Introduction
Hemispherectomy and hemispheric disconnection surgery are indicated for certain groups of patients suffering from medically intractable epilepsy. The surgical technique of hemispherectomy has evolved from complete anatomical hemispheric resection to hemispheric disconnection, requiring minimal tissue removal. Here, we review current hemispherectomy surgical techniques, outcomes, and complications.
Historical Background and Evolution of Hemispherectomy Surgical Techniques
Hemispherectomy is the resection of the entire cerebral hemisphere with preservation of the basal ganglia. This surgical technique is known as anatomic hemispherectomy and was first performed by Dandy in 1928 for glioma surgery. Reference Dandy1 Independently, Lhermitte described the hemispherectomy procedure in the same year. Reference Lhermitte2 In 1938, the first hemispherectomy to treat epilepsy was performed by McKenzie from Canada. Reference McKenzie3 He operated on an adult patient with hemiplegia and intractable epilepsy. Krynauw reported on 12 children suffering from intractable epilepsy and infantile hemiplegia who underwent hemispherectomy. Reference Krynauw4 This report showed excellent control of epilepsy and improvement in behavioral functions. The success of this procedure led to the widespread use of hemispherectomy to treat patients with infantile hemiplegia and intractable epilepsy. Subsequently, the procedure was also used for patients with unilateral hemispheric disorders that cause epilepsy, such as cerebral infarctions, Rasmussen’s encephalitis, and Sturge–Weber syndrome (SWS). Penfield and Rasmussen began performing hemispherectomies at the Montreal Neurological Institute (MNI) in 1952. Reference Rasmussen5 In 1961, White and colleagues reviewed 267 cases that had been reported from several centers. Reference White6 Several animal experiments at that time were done to evaluate hemispherectomy surgical techniques and the impact of the procedure on neurology and electrophysiology. Reference White, Schreiner, Hughes, Maccarty and Grindlay7–Reference White, Schreiner, Hughes, Maccarty and Grindlay9
Hemispherectomy was performed in many centers for more than a decade, but then the procedure started to wane because of delayed complications and the introduction of new antiepileptic medications after the 1960s. Reference Rasmussen5,Reference Oppenheimer and Griffith10 The complications included the development of superficial cerebral hemosiderosis (SCH) and hydrocephalus. The etiology of SCH was thought to result from recurrent delayed bleeding within the large subdural space after the removal of the cerebral hemisphere. Reference Rasmussen5,Reference Falconer and Wilson11 This, in turn, induced inflammation of the ependyma and hemosiderosis. Foramen of Monro blockage was the cause of delayed hydrocephalus. Reference Falconer12 Oppenheimer and Griffith examined three autopsies of patients who died from gradual neurological deterioration after many years of seizure freedom post-anatomic hemispherectomy. Reference Oppenheimer and Griffith10 They observed SCH, ependymitis, hydrocephalus, and hemorrhagic subdural membranes. Despite the effectiveness of hemispherectomy in controlling seizures, the procedure was abandoned in many centers because of the high risk of complications. Between 1952 and 1968, 27 anatomic hemispherectomies were performed in MNI. Of those, 52% developed hydrocephalus and 33% developed SCH with an overall estimated mortality rate of 30–40%. Reference Rasmussen5,Reference Villemure13,Reference Villemure and Luders14
Modification of hemispherectomy techniques was initiated by several surgeons to reduce the incidence of the aforementioned severe complications. Rasmussen introduced functional hemispherectomy that involved subtotal hemispheric resection with preservation of the frontal and occipital lobes. He reported on 40 patients who underwent this modified surgical technique and found that none of them developed SCH after a 4-year follow-up period. Reference Rasmussen5 The rate of seizure-free outcomes from functional hemispherectomy was 85%, similar to that of anatomic hemispherectomy. Reference Rasmussen5,Reference Tinuper, Andermann, Villemure, Rasmussen and Quesney15 Ignelzi and Bucy reported on cerebral hemidecortication in the treatment of infantile hemiatrophy as an alternative to anatomical hemispherectomy. Reference Ignelzi and Bucy16 A modification of the anatomical hemispherectomy technique was initiated by Wilson in 1970. Reference Wilson17 Wilson performed additional surgical techniques to minimize the incidence of SCH and hydrocephalus, which included plugging the foramen of Monro with muscle and suturing the dura mater to the tentorium and falx cerebri. Subsequently, Adam modified and popularized this technique. Reference Adams18 Peacock recommended a prophylactic cerebrospinal fluid diversion procedure in anatomical hemispherectomy. Reference Peacock, Wehby-Grant and Shields19 Other modifications of anatomical hemispherectomy included duroplasty, suggested by Dunn; the use of the omental vascularized flap, introduced by Matheson; and the use of a silicon prosthesis implanted in the cavity after hemispherectomy, presented by Sorano. Reference Dunn, Miles and May20–Reference Sorano and Esposito22 More recently, Winston reported on a cerebral hemicorticectomy technique in which the cortical mantle was entirely removed. Reference Winston, Welch, Adler and Erba23 Carson, from Johns Hopkins, reported on a hemidecortication in which the ventricle was kept closed and the hemispheric lobes cortices were removed in sequences. Reference Carson, Javedan and Freeman24
Functional hemispherectomy, which was introduced by Rasmussen, remains the most important modified approach, paving the way for the concept of less tissue resection with maximal disconnection and leading to the introduction of hemispherotomy. Villemure reported on the lateral hemispherotomy approach around the insula, termed as peri-insular hemispherotomy. Reference Macott, Choi, Rasmussen and Villemure25,Reference Villemure and Mascott26 Modifications to this approach were reported by Shimizu and Maehara. Reference Shimizu and Maehara27 Delalande reported on the vertical hemispherotomy technique. Reference Delalande, Pinard, Basdevant, Gauthe, Plouin and Dulac28,Reference Villemure, Vernet and Delalande29 Schramm introduced hemispheric deafferentation in 1995 and subsequently described transsylvian keyhole functional hemispherotomy. Reference Schramm, Behrens and Entzian30,Reference Schramm, Kral and Clusmann31 To date, this trend toward less tissue removal and increased disconnection has continued and is commonly seen in many centers around the world, resulting in fewer complications, as several reports have confirmed. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32–Reference Koubeissi, Syed, Syed, Jordan, Alshekhlee and Kossoff35 Table 1 summarizes the timeline for the evolution of hemispherotomy.
Table 1: The timeline for hemispherectomy techniques evolution
CSF = cerebrospinal fluid.
Hemispherotomy Indications and Patient Selection
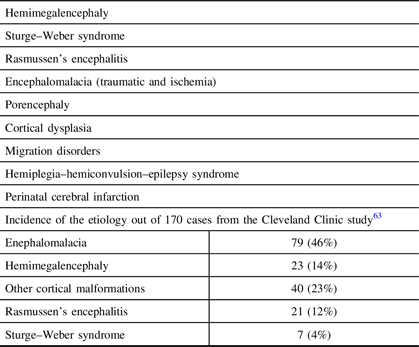
Hemispherotomy is indicated for the treatment of patients with medically intractable epilepsy localized in one cerebral hemisphere. The seizures may originate from one extensive area or multifocal areas within one hemisphere, caused by congenital or acquired conditions. Reference Schramm, Kral and Clusmann31 Congenital diseases include SWS, diffuse cortical dysplasia, tuberous sclerosis, and hemimegalencephaly (HME). The commonly acquired lesions include infantile hemiplegia, hemiatrophy, porencephaly, Rasmussen’s encephalitis, and hemiplegia–hemiconvulsion–epilepsy (HHE) syndrome (Figure 1A and 1B). The groups of patients usually suffer from hemiparesis, delayed cognitive function, and, frequently, hemianopia. Table 2 summarizes the common indications for hemispherotomy.
