Necrotising enterocolitis can be a complication for neonates with CHD. This complex gastrointestinal disease is most seen in the neonatal ICUs, as its highest prevalence is in the preterm infant population. No single aetiology has been identified, but multiple risk factors are well documented in the literature. Reference Singh, Miller, Orgel, Dave, Mackay and Good1,Reference Kataria-Hale, Osborne, Hair, Hagan and Pammi2 One such risk factor is CHD. Reference Spinner, Morris and Nandi3 Infants with cyanotic cardiac lesions are at increased risk for necrotising enterocolitis due to mesenteric hypoperfusion. Enterocytes are aerobic and require sufficient oxygen for survival. Hypoxic conditions deprive the gastrointestinal cells of oxygen resulting in intestinal ischaemia, increased gastrointestinal tract permeability due to enterocyte inflammation, and mucosal barrier injury. Reference Burge, Gunasekaran, Makoni, Mir, Burkhart and Chaaban4
Patients with single-ventricle cardiac anatomy undergo staged palliative cardiac surgery to optimise chances of survival. In the last 10 years, enterally feeding prior to the initial palliative cardiac surgery has become commonplace. A 2016 survey of 46 neonatal CHD institutions found that 65% of centres allow pre-operative feeding in their single-ventricle patients. This percentage of centres has increased substantially since this publication. The first surgery for hypoplastic left heart syndrome, which is a common single-ventricle lesion, is typically either a Norwood or a Hybrid procedure, which is a less invasive stage 1 palliation. It is usually completed within the first 1–2 weeks of life. Reference Slicker, Sables-Baus, Lambert, Peterson, Woodard and Ocampo5,Reference Nordenström, Lannering, Mellander and Elfvin6 Some patients who are not felt suitable for single-ventricle pathway are listed for cardiac transplant.
Neonates that receive pre-operative enteral feeds see benefits in both their pre- and post-operative courses. Post-operatively, patients who received pre-op feeds, have improved fluid management, improved wound healing, decreased duration of mechanical ventilation, and have been associated with less lability in post-operative haemodynamics, often resulting in shorter hospital stays, improved weight-for-age at time of discharge, shorter duration on parenteral nutrition, quicker succession to oral feeds, and, if breastmilk was provided pre-operatively, lower sepsis incidence. Reference Martini, Beghetti and Annunziata7,Reference Herridge, Tedesco-Bruce, Gray and Floh8,Reference Toms, Jackson, Dabal, Reebals and Alten9 Pre-operative benefits of pre-op enteral feeds include increased parental bonding, gut priming, gastrointestinal tract stimulation to prevent gut atrophy, and facilitation of gastrointestinal tract maturation. If fed breastmilk, an additional benefit is a positive impact on microbiome establishment and immune system development. Reference Singh, Miller, Orgel, Dave, Mackay and Good1,Reference Kataria-Hale, Osborne, Hair, Hagan and Pammi2,Reference Slicker, Sables-Baus, Lambert, Peterson, Woodard and Ocampo5,Reference Kelleher, McMahon and James10
Although CHD is a risk factor for necrotising enterocolitis, pre-operatively feeding CHD neonates has not been found to increase rates of necrotising enterocolitis. Reference Cognata, Kataria-Hale and Griffiths11-Reference Sagiv, Tjoeng and Davis13 In an analysis of the National Pediatric Cardiology Quality Improvement Collaborative dataset, of 1740 infants who were fed prior to stage 1 palliative surgery, no statistically significant difference in necrotising enterocolitis between patients fed compared to those not fed per institutional approach was found (p = 0.2). Reference Sagiv, Tjoeng and Davis13 Despite the known benefits and findings of low risk, the National Pediatric Cardiology Quality Improvement Collaborative noted that there was a practice variation in standard feeding neonates pre-operatively. Until 2020, our institution was one of the few National Pediatric Cardiology Quality Improvement Collaborative centres that did not routinely feed single-ventricle CHD patients prior to stage 1 surgery. Feeding pre-operatively was up to provider discretion, with most providers withholding feeds due to concerns for gut ischaemia from coarctation of the aorta, cyanosis, umbilical arterial lines, and risk for necrotising enterocolitis. In early 2020, we convened a multidisciplinary group to complete a literature review as well as collate, review, and compare pre-operative feeding protocols from similar institutions. By the end of February 2020, the multidisciplinary team determined that pre-operatively feeding single-ventricle patients has greater benefit than risk. We collaboratively created a pre-operative enteral feeding algorithm for use in haemodynamically stable neonates after 24 hours of life, weighing 2 kg or more, born at 36 weeks gestational age or older, and were expected to undergo staged cardiac surgery for single-ventricle physiology.
