Clozapine is indicated in treatment-resistant schizophrenia, where it is uniquely effective (National Institute for Clinical Excellence, 2003). Clozapine has been available in the UK for 15 years under the brand name Clozaril®. Two branded generic products have recently been licensed. Standard bioequivalence studies are difficult to conduct for clozapine, where small doses can cause profound hypotension and tachycardia in healthy volunteers; bioequivalence across the dosage range has not been unequivocally demonstrated for the available products (Anon, 2001; Reference Ereshefsky and GlazerEreshefsky & Glazier, 2001).
Clinical equivalence has also been questioned (Anon, 2001). Five papers reporting on outcomes in a total of 131 patients have been published. One, a case series (Reference Mofsen and BalterMofsen & Balter, 2001), reported a high relapse rate and one (Reference Kluznik, Walbek and FarnsworthKluznik et al, 2001), which was sponsored by the patent holder, reported a trend towards deterioration. These papers have been widely cited as proof that switching patients to generic clozapine is a high-risk strategy. The work of Makela et al (Reference Makela, Cutlip and Stevenson2003) (no sponsorship declared), and also of Sajbel et al (Reference Sajbel, Carter and Wiley2001) and Stoner et al (Reference Stoner, Lea and Dubisar2003), both sponsored by a generic manufacturer, did not replicate these findings. This work is less well known.
We report on our experiences of switching all patients in a single mental health trust from Clozaril® (Novartis Pharmaceuticals, Surrey, UK) to generic clozapine.
METHOD
All patients (n=337) were switched from Clozaril® to generic clozapine (Zaponex®; IVAX Pharmaceuticals, London, UK). There were no exclusions.
The following data were collected for each patient:
-
(a) at baseline (1 month before the switch): name, gender, ethnicity, age, duration of treatment with Clozaril®, dose and Clinical Global Impression of Severity of Illness (CGI; Reference GuyGuy, 1976).
-
(b) at follow up (3 months after the switch): dose, CGIs and Clinical Global Impression of change over the past 3 months (CGIc). The CGIc score was chosen as the primary outcome measure, as it is simple to complete and detects change that is clinically meaningful.
Patients who remained on generic clozapine at the point of follow-up were compared with those who dropped out of treatment (independent t-test for continuous data and χ2 for categorical data). Patients who remained on treatment were divided into 3 groups depending on the duration of clozapine treatment at the time of the switch (<18, 18-52, >52 weeks). The CGI severity scores and doses of clozapine before and after switching were compared using paired-samples t-tests. The CGIs score after switching was then subtracted from the baseline score to give an estimate of change. This calculated change score was compared with the clinician-completed CGIc score using Pearson's correlation, a test of internal validity.
RESULTS
Of the 337 patients switched from Clozaril® to generic clozapine, 304 (90.2%) remained on treatment 3 months later; 26 patients (7.7%) stopped treatment; 5 (1.5%) moved out of the area and 2 (0.6%) died. Completers had been on treatment for longer at the point of switch (mean 62.6 v. 23 months, t=3.778, P<0.001) and were receiving a higher dose (mean 443 mg/day v. 340 mg/day, t=2.559, P=0.011) than those who stopped treatment. There were no differences with respect to age or gender.
Mean CGIs scores before and after the switch were: patients treated for <18 weeks (3.74, 3.37, t=1.17, P=0.25); 18-52 weeks' treatment (3.86, 3.41, t=1.991, P=0.056); >52 weeks' treatment (3.42, 3.19, t=3.658, P<0.001); and for the whole group (3.49, 3.23, t=4.242, P<0.001).
Significant dose increases were seen in those who had been treated for <18 weeks (mean 327 mg before, 380 mg after, t=3.732, P=0.001). No significant dosage adjustments were seen in other patients.
The CGIc scores after switching are shown in Fig. 1. The CGIc score was correlated with the calculated change score (Pearson's correlation=0.341, P<0.01). Using a 1-point difference from the anchor point of 4 (no change) as a measure of clinically significant change in mental state, overall 19 patients deteriorated, 193 stayed the same and 92 improved.