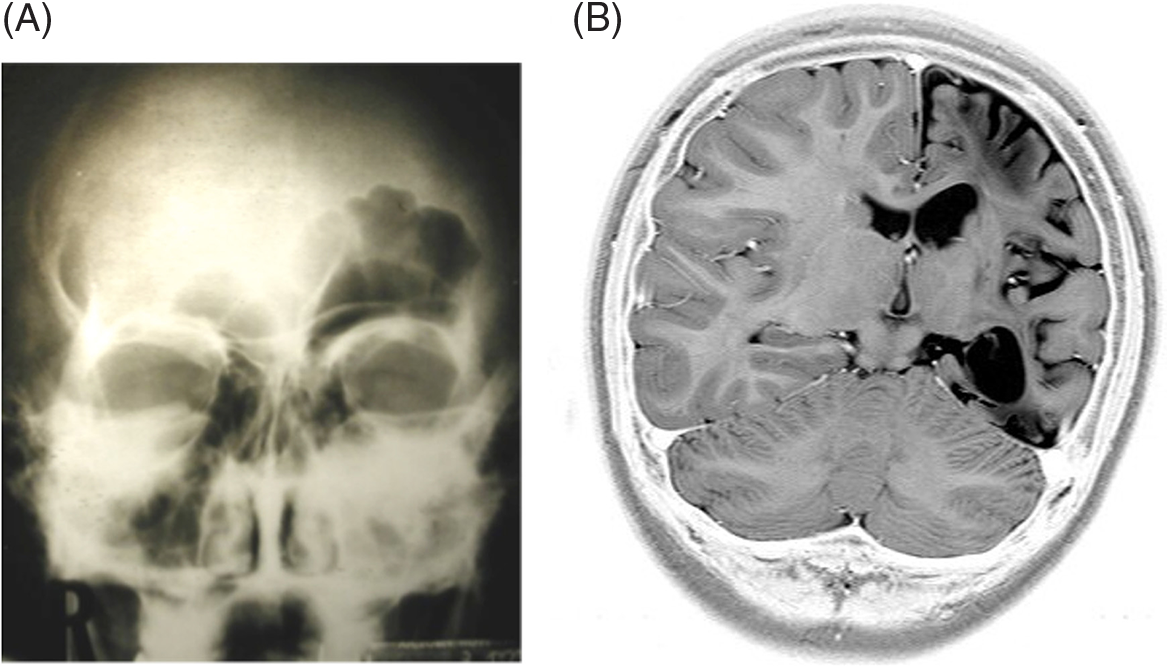
Figure 1: Skull X-ray and MRI brain. (A) Skull X-ray showing prominent frontal sinus on the left side that corresponds to the side of atrophy as a radiological sign before the era of CT and MRI brain. (B) MRI brain, coronal view depicting left hemispheric atrophy.
Table 2: Common indications of hemispherectomy and incidence of etiologies
A unilateral hemispheric lesion in early childhood or infancy is a common indication for hemispherotomy and is associated with a high success rate. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32 In general, the procedure is performed less frequently in adults. Hemispherotomy is also indicated in catastrophic epilepsy in infants or young children with severe medically uncontrolled epilepsy resulting from cerebral damage or inflammatory processes. Reference Lettori, Battaglia and Sacco36–Reference Duane, Ng, Rekate, Chung, Bodensteiner and Kerrigan38 The procedure involves disconnection combined with tissue resection of nonfunctioning or malfunctioning parts of one hemisphere. Most patients with a perinatal insult or congenital malformation suffer from spastic hemiparesis, and their language has been transferred to the other hemisphere. The surgery will result in loss of fine motor functions of the contralateral hand and impaired gait; however, most patients may walk and move their hands to a certain degree. Moreover, the procedure usually results in improvements both in cognitive function and in underlying encephalopathy. In summary, hemispherotomy is best indicated in patients who fulfill the following criteria: the presence of medically intractable epilepsy, contralateral motor dysfunction, psychomotor delay, and a normal contralateral hemisphere with clear electroencephalographic (EEG) and magnetic resonance imaging (MRI) abnormalities in the affected hemisphere.
Contraindications
Hemispherotomy is not indicated in patients with bilateral hemispheric epilepsy. Isolated ictal activity from the contralateral hemisphere is acceptable and is seen in about two-thirds of the patients. This phenomenon could be secondary from the affected hemisphere. The rate of seizure freedom is shown to be high in patients with suspected bilateral epileptic independent activities. Reference Doring, Cross, Boyd, Harkness and Neville39 The presence of minor MRI abnormalities in the contralateral hemisphere is not considered to be a contraindication for hemispherotomy. Reference Hallbook, Ruggieri and Adina40 Although the presence of good visual field function is considered as a contraindication, some series have shown that children might have a good adaptation to the presence of complete hemianopsia. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32,Reference Schramm and Winn41 A normal motor function is a known contraindication for complete hemispherotomy. Mental retardation is not a contraindication for hemispherotomy in current practice. Reference Schramm and Winn41
Prehemispherotomy Evaluation
The main aim of the preoperative assessment is to localize the epileptiform activities originating from a diffuse area in the diseased hemisphere and to ensure that the contralateral hemisphere will carry an acceptable level of postoperative neurological function. Careful clinical assessment of the affected neurological function is mandatory before deciding on surgery. Taking a detailed history that covers the perinatal period, family history, and assessment of the motor and sensory functions is an important initial step. A formal ophthalmological examination can verify the presence and degree of visual field defects based on the patient’s age. Neuropsychological evaluation can quantify the postoperative effect of hemispherectomy on patient cognition and mental functions. Video EEG monitoring of seizures and high-resolution MRIs remain the mainstays of preoperative assessment. The MRI evaluates for structural abnormalities, and thin-cut volumetrics with three-dimensional reconstructions reveal more subtle structural abnormalities. Reference Ruggieri, Najm and Wyllie42 Any abnormality in the contralateral hemisphere should be evaluated as a source of other epileptogeneses. Interictal positron emission tomography (PET) imaging is helpful for delineating the metabolic function in both hemispheres to confirm lateralization of the seizure origin. Single-photon emission computed tomography (SPECT) is generally used to identify abnormalities during the ictal period. A functional MRI (fMRI) is useful for patients in a particular age group to define language lateralization presurgically. In addition, diffusion tensor imaging and intraoperative MRIs may increase the rate of complete disconnection. Reference Kim, Seo, Schroff, Chen, Lee and Baumgartner43 Magnetoencephalography (MEG) that lateralizes spike waves to the affected hemisphere could correlate with better outcomes. Reference Torres, Fallah and Ibrahim44 An intracarotid amobarbital (WADA) test can demonstrate hemispheric dominance for language and assess the possibility of language transfer to the contralateral hemisphere. Children younger than 4 years of age may not require language lateralization assessment; they are more likely to have language transfer to the other hemisphere even after hemispherotomy. Reference Menard, Le Normand, Rigoard and Cohen45–Reference Vining, Freeman and Pillas47 In addition, the WADA test may help researchers differentiate between epileptic activities spread from the diseased hemisphere and activities from the contralateral hemisphere.
It is critical for all candidates to undergo continuous video EEG monitoring for ictal and interictal epileptic activities. The presence of diffuse ictal activities in the diseased hemisphere with no significant contralateral epileptic activities is of positive prognostic value. The expected neurological dysfunction post-hemispherotomy should be explained to the parents and the patient. The fact that the hemianopsia could prevent driving in the future should be disclosed, if relevant. The main goals of surgery are the elimination of seizures, the improvement of encephalopathy, and the recovery of cognitive functions.