The aim of this study is to monitor the impact of our practice change with the primary outcome of necrotising enterocolitis incidence from birth to 2 weeks post-operative cardiac surgery. Through monitoring, we can objectively address the concerns causing hesitation to pre-operatively feed patients slated to undergo stage 1 Norwood or a Hybrid palliation prior to neonatal discharge from our institution.
Methods
Study design
This is a single-site, retrospective cohort study including patients from 1 March, 2018 to 1 July, 2022. The institution is a large volume, tertiary referral centre, paediatric non-profit hospital. For inclusion, parents had provided informed consent to Study #16050323 under the Phase II National Pediatric Cardiology Quality Improvement Collaborative registry for care of children with hypoplastic left heart syndrome and similar CHD requiring staged single-ventricle surgeries and intensive care as a neonate.
Study cohort
Consistent with a retrospective cohort study design, our initial cohort list came from a report pulled in Fall 2022 with the following inclusion criteria: (a) admitted to a level IV neonatal ICU within the first week of life between 1 March, 2018 and 1 July, 2022. (b) Qualified for stage 1 Norwood, hybrid palliation cardiac surgery, or cardiac transplant during their neonatal hospitalisation at our institution. All gestational ages and birth weights were included. Exclusion criteria were only those families that declined participation in the National Pediatric Cardiology Quality Improvement Collaborative registry study.
The cohort was divided into two groups – Pre-Feeding Algorithm Group, patients born on or before 30 June, 2020, and, a Post-Feeding Algorithm Group, patients born 1 July, 2020 or later. In the pre-feeding algorithm group, provider discretion determined which patients were enterally fed and which were not. In the post-feeding algorithm group, objective criteria were developed using literature review and expert consensus to create an institutional-based single-ventricle pre-operative feeding algorithm (Fig. 1). To be included in the algorithm, patient had to be deemed haemodynamically appropriate (reassuring vital signs and perfusion, no vasoactive support, appropriate near-infrared spectroscopy (>40 and/or venous saturations), diastolic blood pressure consistently greater than 30 mmHg, non-acidotic with C-reactive protein < 4 and lactate < 2, and be over 2 kg and 36 weeks gestational age. In addition to this objective haemodynamic criterion, infants were monitored closely for signs of feeding intolerance.

Figure 1. Feeding Algorithm. Copyright © 2020 The Children’s Mercy Hospital. All rights reserved. Used with Permission.
Additionally, we aimed to further understand the impact of patient demographics and patient characteristics on those receiving enteral feeds pre-operatively at any time period. Patients in the not fed (NPO) group were not fed prior to their cardiac surgery. Patients in the Preop Fed or Enteral Feeding (EN) group received some volume of enteral feeds prior to their cardiac surgery. Patients were not randomly selected to be pre-operatively fed or not fed, rather patients followed the standard of care by the clinical teams at time of admission. See Figure 2 for group stratification.
