes led to a more significant benefit in FBS and HbA1c. The same benefits were observed in FBS when oral Mg supplement was provided in the long term.
In 2017, a meta-analysis was conducted to evaluate the effect of oral Mg supplementation on T2DM-associated cardiovascular risk factors(Reference Verma and Garg45). There appears to be suggestive evidence of the benefit of oral Mg supplementation on the fasting plasma glucose level. A beneficial effect was observed in diabetic participants with hypomagnesemia(Reference Verma and Garg45). Another meta-analysis that assessed the effects of Mg supplementation on insulin sensitivity and glucose control in diabetic and non-diabetic individuals indicated a significant effect on the HOMA-IR index but not on plasma glucose, HbA1c and insulin levels. However, Mg supplementation intake for more than 4 months can improve the HOMA-IR index and fasting glucose in diabetic or non-diabetic individuals(Reference Simental-Mendía, Sahebkar and Rodríguez-Morán46). Another meta-analysis with the goal of reviewing the effect of Mg supplementation on glucose metabolism in people with or at risk of diabetes revealed Mg supplementation could reduce fasting plasma glucose in people with diabetes and improve plasma glucose levels after a 2 h oral glucose tolerance test and the HOMA-IR index(Reference Veronese, Watutantrige-Fernando and Luchini47). Since the mentioned meta-analysis provides a snapshot of knowledge at the time of incorporating of data from studies identified during the latest search, newly identified studies can change the conclusion of those reviews. So, we aimed to include all of the controlled and clinical trials to summarise current findings on the effect of oral Mg supplementation on glycaemic control in T2DM patients. Results from such investigations can produce evidence with greater clarity in the applicability of oral Mg supplementation on glycaemic control in T2DM patients and enable health professionals to make specific recommendations for incorporating Mg supplementation into the habitual diets in this context.
Due to the divergence in all mentioned meta-analysis, some points should be taken into account. In our analysis, most of the participants presented normo-magnesemia. Hence, the possible beneficial effect of oral Mg supplementation might be minimised in this population. Oral Mg supplementation in individuals with hypo-magnesemia can be more efficient than others(Reference Guerrero-Romero, Jaquez-Chairez and Rodriguez-Moran48). Also, the most common approach for evaluating Mg status is serum Mg concentration as a non-invasive, feasible and inexpensive test. But serum Mg concentration is kept under tight control, and also it has a little correlation with total body Mg concentrations in tissues. Thus, it is not a sensitive evaluation except for severe deficiency. In addition, there are individuals, in particular those with a subtle chronic Mg deficiency whose serum Mg levels are within the reference range but still may have a deficit in total body Mg. And vice versa: some people, though very few, have low serum Mg levels but a physiological Mg body content(Reference Jahnen-Dechent and Ketteler49).
Another possible explanation for the mentioned divergence in findings may be that, although the Mg serum can increase during a period of supplementation, the complete equilibrium in intracellular levels and observe beneficial effects may be obtained during a more extended period of intervention(Reference Marques, Klein and da Cunha50). Although serum Mg levels might reflect the dietary Mg intake, we should keep in mind that in T2DM patients there is a wide range of non-dietary factors such as serum Ca:Mg ratio and anti-hypertensive drugs including diuretics affect Mg homoeostasis(Reference Ozcaliskan Ilkay, Sahin and Tanriverdi51). Furthermore, since the homoeostasis of the Mg is strictly regulated by the renal function(Reference Navarro-González, Mora-Fernández and García-Pérez52), there could be a possible favourite effect of oral Mg supplementation patients with impaired renal function.