Hemispherotomy Surgical Techniques
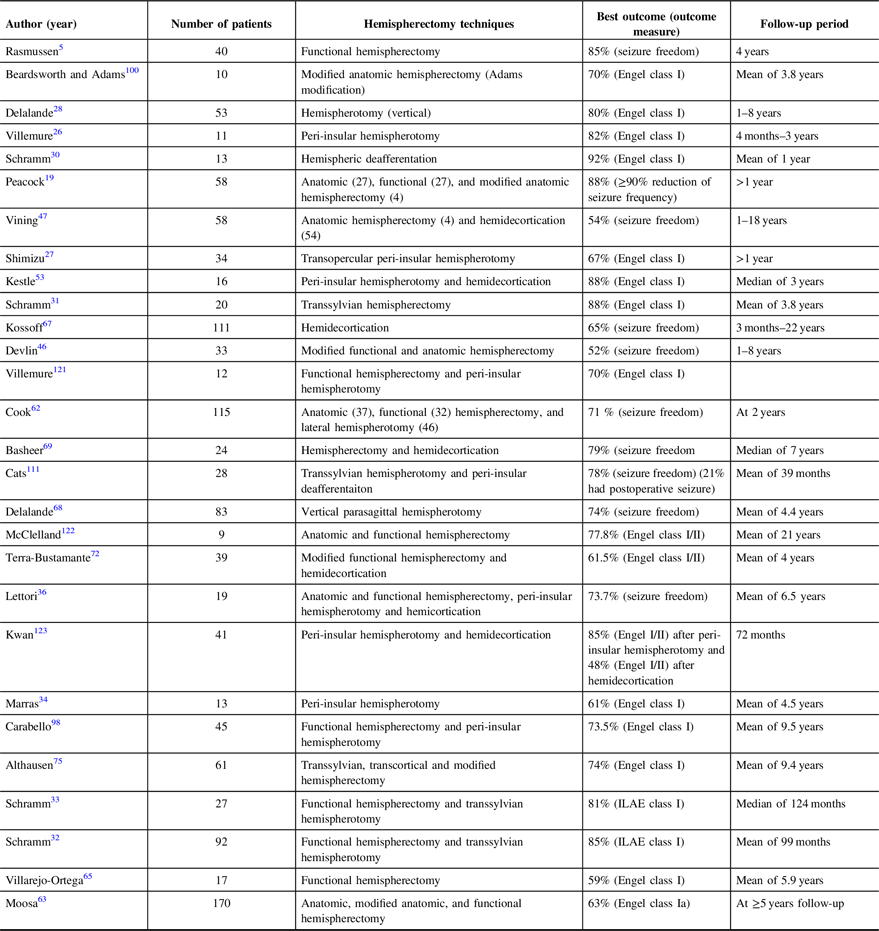
The different surgical techniques for hemispherotomy follow the principle of functional hemispherectomy with minimized tissue resection and maximized disconnection. Image guidance technology may assist surgical disconnection and intraoperative ultrasound may help to ensure complete anatomical disconnection. In addition, in certain centers, intraoperative MRIs may help assess the completeness of disconnection. Morino and colleagues performed anatomical analyses of different hemispherotomy procedures in 2002. Reference Morino, Shimizu, Ohata, Tanaka and Hara48 This cadaveric study showed that the different procedures shared four important surgical steps, including disruption of internal capsule and corona radiata, resection or disconnection of mesial temporal structures, transventricular corpus callosotomy, and disruption of the frontal horizontal fibers. Extensive review of the different forms of hemispherotomy surgical techniques is beyond the scope of this review. In this section, we will shed light on the main surgical techniques used and describe in detail our surgical techniques for hemispherectomy. Table 3 summarizes hemispherectomy/hemispherotomy techniques.
Table 3: Summary of different studies seizure outcome analysis after different hemispherectomy techniques
Anatomic Hemispherectomy
This procedure consists of the complete removal of the entire cerebral hemisphere with or without the removal of basal ganglia. This procedure is still in use in current practice. In certain centers, anatomic hemispherectomy is performed for HME and for reoperation after failed functional hemispherectomy. Reference Hadar and Bingaman49 The procedure starts with the removal of the temporal neocortex, using a posterior resection line at the distal Sylvian fissure, followed by division of the temporal white matter and temporal stem lateral to the hippocampus, leaving the amygdala, hippocampus, and parahippocampal gyrus. Then the uncus is removed, followed by removal of the amygdala and the hippocampus. The posterior resection of the hippocampus and the parahippocampal gyrus is carried out until the level of quadrigeminal cistern and hippocampus tail at the trigone. The superior circular sulcus is exposed as the start of the suprasylvian step, and the middle cerebral artery branches are divided. This is followed by the creation of a window to divide the corona radiata and open the lateral ventricle. The foramen of Monro is plugged with cottonoid to prevent blood from entering the ventricular system, followed by transventricular callosotomy from the rostrum to the splenium. Posterior dissection of the cingulate gyrus is carried out to expose the tentorium and the most posterior part of the parahippocampal gyrus. Subsequently, the fornix is divided at the hippocampus tail. The posterior cerebral artery is encountered and divided. At the level of genu, the mesial frontal lobe is dissected, dividing the branches of the anterior cerebral artery and the pia mater along the falx down to the frontal base. The gyrus rectus is removed, and the anterior cerebral artery is protected. Subpial dissection of the frontal base is performed up to the level of the optic nerve and internal carotid artery. At this stage, all bridging veins of the hemisphere are divided. The hemisphere is then removed en bloc. Hemostasis is secured, and the cavity is irrigated with normal saline. The dura is closed, followed by closure in layers. Of note, there are some modifications for anatomic hemispherectomy techniques as mentioned earlier; however, they are not used in current practice. Reference Nagel, Elbabaa, Hadar, Bingaman and Luders50,Reference Girvin and Baeesa51
Functional Hemispherectomy
The concept of this procedure was described by Rasmussen and has continued into the modern era. The technique is based on less tissue resection and maximal disconnection. It consists of certain steps, including standard temporal lobectomy, central resection, corpus callosotomy, and frontal, parietal, and occipital lobe disconnection. We describe herein the surgical technique of functional hemispherectomy performed by John Girvin (the senior author), which he inherited from the Rasmussen and Penfield eras and which continues to be performed in many institutions. Reference Girvin and Baeesa51 This description of the technique should not be construed as implying that this is necessarily the best technique of carrying out functional hemispherectomy nor that it is superior to the more recently introduced technique of hemispherotomy. We provide the description as evidence of a technique that has been successfully applied for nearly 30 years.
After general anesthesia under endotracheal intubation, the head is turned to one side and fixed with a head clamp. The skin incision is marked using a reverse question mark starting 1 cm anterior to the tragus and ending between the superior hairline and the widow’s peak at the anterior hairline as shown in Figure 2. In our practice, the size of the exposure depends on the underlying pathology. In a case of infantile hemiplegia or poststroke encephalomalacia with an enlarged ventricle, the size of the exposure is usually smaller, and less cerebral tissue removal is done, in general, compared with other cases, such as HME, in which the anatomy is distorted, and the ventricle is small. After bone exposure, a craniotomy is performed to expose the perisylvian area and central Rolandic region. The dura is opened, and the suprasylvian and infrasylvian regions are exposed. The initial stage of the procedure is a standard radical temporal lobectomy. Reference Al-Otaibi, Baeesa, Parrent, Girvin and Steven52 The posterior resection line of the temporal neocortex is located at the distal end of the Sylvian fissure. After the lateral temporal neocortex is removed, attention is focused on the removal of mesial temporal structures. The uncus and amygdala are removed using an ultrasonic aspirator, and the hippocampus and parahippocampal gyrus are resected. The posterior resection limit is located at the hippocampal tail at the trigon region.