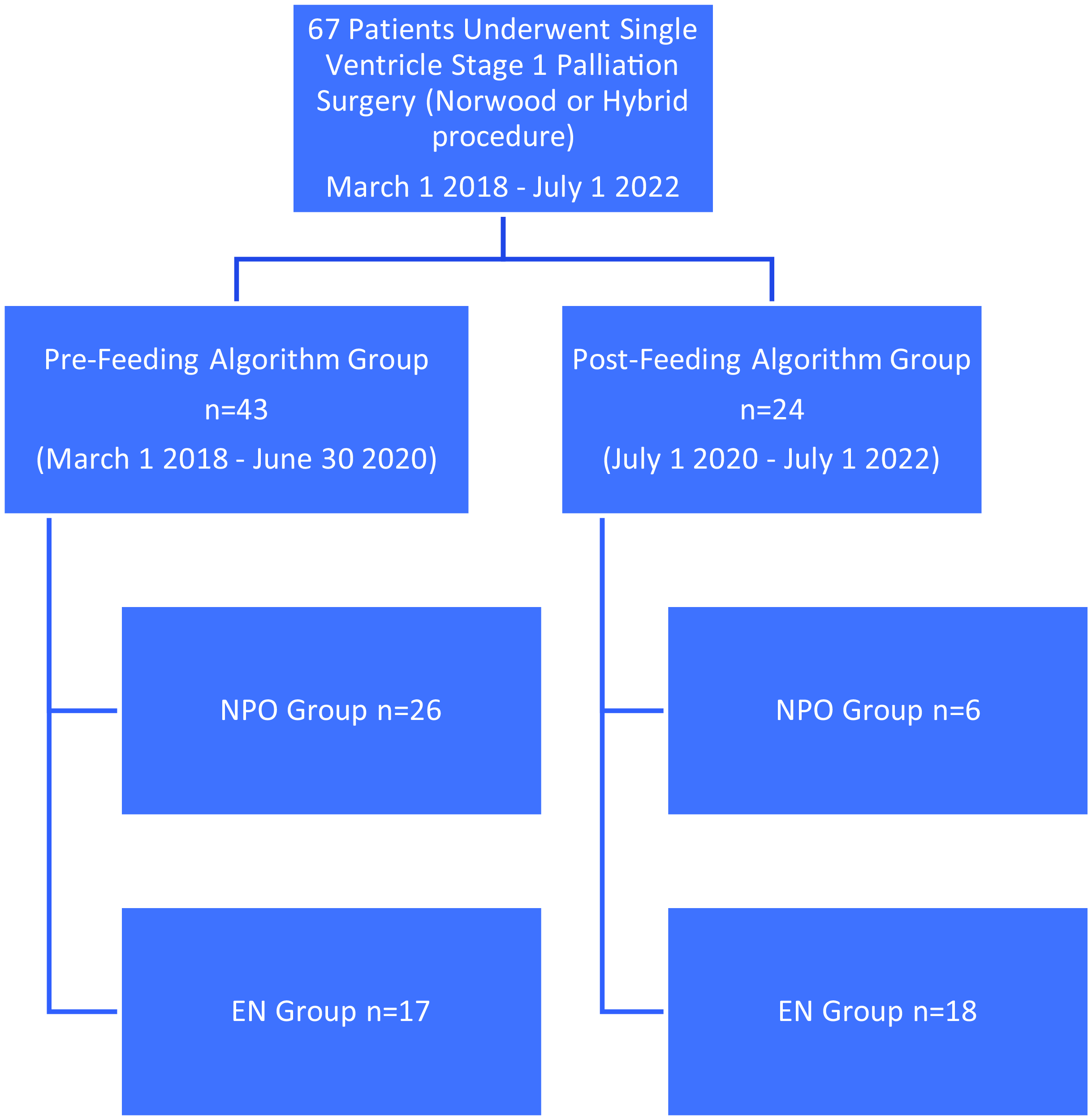
Figure 2. Patient flow chart.
Outcome measure
The primary outcome of interest was diagnosis of modified Bell stage IIa or higher necrotising enterocolitis, either pre-operatively or in the immediate post-cardiac surgery period. Reference Bell, Ternberg and Feigin14,Reference Walsh and Kliegman15 We defined the immediate post-op status as 2 weeks (14 days) following cardiac surgery. Necrotising enterocolitis stage IIa or higher requires patients to show a radiologic sign of necrotising enterocolitis for diagnosis. Reference Kelleher, McMahon and James10 The measure for necrotising enterocolitis was confirmed by reviewing the original radiology report in addition to the treating team’s progress note.