Other points that could modify the response of the glycaemic parameters to oral Mg supplementation are different formulations/salts of the Mg, which could be responsible for different bioavailability of the Mg and heterogeneity in the results. Bias in the results can also be introduced as a result of factors affecting the glycaemic control. These factors often manifest differently among the racial and ethnic groups and can have individual variations across a person lifespan(Reference Harris, Eastman and Cowie53). Differences in the various dietary compliance and energy intake(Reference He, Xie and Ma54), the gut microbiome(Reference Sreng, Champion and Martin55), lifestyle factors and medications(Reference Chrvala, Sherr and Lipman56–Reference Lipska, Krumholz and Soones58), glycaemic index and rate of the intestinal digestion and absorption of carbohydrate(Reference Clar, Al-Khudairy and Loveman59) and diversified used approaches for glycaemic control measurements(Reference Herman60) may also contribute to the different clinical response.
At last, any clinician who will interpret our results should bear in mind that some of the medical conditions such as end-stage renal disease or pregnancy and supplements and medications including vitamins E, Ribavirin and interferon-α, generally can present a falsely low HbA1C levels(Reference Radin61). Variants of haemoglobin could also be considered as potential interferes that could affect the measurement of HbA1C(Reference Goel, Tripathi and Sen62).
Several mechanisms have been proposed for the favourite effects of Mg on glycaemic control. Mg is the main co-factor in all enzymes of glycolysis(Reference Barbagallo and Dominguez44) and is also necessary to regulate of insulin signalling, in the phosphorylation of the insulin receptor kinase, in the post receptor action of insulin and in insulin-mediated cellular glucose uptake(Reference Barbagallo and Dominguez44). Another possible link between Mg deficiency and abnormal glycaemic control is reduced glucose utilisation in the cells, following the post-receptor insulin resistance, which can worsen the reduced insulin sensitivity(Reference Barbagallo and Dominguez44). On the other hand, the relation between Mg deficiency and reduced insulin sensitivity is the presence of oxidative stress and/or inflammation(Reference Nasri, Shirzad and Baradaran63). Oxidative stress is often increased in metabolic disorder such as T2DM, as a condition associated with Mg deficits(Reference Barbagallo and Dominguez44,Reference Madihi, Marikhi and Nasri64) .
The present meta-analysis has some limitations, such as high heterogeneity among the included studies. The pair-wise analysis was not applicable, while dose and duration differed across trials. Moreover, the effects of the confounding variables, including the genetic background and lifestyle factors, on the efficacy of oral Mg supplementation were ignored. Therefore, the results should be interpreted with caution.
The strength of the present study was that we provided the correct analysis in the homogenous populations against an analysis of the pooled individual data when the need for this method is obviated.
Safety
Although the positive effects of oral Mg supplementation on health have been reported, a particular concern about its administration in some medical condition, including chronic kidney disease and end-stage renal disease, should be considered(Reference Kanbay, Goldsmith and Uyar65,Reference Spiegel66) . Also, it might be unsafe for patients who take specific diuretics and heart medications(Reference Sultan, Jahangir and Gussak67).
Implications for practice
Although the current meta-analysis suggests that oral Mg supplementation might benefit the glycaemic control in T2DM patients, so far, the RCT are not sufficient for making robust guidelines for clinical practice.
Implications for research
Moving forward, there is a place for larger, longer, pragmatic clinical trials, which would be necessary to rely on simpler and less sensitive outcome measures. Another outcome to consider is whether any beneficial effects are maintained.
Conclusion
Oral Mg supplementation could have a beneficial effect on glycaemic control in T2DM patients. Yet, the clinical trials are not sufficient to make guidelines for clinical practice.
Acknowledgements
We appreciate Dr. Chang Xu, Chinese Evidence-Based Medicine Center and Chinese Cochrane Center of West China Hospital at Sichuan University, for his help in the methodology of robust error meta-regression.
This study received no funding.
O. A. and S. M. designed this study. O. A., S. M. and M. Z. contributed to the conduct of the search. O. A. and M. A. H. K. performed the statistical analysis and interpreted the results. M. M., S. N. and A. L. wrote the initial manuscript. S. M. and M. M. critically revised the manuscript and contributed to the subsequent drafts of the manuscript. All authors approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.