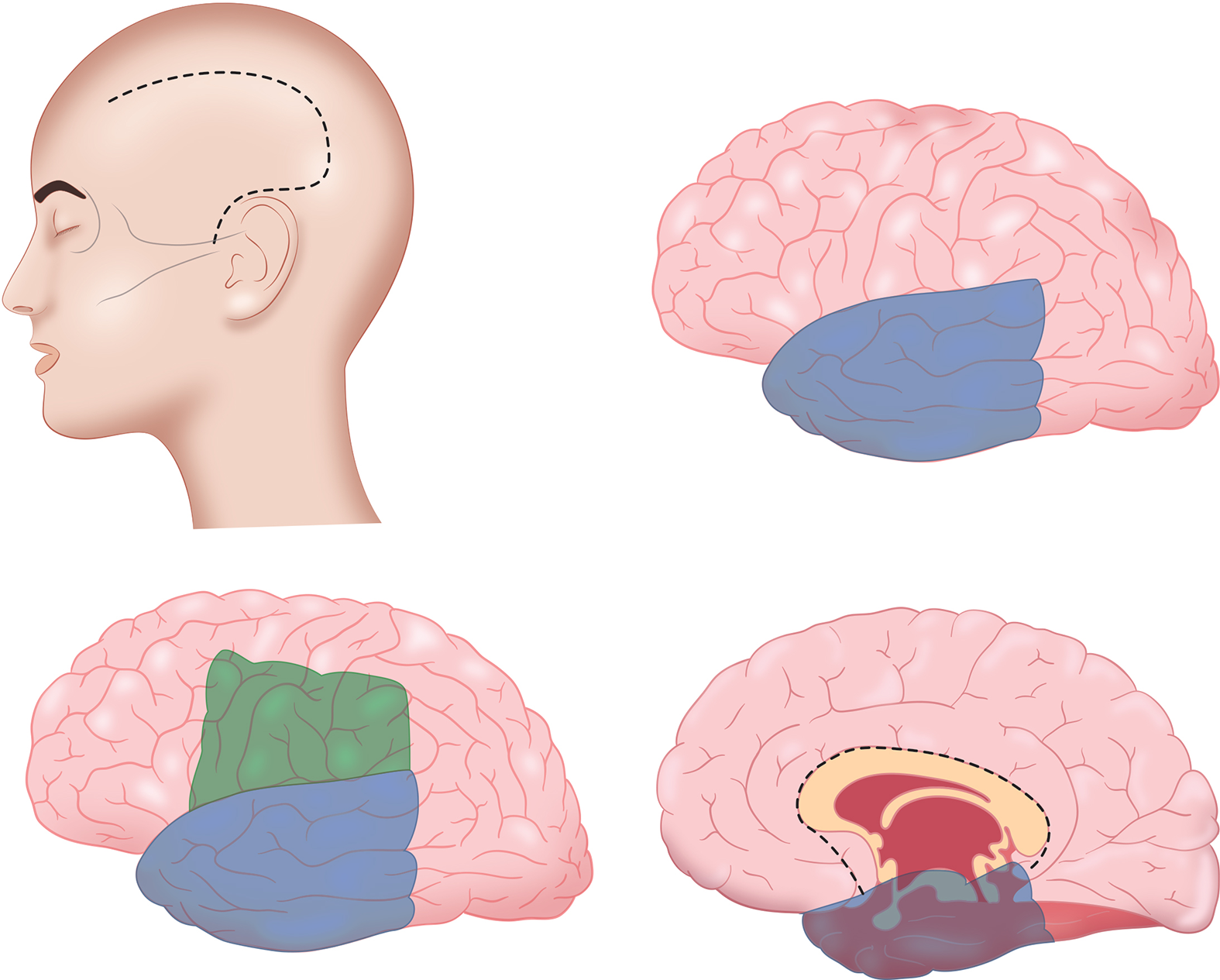
Figure 2: Schematic demonstration of the functional hemispherectomy technique performed by the senior author (JG), from skin incision to the hemispheric disconnection techniques.
The suprasylvian stage is the second step of this procedure, consisting of creating a “window” through the cerebral mantle at the central Rolandic area. The lower limb of this “Rolandic window” is the Sylvian fissure after removal of the frontal and parietal opercula. The resection involves the middle and inferior frontal gyri as well as the inferior parietal lobe to provide enough access to the roof of the lateral ventricle. The Rolandic window can be enlarged based on the cerebral hemisphere status, as in HME. Anterior extension of this window is to the level of the corpus callosum rostrum and posteriorly to the splenium level or applying other landmarks by using the sphenoid ridge level as the landmark for the window anterior limb and the posterior resection line of the temporal lobe as the landmark for the posterior limb. Dissection is made from the insular superior circular sulcus through the corona radiate toward the ventricle. The white matter is removed, and the lateral ventricle is exposed. The foramen of Monro is plugged with cottonoid to prevent blood and tissue debris from entering the contralateral and third ventricle. At this stage, care must be taken to secure the anterior, middle, and posterior cerebral artery branches that supply the frontal, parietal, and occipital lobes to prevent infarction of the disconnected tissue, which can lead to postoperative cerebral tissue swelling.
The third stage consists of a transventricular corpus callosotomy. The midline is identified at the junction of the septum pellucidum and the roof of the ventricle. Sectioning of the corpus callosum is carried out to the genu anteriorly and to the splenium posteriorly. Pericallosal arteries are used as landmarks for the midline. The fourth stage consists of disconnection of the frontal lobe fibers. This is accomplished by extending the anterior limb of the Rolandic window medially through the orbitofrontal cortex to the interhemispheric fissure. The olfactory tract and arterial supplies to the remaining orbitofrontal cortex are preserved. Using subpial dissection and the anterior cerebral artery as a landmark, the most medial aspect of the orbitofrontal incision line is connected to the corpus callosotomy incision. The final stage is to disconnect the posterior parietal and occipital cortices. This is achieved by extending the posteromedial aspect of the temporal lobectomy superiorly to join the posterior callosotomy incision. Hemostasis is secured and the dura is closed, followed by closure in layers in the usual fashion.
Peri-insular Hemispherotomy
This technique was described by Villemure to accomplish complete hemispheric disconnection with minimal tissue resection. Reference Villemure and Mascott26 The disconnection in this procedure is achieved through a peri-insular window. The insular cortex is aspirated during this approach from suprasylvian and infrasylvian access. The internal capsule fibers are divided using the suprasylvian window to expose the ventricle, followed by corpus callosotomy and disconnection of the frontal fibers. The mesial temporal structures are resected through an infrasylvian access. The advantages of this procedure are minimal exposure and reduced operative time, and blood loss. Such a procedure seems to be easily performed in patients with enlarged ventricles, such as in cases of encephalomalacia and porencephaly. Nevertheless, it also has been successfully performed in children with normal or small ventricles. Reference Kestle, Connolly and Cochrane53
Hemispheric Differentiation
This procedure was described by Schramm, as mentioned earlier. Reference Schramm, Behrens and Entzian30 It is similar to the peri-insular hemispherotomy procedure. The procedure starts with either a selective amygdalohippocampectomy through the superior temporal gyrus or an anterior temporal lobectomy. Disconnection of occipital, parietal, and frontal lobes is achieved through intraventricular access. This procedure was subsequently further modified by Schramm and became keyhole hemispherotomy.