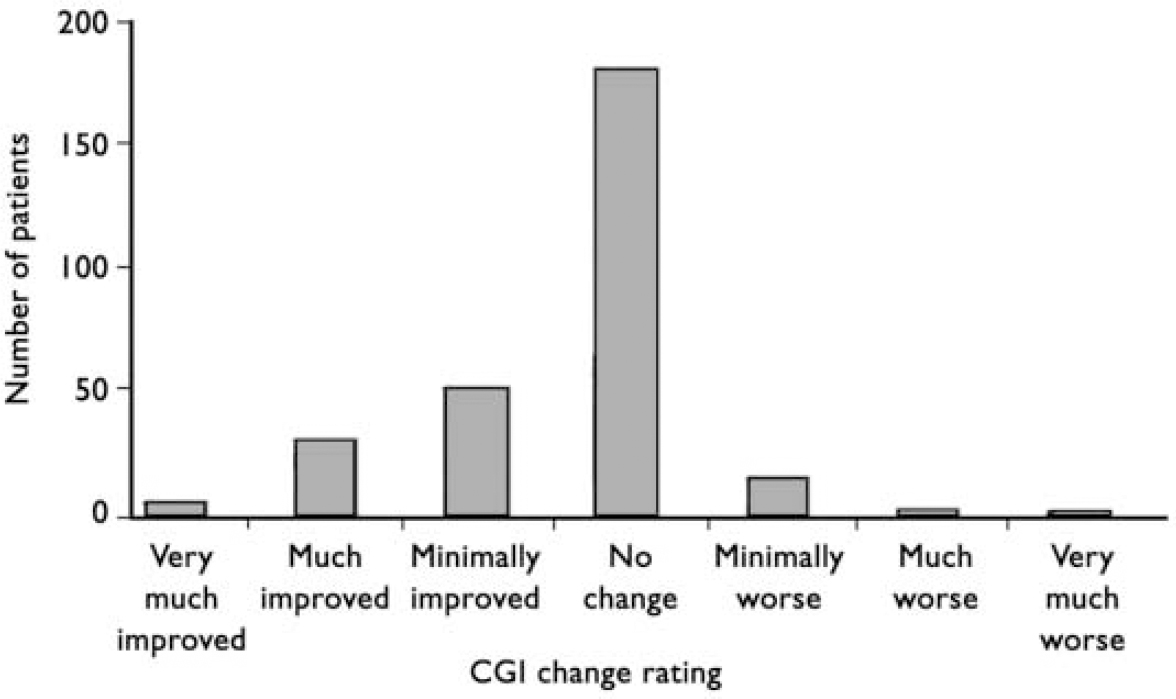
Fig. 1 Clinical Global Impression (CGI) change scores 3 months after switching from Clozaril® to generic clozapine. Overall, 16 patients were rated as minimally worse and 3 as much worse. Of the 3 patients rated to be much worse, 2 were known to be partially or non-compliant and I was chronically physically unwell; 8 of the 16 patients rated as minimally worse had ‘spontaneous explanations’ recorded on their rating form, such as family bereavement, compliance in doubt, acutely physically unwell and lost mental health review tribunal.
DISCUSSION
We found no evidence of dosage escalation or clinical deterioration in patients switched from branded Clozaril® to generic clozapine. This is consistent with the findings of Sajbel et al (2003), Stoner et al (Reference Stoner, Lea and Dubisar2003) and Makela et al (Reference Makela, Cutlip and Stevenson2003), but in contrast to those of Kluznik et al (Reference Kluznik, Walbek and Farnsworth2001) and Mofsen & Balter (Reference Mofsen and Balter2001). Collectively, these studies report on outcomes in a total of 131 patients, less than half the number in our cohort. Individually, they lack the power to detect even large treatment effects. Their different findings can easily be explained by combinations of small sample size, heterogeneous patient groups, the use of different outcome measures and patient selection, sponsorship and publication bias.
Almost 8% of patients discontinued clozapine after switching but before the 3-month follow-up period was complete. Although this attrition rate seems high, it is consistent with the meta-analysis of Wahlbeck et al (Reference Wahlbeck, Cheine and Essali1999); 14.8% of patients in short-term randomised controlled trials and 39% of patients randomised to treatment with clozapine in long-term randomised controlled trials ‘left the study early’.
Patients who were still taking clozapine 3 months after switching to the generic preparation tended to improve. This improvement was highly statistically significant but clinically small. Our results do not constitute proof that the generic preparation is superior to Clozaril®, simply that it is not inferior.
By using a CGIc score of much or very much worse as a proxy for relapse, three patients could be considered to have relapsed. In addition, 16 patients were rated as minimally worse. Wahlbeck et al (Reference Wahlbeck, Cheine and Essali1999) found that 7.5% of patients in long-term studies relapsed. Our findings are consistent with this.
As expected, there was upwards dosage drift in the group of patients who had been treated for <18 weeks at baseline. Such patients are being initiated and stabilised on treatment. There was no dosage drift in those who had been treated for >18 weeks at baseline.
Implications for clinical practice
Large numbers of patients around the world have been switched to generic preparations of clozapine (Reference Ereshefsky and GlazerEreshefsky & Glazer, 2001). The number of publications reporting on outcome is very small. Our study alone triples the number of patients for whom data are available. It may be true that generic preparations are not proven exactly bioequivalent to branded Clozaril® (Reference Lam, Ereshefsky and ToneyLam et al, 2001; Reference Mofsen and BalterMofsen & Balter, 2001) but it is not clear that any differences that do exist are clinically important. The studies of Kluznik et al (Reference Kluznik, Walbek and Farnsworth2001) and Mofsen & Balter (Reference Mofsen and Balter2001) were widely cited by the original patent holders in a campaign aimed at protecting their monopoly. The selective use of studies reporting on the efficacy and safety of drugs makes evidence-based decision-making impossible. The methods used by the pharmaceutical industry must be challenged.
Limitations
The CGIc scores might not detect small changes in psychopathology, thus underestimating the number of patients whose mental state changed after the switch. Patients were followed-up for only 3 months after switching; nothing is known about outcomes beyond this point. Changes in other prescribed medicines or life events that may have affected outcome were not controlled for.
Acknowledgements
Thanks to psychiatrists and keyworkers at Oxleas NHS Trust and to Tracy Isaac for help with data collection.




eLetters
No eLetters have been published for this article.