Measures
Demographics evaluated included date of birth, patient age at time of surgery, race/ethnicity, sex assigned at birth, gestational age and stage 1 palliation surgery date. Feeding route (oral or feeding tube-dependent, or tube-assisted), types of enteral feeds (breastmilk or formula), maximum enteral feeding volume in millilitres per kilogram (mL/kg/day), and presence of an umbilical arterial line were recorded. Maximum enteral feeding volumes were found in the intake table completed by bedside nursing in real time. See Table 1 for results. A unique variable in our study dataset includes renal near-infrared spectrometry data to assess its utility in monitoring feeding tolerance, while the neonate was being fed pre-operatively. Reference DeWitt, Charpie, Donohue, Yu and Owens16 Near-infrared spectroscopy values while patient was being pre-operatively enterally fed were reviewed. A cut-off of 40% was selected as the criterion definition of low renal/somatic near-infrared spectroscopy. This measure was operationalised in the dataset as low near-infrared spectroscopy: if the patients’ near-infrared spectroscopy decreased to < 40% for three consecutive measurements while being enterally fed. This was sampled every hour from vital sign charting.
Table 1. Results of n = 67 infants across the pre-feeding algorithm with patients born 1 March, 2018 through 30 June, 2020, 2 years prior to implementation of a pre-operative feeding algorithm, and, the post-feeding algorithm group, patients born between 1 July, 2020 and 1 July, 2022. Three outliers removed for analysis – went above the 40 ml/kg to full feeds (estimated 100 ml/kg/day). NEC = necrotising enterocolitis

Procedures
Data were collected using an electronic medical record retrospective chart review. Statistical analysis was completed using SPSS version 24 to evaluate descriptive statistics for demographics, to compare differences between the pre-feeding and post-feeding algorithm groups and to compare all patients fed pre-operatively (EN group) to all patients not fed pre-operatively (NPO group). Variables were assessed for kurtosis and skewness for significant cases outliers. Independent samples t-tests, Fisher’s exact test (two-sided), and Pearson’s Chi-square (two-sided) were used to evaluate statistical significance for continuous and categorical variables.
Results
Seventy-five per cent (18/24) of neonates in the post-feeding algorithm group met the criteria and were fed. No infants in the post-feeding algorithm group had any gastrointestinal abnormalities which may have impaired accuracy of near-infrared spectroscopy monitoring. Twenty-four neonates had recorded near-infrared spectroscopy values, and 12.5% (n = 3) had episodes of near-infrared spectroscopy < 40 x3; however, none of these patients had necrotising enterocolitis within the pre-operative or 14 days post-operative period. Three patients who received full enteral feeds (defined as > 100ml/kg) prior to surgery were excluded in the analysis due to significant outlier and skewing comparative analysis.
There were a few statistically but non-clinically significant differences noted between groups (gestational age and race). Importantly, there was no difference in incidence of necrotising enterocolitis between the not fed and the enteral nutrition groups (Table 2). All patients in all groups received prostaglandin infusion prior to cardiac surgery.
Table 2. Patient demographics, characteristics, and outcomes if the patient was not fed pre-operatively (NPO group) or enterally fed (EN group) across any time period

NEC = necrotising enterocolitis; BTT = Blalock–Taussig–Thomas; NICU = neonatal ICU; HLHS = hypoplastic left heart syndrome.
Enteral feeding while having a umbilical arterial line did not have a significant influence on necrotising enterocolitis incidence. 64.7% (22/34) of neonates with an umbilical arterial line in place were fed pre-operatively.
Analysis included the assessment of pre-operative enteral feeding algorithm compliance. In the 2 years following implementation of our pre-op feeding algorithm, our centre was approximately 75% compliant with adhering to the algorithm.
Discussion
Enteral feeding has several benefits for both pre-operative and post-operative infants with CHD. Those benefits include gut microbiome priming, shorter hospital stays, and decreased mechanical ventilation days, to name a few. By implementing a pre-operative enteral feeding algorithm, we found that we were able to nearly double the percentage of neonates that received enteral feedings pre-operatively without a change in incidence of necrotising enterocolitis. There was no statistically significant impact on necrotising enterocolitis between the pre-algorithm group and the post-algorithm implementation group.