Keyhole Transsylvian Hemispherotomy
This technique consists of a transsylvian hemispherotomy using a small craniotomy (4 × 4 cm) centered at the Sylvian fissure, as described by Schramm. Reference Schramm, Kral and Clusmann31 The procedure depends on transsylvian exposure of the insular circular sulcus and the entry into the ventricle to achieve callosotomy. This is followed by disconnecting frontobasal fibers and an infrasylvian amygdalohippocampectomy. The insular cortex is removed using subpial dissection technique. Schramm did not recommend this technique for HME; it is preferred for cases with enlarged ventricles and less distorted anatomy. The advantages include less operative time and less blood loss. The mean operating time was 3.6 h as compared to a mean time of 6.3 h for functional hemispherectomy. Reference Schramm, Kral and Clusmann31
Transopercular Hemispherotomy
Shimizu and Maehara described the achievement of adequate disconnection through frontoparietal opercular access. Reference Shimizu and Maehara27 The upper insular cortex is then removed at this stage and the remaining is removed during the approach to temporal horn. After resecting the opercula, the ventricle is accessed, and then a callosotomy is done followed by disconnection steps as in a functional hemispherectomy. In this approach, the mesial temporal structures are resected through an access point at the medial insula.
Vertical Hemispherotomy
The term hemispherotomy was coined by Delalande. Reference Delalande, Pinard, Basdevant, Gauthe, Plouin and Dulac28 The procedure is carried out through a vertical skin incision at the midline, followed by retraction of the cerebral hemisphere and sectioning of the corpus callosum. This is followed by disconnection of the internal capsule fibers and establishment of a corridor for the mesial temporal structures’ resection. In this procedure, the disconnection is performed through white matter access. The insular cortex is disconnected laterally from the basal ganglia. The surgical approach to the lateral ventricle can be reached either through a transparenchymal or interhemispheric approach, as shown in Figures 3 and 4. Reference Kawai, Morino and Iwasaki54 Recently, endoscopy was introduced into this form of hemisphorotomy. Reference Sood, Marupudi, Asano, Haridas and Ham55,Reference Chandra, Kurwale, Garg, Dwivedi, Malviya and Tripathi56 Chandra et al. reported on five children with perinatal strokes, HME, and Rasmussen syndrome who underwent endoscopic hemispherotomy. Access was achieved through an interhemispheric paramedian approach to do corpus callosotomy and subsequently access the lateral ventricle. Then, an intraventricular disconnection was carried out. The intraoperative MRI confirmed the disconnection. The estimated blood loss was 80 ml and the mean operating time was 220 min. The seizure control outcome at the mean follow-up of 10.2 months was comparable to other types of surgical techniques. Reference Chandra, Kurwale, Garg, Dwivedi, Malviya and Tripathi56 Recently, in 2019, Wagner et al. described a cadaveric feasibility experiment for the utilization of endoscopes in hemispherotomy. Reference Wagner, Vaz-Guimaraes, Camstra and Lam57 This was then used clinically on two pediatric patients who suffered from perinatal stroke and exhibited large lateral ventricles. No blood transfusions were required. Postoperatively, an external ventricular drain was removed in 2 days, and patients were discharged with no new neurological deficits and no need for in-hospital postoperative rehabilitation.
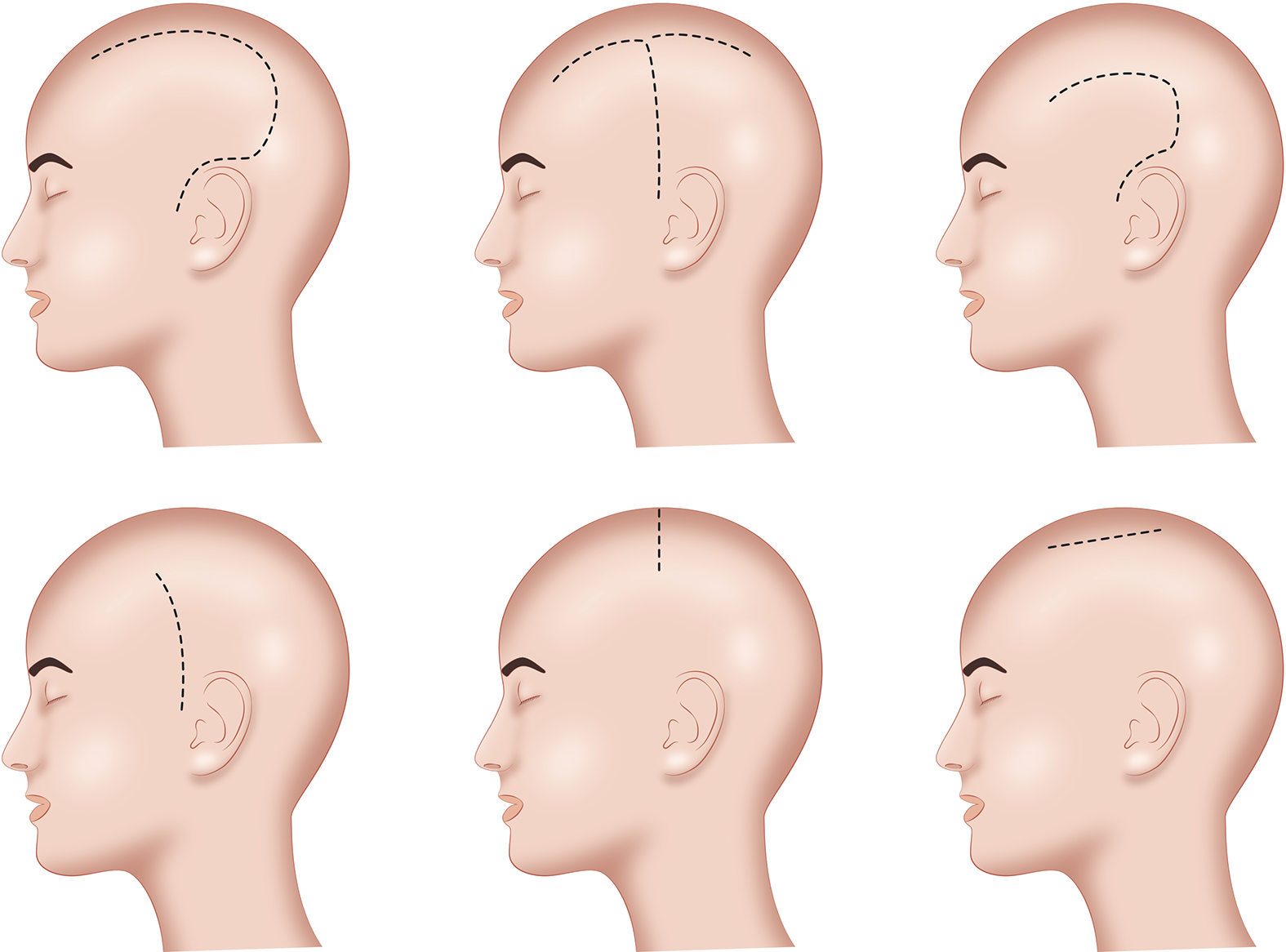
Figure 3: Schematic demonstration of different skin incisions that have been utilized for different hemispherotomy techniques.
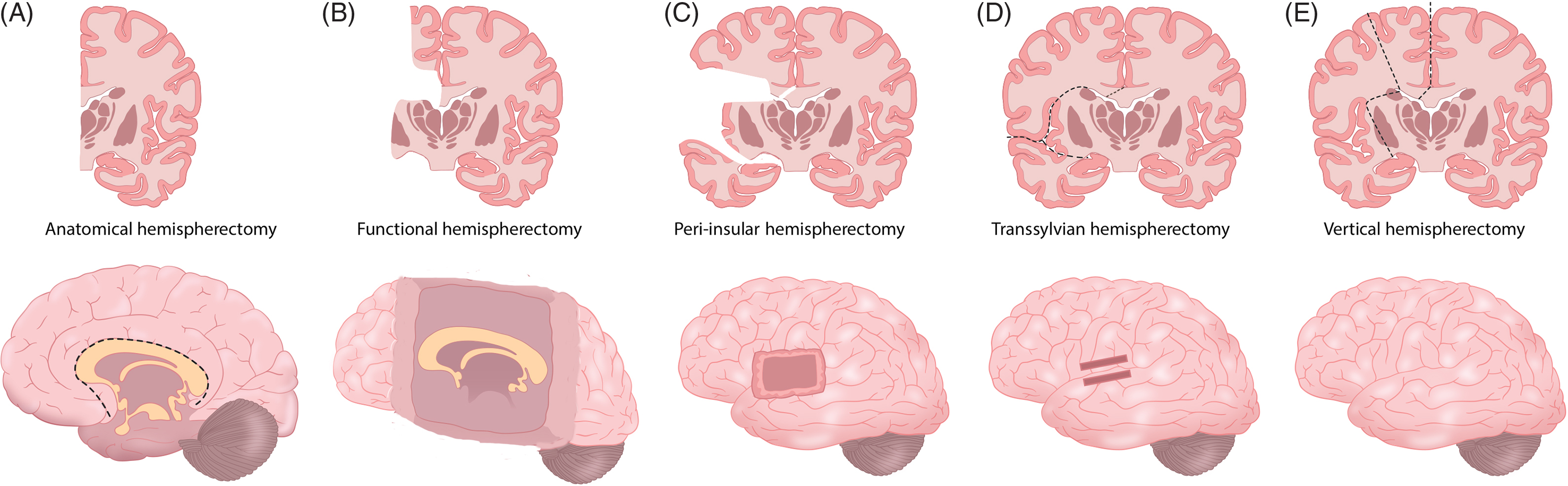
Figure 4: Schematic demonstration for the main surgical techniques from the anatomic hemispherectomy (A) to different hemispherotomy technical methods (B–E).
Hemidecortication
Since the early description of this procedure by Iqnelzi and Bucy, there were no significant changes in the technique for decades. Reference Ignelzi and Bucy16 The procedure consists of removal of the entire cortex of the hemisphere, leaving the ventricle closed to give an advantage. However, the temporal horn is opened, which would allow some blood and debris to enter the ventricle. The blood loss and the incidence of incomplete disconnection might be greater with this procedure. Reference Schramm58 In cases of HME and other migrational disorders, heterotopia within the white matter might preclude an adequate disconnection of the diseased hemisphere.
Selecting Hemispherotomy Techniques for Certain Disorders
In general, there is no clear evidence that certain hemispherotomy techniques are more suitable to certain pathologies and diagnoses. However, we explore here certain recommendations drawn by the experience of other surgeons throughout the evolution of different surgical techniques. It is recommended that one select an anatomic or functional hemispherectomy for cases with HME, due to the presence of distorted anatomy and small ventricles. Reference Schramm and Winn41,Reference Schramm and Behrens59,Reference O’Brien, Basu, Williams and May60 In such disorders, a functional hemispherectomy would allow greater exposure to a number of anatomical landmarks. In cases with SWS, the diffuse leptomeningeal angiomas often lead to increased blood loss throughout the procedure. Therefore, hemidecortication is not an appropriate technique in this setting. Performing a staged procedure may reduce blood loss. Reference Kestle, Connolly and Cochrane53 In one report, death resulted from uncontrolled bleeding in a patient with SWS. Reference Carson, Javedan and Freeman24 In cases with Rasmussen’s encephalitis, early intervention may increase the chance of early transfer of function, even though the procedure worsens the existing hemiparesis caused by the disease. Reference Vining, Freeman and Pillas47 Recently, subtotal hemispherectomy was performed in 23 children through sparing the primary sensorimotor cortex, with excellent results. Reference Chugani, Asano, Juhasz, Kumar, Kupsky and Sood61 However, further investigation of this technique in larger groups of patients is required to fully evaluate the effectiveness and the best candidates for such a procedure.
In a report of a series of 115 cases treated with different types of procedures, not one of the anatomic hemispherectomy cases required reoperation for recurrent seizures. Reference Cook, Nguyen and Hu62 However, it was associated with the longest hospitalization and hydrocephalus. The fewest numbers of procedural side effects were found with a modified lateral hemispherotomy. It is important to note that the functional hemispherectomy and modified hemispherotomy have steep learning curves. Therefore, the selection of the procedure should be based on the surgeon’s experience and preference.
Outcome and Predictors
Hemispherectomy is one of the most successful procedures to treat intractable epilepsy. Patient selection is an important factor that directly influences the outcome. Although all types of hemispherectomy techniques induce the same anatomical and physiological effects, the outcomes vary slightly between those techniques. Reference Villemure and Mascott26,Reference Shimizu and Maehara27,Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32,Reference Moosa, Gupta and Jehi63–Reference Villarejo-Ortega, Garcia-Fernandez and Fournier-Del Castillo65 Nevertheless, in some studies, the long-term follow-up outcomes of different techniques are similar. Reference Moosa, Gupta and Jehi63 The success rate of hemispherectomy in the cross-sectional published series ranges between 52% and 80%. Reference Lettori, Battaglia and Sacco36,Reference Hallbook, Ruggieri and Adina40,Reference Devlin, Cross and Harkness46,Reference Moosa, Gupta and Jehi63,Reference Jonas, Nguyen and Hu66–Reference Boshuisen, van Schooneveld and Leijten73 Hemispherotomy is commonly done in children in contrast with adults. This is likely due to the fact that the procedure is usually done during early childhood and rarely to be done during adulthood in many centers. There are certain limitations in adults, such as with language and cognitive functions, that may prevent doctors from doing the procedure in this age group. Reference McGovern, Moosa and Jehi74 There are reported case series in adults that showed a relatively comparable outcome to the pediatric age group in well-selected cases. Reference Schramm, Delev, Wagner, Elger and von Lehe33,Reference McGovern, Moosa and Jehi74,Reference Althausen, Gleissner and Hoppe75 In terms of the outcome over years, our group reviewed the outcomes of 53 patients who underwent functional hemispherectomy and, of those, 46.7% were seizure-free without auras after the third year following surgery, indicating a decline in seizure freedom over time. Reference Alsemari, Al-Otaibi and Baz76 In 2013, Moosa and colleagues from the Cleveland Clinic Foundation reported on the largest series (170 pediatric patients) of hemispherectomies. Reference Moosa, Gupta and Jehi63 The study was conducted using survival analysis with control of the follow-up duration variables. The seizure-free rate was 63% at 5 years. The most important factor that affected the outcome was the presence of early postoperative seizure and bilateral abnormalities in PET scans. Interestingly, the presence of bilateral MRI abnormalities did not affect the outcomes. These findings are similar to the findings of an early study of 110 children from Cleveland. Reference Hallbook, Ruggieri and Adina40 The results of these studies indicated that contralateral MRI abnormalities might not be used as a contraindication for hemispherectomy, although it is a common source of concern during patient selection. In contrast, another study of 43 children demonstrated that contralateral MRI abnormalities did affect outcomes. Reference Boshuisen, van Schooneveld and Leijten73 In a study of 18 cases, bilateral PET abnormalities did not affect the outcomes in contrast to the Cleveland study findings. Reference Gonzalez-Martinez, Gupta and Kotagal37 In eight HME cases studied by Rintahaka and colleagues, four had bilateral PET abnormalities, and the authors concluded that this finding correlated with a poorer prognosis. Reference Rintahaka, Chugani, Messa and Phelps77 The PET findings could suggest the presence of epileptogenic foci in the apparently healthy hemisphere that affect the outcomes in contrast with MRI structural abnormalities.