Our primary outcome, necrotising enterocolitis stage IIa or greater happening between birth and 2 weeks following the Norwood or Hybrid cardiac surgery, occurred in 9% (6/67) of our total cohort. It is challenging to compare this number to a specific necrotising enterocolitis rate because of our unique, specific time frame selected for data collection. However, looking at the total necrotising enterocolitis rates in the single-ventricle CHD population, this rate is comparable to what other researchers have found. Reference Spinner, Morris and Nandi3,Reference Kelleher, McMahon and James10,Reference Becker, Hornik and Cotten17 The results of this study are consistent with prior studies finding pre-operatively feeding trophic volumes does not increase pre-operative or immediate post-operative necrotising enterocolitis in CHD patients who undergo stage 1 palliation cardiac surgery. This is further evidence reinforcing that pre-operative feeding is safe, and beneficial. No clinically, statistically significant differences were found between any group demographics or clinical characteristics.
Of the six patients who were diagnosed with necrotising enterocolitis, three were fed pre-operatively. Two cases occurred prior to cardiac surgery, and four cases occurred within 2 weeks following cardiac surgery. The two patients who had necrotising enterocolitis pre-operatively had cardiac surgery on day of life 25 and 34. Typically, stage 1 palliation occurs within the first week or two of life. There is a growing body of “observational inference” that the longer patients wait for stage 1 palliation, the greater the necrotising enterocolitis risk. Reference Burge, Gunasekaran, Makoni, Mir, Burkhart and Chaaban4,Reference Kelleher, McMahon and James10 The time between birth and cardiac surgery is tenuous. The neonatal body is adjusting to life in the extrauterine world, including using lungs and the gastrointestinal tract for the first time. With a structural cardiac difference as seen in single-ventricle patients, there is the compounded impact of systemic hypoperfusion on this new body. Dips in haemodynamic stability while awaiting cardiac intervention may cause an insult, setting the scene for gut ischaemia, or necrotising enterocolitis.
Our decision to only include necrotising enterocolitis that occurred between birth and 2 weeks following cardiac surgery was due to the work of Kelleher, McCahon, and James. They describe in their review article published in fall 2021 that post-operative necrotising enterocolitis has countless contributing factors. So many that assessing the impact of pre-operative enteral feeds alone on necrotising enterocolitis incidence occurring anytime during a multi-month hospitalisation is extraordinarily challenging and unlikely to be accurate. Reference Kelleher, McMahon and James10 In other words, it would be misleading to draw a conclusion that pre-operative enteral feeding significantly impacts necrotising enterocolitis occurring months after cardiac surgery. Of note, our institution does have post-operative enteral feeding algorithms which are followed dutifully in the paediatric cardiac ICU. Post-op enteral feeding algorithms have been found to decrease post-operative necrotising enterocolitis incidence. Reference Kelleher, McMahon and James10,Reference Cognata, Kataria-Hale and Griffiths11 In the cardiac ICU post-operative algorithms, feeding advancement occurs at a faster rate when neonates have been fed pre-operatively.
In this study, we did not assess bolus versus continuous feeding on necrotising enterocolitis incidence. At trophic volumes, the impact of bolus versus continuous feeds on mesenteric blood flow and oxygenation consumption are indistinguishable. There were also no significant findings regarding feeding route, oral as opposed to using a feeding tube. We suspect there would be a difference at greater volumes, but our study did not assess this. We did however evaluate if the presence of an umbilical artery line increased necrotising enterocolitis incidence. Consistent with past literature findings, we did not find an increased necrotising enterocolitis incidence in patients with an umbilical arterial line. Reference Havranek, Johanboeke, Madramootoo and Carver18,Reference Surmeli Onay, Velipasaoğlu, Tutal and Sarılar19
Breastmilk is known to decrease necrotising enterocolitis risk. In our study, most patients received breastmilk. Breastmilk is well known to be protective against necrotising enterocolitis. Reference Nordenström, Lannering, Mellander and Elfvin6,Reference Kelleher, McMahon and James10,Reference Cognata, Kataria-Hale and Griffiths11 The high rate of breastmilk utilisation likely positively influenced our necrotising enterocolitis rates.