The presence of early postoperative seizures correlates with poor outcomes. Reference Moosa, Gupta and Jehi63,Reference Mani, Gupta and Mascha78–Reference Koh, Nguyen and Asarnow80 Therefore, patients with early postoperative seizures must be investigated for surgical failure. Other factors, such as gender, ages at surgery and seizure onset, underlying pathology, seizure semiology, surgery side, and interictal abnormalities, did not have any correlation with seizure freedom. Reference Moosa, Gupta and Jehi63,Reference Jonas, Nguyen and Hu66,Reference Boshuisen, van Schooneveld and Leijten73 Kossoff and colleagues from Johns Hopkins studied 111 patients who underwent hemidecortication. The authors demonstrated that poorer outcomes were found in patients with HME and cerebral migrational disorders. Reference Kossoff, Vining and Pillas67 By contrast, other studies describing other forms of hemispherectomy did not reveal this finding. Reference Hallbook, Ruggieri and Adina40,Reference Moosa, Gupta and Jehi63,Reference Jonas, Nguyen and Hu66,Reference Delalande, Bulteau and Dellatolas68,Reference Boshuisen, van Schooneveld and Leijten73 This might indicate that hemidecortication is not the optimal procedure in cases of migrational disorders. In a series of 96 pediatric cases, the presence of HME and surgery type was demonstrated to influence seizure control outcomes. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32 In a recent report, early surgery was associated with higher seizure remission (90%) as compared with late surgery (60%). Reference Althausen, Gleissner and Hoppe75 On the other hand, hemispherectomy in adults was shown to have similar success by comparison with the procedure in children in selected groups of patients. Reference Spencer and Huh71,Reference Cukiert, Cukiert and Argentoni81 The seizure freedom rate from the pooled data of 1528 patients was found to be 73%. Reference Hu, Zhang, Zhang, Shao and Zhang82 In this systematic review and meta-analysis of 56 studies, the main predictors of less favorable outcome were the presence of generalized seizure, developmental malformation etiology, non-lateralizing EEG, and contralateral MRI abnormalities. Reference Hu, Zhang, Zhang, Shao and Zhang82
Both early and recent literature have shown that motor function in the long-term post-hemispherectomy will remain unchanged in the majority of patients, and few patients will show either worsening or improvement in motor function. Reference Devlin, Cross and Harkness46,Reference McKissock83,Reference Mc84 Certain groups of patients develop additional motor deficits resulting in spasticity and limb deformities. Reference Moosa, Gupta and Jehi63,Reference Hu, Zhang, Zhang, Shao and Zhang82 As seen in both animal and human studies, brain plasticity allows the reorganization of certain motor and sensory functions in the healthy hemisphere. Reference Gramsbergen and Ijkema-Paassen85–Reference Huttenlocher and Raichelson88 Cerebral reorganization and early improvement in motor function are better seen after early surgical intervention (during the first year of life). Reference Holloway, Gadian, Vargha-Khadem, Porter, Boyd and Connelly89–Reference Holthausen and Strobl92 In one study using transcranial magnetic stimulation (TMS), the degree of motor cortex reorganization did not correlate with the degree of motor dysfunction. Reference Kastrup, Leonhardt, Kurthen and Hufnagel93 Jonas and colleagues demonstrated that patients with HME do worse in several domains, presurgically and postsurgically, than patients suffering from other pathologies, such as cortical dysplasia, infarcts, and encephalitis. Reference Jonas, Nguyen and Hu66 Recently, children with developmental disorders were found to show less improvement after hemispherectomy than those with acquired pathologies. Reference van der Kolk, Boshuisen and van Empelen94 The overall functional outcome might be dependent on several factors, such as underlying pathology, degree of presurgical cognitive decline, duration and severity of epilepsy, and postsurgical seizure freedom.
Many studies showed mild to modest cognitive function improvement after hemispherectomy and remission of epilepsy-induced encephalopathy. Reference Schramm and Winn41,Reference Devlin, Cross and Harkness46,Reference Jonas, Nguyen and Hu66,Reference Althausen, Gleissner and Hoppe75,Reference Wyllie, Comair, Kotagal, Raja and Ruggieri95–Reference Pulsifer, Brandt, Salorio, Vining, Carson and Freeman99 The decline in intellectual function is stabilized after surgery, and progressive postsurgical improvement has been observed. Reference Rasmussen5,Reference Villarejo-Ortega, Garcia-Fernandez and Fournier-Del Castillo65,Reference Beardsworth and Adams100,Reference van Schooneveld, Jennekens-Schinkel, van Rijen, Braun and van Nieuwenhuizen101 Schooling may improve after the procedure, but this is highly dependent on the preoperative cognitive function. The quality of life was found to be significantly improved in proportion to cognitive function. Reference Battaglia, Veggiotti and Lettori102 On the other hand, there is no significant loss of language function after surgery in either hemisphere. Reference Peacock, Wehby-Grant and Shields19,Reference Vining, Freeman and Pillas47 This suggests a reorganization of language before surgery. Speech-related fMRI activation studies demonstrate that distinct subregions of Broca’s area and their contralateral homologs could conduct language function. Reference Liegeois, Connelly, Baldeweg and Vargha-Khadem103
Although the surgery often results in homonymous hemianopsia, studies demonstrated the presence of residual visual function despite the loss of the occipital cortex and striatal fibers. Reference Werth104 This could be explained by the reorganization of the contralateral cortex, such as an expansion of the peripheral vision cortical representation area. Another explanation is that other brain structures, such as the midbrain (superior colliculi and pretectum), can mediate this residual visual function. Reference Haak, Langers, Renken, van Dijk, Borgstein and Cornelissen105,Reference Ptito, Fortin and Ptito106 Hemispherectomy in early life may allow the reorganization of cerebral function and adaptation during this stage of development. The auditory function test after hemispherectomy was more sensitive for the right hemisphere than for the left, and the stronger contralateral ear-hemisphere connection dominates and masks the weaker ipsilateral ear-hemisphere. Reference de Bode, Sininger, Healy, Mathern and Zaidel107 Interestingly, dichotic listening was found to be superior in post-hemispherectomy patients as opposed to normal control subjects. Reference Wester, Hugdahl and Asbjornsen108 Zatorre and colleagues found a preservation of auditory spatial localization after hemispherectomy and suggested that this function is mediated by cortical systems in the remaining hemisphere, with or without subcortical structure involvement. Reference Zatorre, Ptito and Villemure109 Recently, spatial attention was demonstrated to be transferred to the remaining hemisphere. Reference Zhu, Cui and Zhu110 Table 3 summarizes the outcomes of different studies in terms of seizure control.
Hemispherectomy may fail to control seizures for two reasons: the incomplete disconnection that is frequently seen in the region of the corpus callosum and the presence of bilateral epileptogenic activity. Incomplete disconnection can be found in up to 30% of cases. Cats and colleagues found that the presence of insular cortex postoperatively is the statistically significant factor associated with a postoperative seizure. Reference Cats, Kho, Van Nieuwenhuizen, Van Veelen, Gosselaar and Van Rijen111 Reoperation was found to be successful in controlling seizures; however, the success rate is often lower than that of the initial procedure. Overall, the reoperation rate in the literature varies between 5% and 19%. Reference Peacock, Wehby-Grant and Shields19,Reference Shimizu and Maehara27,Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32,Reference Schramm and Winn41,Reference Schramm58,Reference Peacock112 Vadera and colleagues recently reported on 36 patients who underwent a redo hemispherectomy, and of those, 19% were seizure-free after anatomic hemispherectomy, 45% had a ≥90% seizure reduction, and 36% did not improve. Reference Vadera, Moosa and Jehi113 In this report, the generalized epileptic activity in the EEG was associated with a poor prognosis.
Complications and Adverse Effects
We suggested the use of “adverse effects” to discuss the expected postoperative loss of cortical functions, such as motor skills and visual acuity. Complications are the unexpected postoperative events that include, for example, infection, bleeding, and deterioration of neurological status. Historically, the most severe complications were SCH and hydrocephalus. The findings were that SCH happened in approximately 33% of patients, with an overall estimated mortality rate of 30% to 40%. In addition, hydrocephalus occurs in 52%. Reference Rasmussen5,Reference Villemure13,Reference Villemure and Luders14 Newer literature has indicated a decline in the incidence of SCH. To our knowledge, the last reported case of hemispherectomy-related SCH was in 1996. Kalkanis and colleagues reported on a 52-year-old woman who had a neurological deterioration attributed to SCH 36 years after hemispherectomy. Reference Kalkanis, Blumenfeld and Sherman114
In 2013, the incidence and risks of hydrocephalus after hemispherectomy were reviewed in a multicentric study by Lew and colleagues and the post-hemispherectomy hydrocephalus workgroup. Reference Lew, Matthews, Hartman and Haranhalli115 The study analyzed data from 690 patients collected from 15 pediatric epilepsy centers in the USA, Japan, and China. Of those patients, 162 (23%) had been treated for postoperative hydrocephalus. A multivariate regression analysis showed that the risk factors for hydrocephalus are anatomic hemispherectomy and previous brain surgery. In a univariate analysis, the use of hemostatic agents and resection of the basal ganglia and/or the thalamus was found to be a risk factor as well. Reference Lew, Matthews, Hartman and Haranhalli115 The strongest predictor was anatomic hemispherectomy with a higher rate of hydrocephalus. The etiology of early postoperative hydrocephalus can be explained by the wide exposure of the ventricles and the presence of blood products and tissue debris in the surgical cavity that may have blocked the cerebrospinal fluid (CSF) pathway and absorption. On the other hand, a delayed hydrocephalus that occurs many years after the procedure is difficult to explain. In a report on two patients, hydrocephalus occurred 27 and 34 years after hemispherectomy. Reference Strowitzki, Kiefer and Steudel116 The cause of delayed hydrocephalus might be related to the loss of brain volume from axonal degeneration and scar tissue formation that altered compliance, leading to hydrocephalus. Serial MRIs with extended follow-up exams might give a better understanding of the progressive slow cerebral and CSF dynamics changes. Table 4 summarizes the incidence of hydrocephalus and mortality in different hemispherectomy techniques.
Table 4: The incidence of hydrocephalus and mortality from analysis of different recent large case series
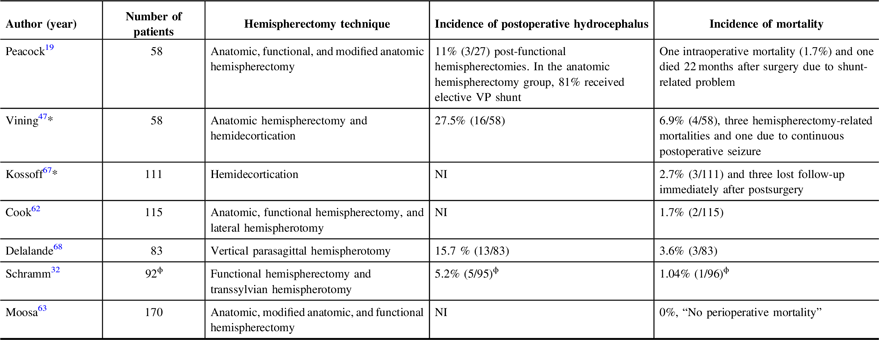
NI = not indicated.
* These studies share a significant number of the same patients’ data.
ϕ Ninety-six patients were operated on and out of those 92 were eligible for the study inclusion criteria. Morbidity and mortality were calculated on the basis of 96 patients.
Intraoperative complications include blood loss, coagulopathy, electrolyte imbalance, hypothermia, and hypovolemia. Blood loss was found to be more extensive in longer procedures, and more tissue removal was required than in procedures with less exposure and less operative time. Reference Schramm, Kral and Clusmann31 HME was found to be associated with greater blood loss. Reference Cook, Nguyen and Hu62 Immediate postoperative complications are electrolyte imbalance and diabetes insipidus, as the most common postoperative endocrinopathy features. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32 Fever without origin is the most common early postoperative complication that is transient; it may continue for the first 10 days due to aseptic hematogenous meningitis. Corticosteroids might help in reducing the symptoms of fever and headache resulting from aseptic meningitis. Subdural or epidural hematomas may occur, and wound infection seems to appear more frequently with large craniotomy approaches and long procedure times. Reference Adams18,Reference O’Brien, Basu, Williams and May60 Delayed complications are recurrence of seizures and late-onset hydrocephalus. Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32,Reference Jonas, Nguyen and Hu66 The expected postoperative adverse effects are motor weakness, speech dysfunction, and contralateral visual field loss. The mortality rate in older series was 6–8%; however, in recent series, with the use of modified hemispherectomy techniques, the mortality rate has dropped to 0–4%. Reference Rasmussen5,Reference Peacock, Wehby-Grant and Shields19,Reference Shimizu and Maehara27,Reference Schramm, Kuczaty, Sassen, Elger and von Lehe32,Reference Vining, Freeman and Pillas47,Reference Moosa, Gupta and Jehi63,Reference Delalande, Bulteau and Dellatolas68,Reference Basheer, Connolly, Lautzenhiser, Sherman, Hendson and Steinbok69 The most common causes of mortality are bleeding, hydrocephalus, and brain edema. Despite its low mortality rate, hemispherectomy is one of the procedures in epilepsy surgery associated with the highest morbidity and mortality. Reference Wiebe and Berg117
Conclusion
Hemispherectomy is an effective procedure to treat epilepsy. The procedure began as complete hemispheric removal and has undergone significant modifications to become a disconnective procedure resulting in the reduction of morbidity and mortality rates. Early hemispherectomy is advisable to stabilize and improve a decline in cognitive function and allow early post-procedure cerebral reorganization. The predictors that affect outcome might be the presence of bilateral MRI/PET abnormalities, non-lateralizing EEG, the presence of generalized seizure, developmental malformation etiology, and the occurrence of early postoperative seizures. HME was associated with higher morbidity and an incomplete disconnection rate. Therefore, it must be approached with caution, and more tissue resection is advisable in such cases. Based on this review, we suggest that future studies should focus on the predictors that influence outcomes based on the underlying pathology and the type of procedure used. The evolution of hemispherectomy will continue as new radiological and diagnostic methods increase our understanding of epileptic syndromes and pathology substrates.
Disclosure
The authors have nothing to disclose.
Statement of Authorship
FA: wrote the manuscript and designed illustrations. RA, SA, and SB: reviewed and edited the manuscript. DS: reviewed, edited, and contributed to the final manuscript production. JG: contributed to manuscript writing, editing, and illustration design. All authors critically reviewed the manuscript and approved the final version.