This study is unique in that we documented feeding volume in millilitres per kilogram (ml/kg/day). Most published studies do not document exact feeding volumes but rather vaguely categorised all volumes as “trophic.” The definition of trophic is not standardised. Trophic refers to feeds less than full volume, usually indicating feeds at 25–50% full volume, but that is up to provider interpretation. In this study, we analysed the data using feeding volume as a continuous variable. Our cohort was too small to power statistical analysis between maximum pre-operative feeding volumes and necrotising enterocolitis. However, the clinically significant trend observed was that infants fed closer to and greater than 40 ml/kg/day had higher risk of developing necrotising enterocolitis during their total hospitalisation at our institution. Infants fed pre-operative volume of 30 ml/kg or less had a lower necrotising enterocolitis incidence. We are discussing altering our pre-operative enteral feeding algorithm to recommend a maximum of 30 ml/kg/day instead of 40 ml/kg/day. When following the feeding algorithm, no patient is required to be fed the maximum allowance, but the patient may be if deemed beneficial by the medical team.
Near-infrared spectroscopy is a non-invasive technology that continuously evaluates regional oximetry. Reference Owens, King, Gurney and Charpie20 To date, there is much research demonstrating the safety of pre-operative enteral feeding. A clinical question is if near-infrared spectroscopy could be used as a non-invasive surrogate marker to assess mesenteric perfusion. A future research direction is to investigate this relationship further. Could near-infrared spectroscopy help mediate necrotising enterocolitis risk while being enterally fed? Based on our experience, we suspect the answer is yes. Changes in systemic oxygen delivery while awaiting cardiac intervention may set the scene for gut ischaemia, or necrotising enterocolitis. Reference Nordenström, Lannering, Mellander and Elfvin6,Reference Kelleher, McMahon and James10 As an institution, we will continue to monitor feeding tolerance by using a near-infrared spectroscopy ≤ 40 threshold for when to begin discussions of ceasing, or at least decreasing, enteral feeding to thwart necrotising enterocolitis. Clinical judgement and provider discretion will be heavily relied on when making these clinical decisions.
The multidisciplinary approach to the application of this feeding algorithm had an overall positive impact and acceptance. In addition to the criteria used to determine feeding plans, its implementation insured that tangible discussions about enteral nutrition, feeding route, and tools for monitoring signs of intolerance occurred on daily collaborative, family-centred care team rounds.
Limitations
Limitations of this study include the inherent limitations of a retrospective study, notably the retrospective chart review data collection method. Another limitation was the use of convenience sampling by timing for admission to our institution without random selection to groups. The sample size was also very small to evaluate a diagnosis as rare as necrotising enterocolitis.
Conclusion
Implementation of a pre-operative enteral feeding algorithm was successful, and following this algorithm was safe for our patients. We nearly doubled the percentage of patients being fed pre-operatively without increasing necrotising enterocolitis. This study supports pre-operative enteral feeding neonates with CHD prior to stage I Norwood or Hybrid surgeries. The benefits of pre-operative trophic enteral feeds outweigh the risks. Ongoing practice change and evaluation are being conducted to give patients the most benefits from enteral feeds while simultaneously minimising the necrotising enterocolitis risk.
Acknowledgements
The authors thank Adrienne Olney, MS, and Ryan Thompson for their assistance with statistical evaluation and analysis. The authors also thank Dr William Gibson, DO, Cardiothoracic Surgeon at Children’s Mercy, for his expert manuscript review.
Lucy Pappas completed data collection under the supervision of Dr John Daniel. Lucy Pappas was the primary author of the manuscript. Lori Erickson completed the data analysis. Amy Ricketts, Aaron Hahn, Matthew Moehlmann, and John Daniel provided expert review of the manuscript in preparation for publication.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.







