Terson syndrome has historically been defined as the association of vitreous hemorrhage and spontaneous subarachnoid hemorrhage (SAH). It is named after Albert Terson, a French ophthalmologist who described it in 1900.Reference Gutierrez Diaz, Jimenez Carmena, Ruano Martin, Diaz Lopez and Muñoz Casado 1 , Reference Terson 2 Initially, subarachnoid blood was thought to directly transmit into the eye through the optic nerve. However, the current understanding is that the retinal hemorrhages occur as a result of the sudden increase in intracranial pressure that occurs with an SAH. This pressure is transmitted to the optic nerves because of the communication of the subarachnoid space within the optic nerve sheath. This in turn leads to compression and obstruction of the retinal veins and retinochoroidal anastomoses. The resulting acute rise in intraocular venous pressure causes venous stasis and distension followed by rupture of the fine papillary and retinal capillaries.Reference Ogawa, Kitaoka, Dake and Amemiya 3 This definition has been further supported by recent case reports of iatrogenic Terson syndrome following an acute rise in intracranial pressure after endoscopic third ventriculostomy.Reference Hoving, Rahmani, Los and Renardel de Lavalette 4 , Reference Reddy, Rodriguez, Alsunbul, Ling, Kosick and Reddy 5 Furthermore, recent clinical histopathological studies have confirmed that Terson syndrome does not only lead to vitreous hemorrhage, but can also lead to bleeding into the subhyaloid, retinal, and subretinal spaces.Reference Arroyo and Bula 6 , Reference Ko and Knox 7 As a result, the updated definition of Terson syndrome now reflects hemorrhaging into any of these spaces secondary to acute rise in intracranial pressure.
A systematic and prospective evaluation of the incidence of Terson syndrome has never been performed in Canada. Furthermore, it has been suggested that the presence of Terson syndrome may carry a worse prognosis.Reference McCarron, Alberts and McCarron 8 - Reference Sung, Arnaldo, Sergio, Juliana and Michel 10 The goal of this study is to raise awareness of Terson syndrome by prospectively evaluating the incidence of Terson syndrome in a Canadian tertiary care hospital and to evaluate the use of Terson syndrome as a prognostic tool by comparing the neurological morbidity and mortality of patients with and without Terson syndrome.
Methods
Consecutive patients admitted to the Hamilton General Hospital from May 2012 to May 2013 with a diagnosis of SAH were offered inclusion into the study. Patients with intracranial bleeding other than spontaneous SAH were excluded. Patients with other potential causes of vitreous and/or retinal hemorrhages (e.g., severe diabetic retinopathy, sickle cell retinopathy, central retinal vein occlusion) were also excluded. As soon as medical clearance was obtained, funduscopic examinations were carried out to all included patients with an indirect ophthalmoscope under tropicamide mydriasis.
The primary outcome measure was the presence or absence of Terson syndrome in at least one eye among all patients included in the study. Secondary outcome measures were subsequently compared between patients with Terson syndrome versus those without, including: Glasgow Coma Scale (GCS) and Hunt/Hess (H&H) score upon arrival to the hospital; Fisher score of the SAH; modified Rankin score upon discharge; and all-cause mortality. Baseline characteristics, including, age, sex, preexisting SAH risk factors, and location of any identified ruptured aneurysm(s) were also recorded for all patients.
The incidence of intraocular hemorrhages was calculated by dividing the number of patients with Terson syndrome to the total number of patients in the study. The median and interquartile range and the nonparametric Mann-Whitney U test (rank-sum test) were performed to compare the secondary outcome measures between patients with Terson syndrome versus those without Terson syndrome. A logistic regression was used to evaluate the ability of the GCS and H&H scale to predict the outcome of having Terson syndrome. Odds ratios with confidence intervals were used to compare all-cause mortality in both groups. This study was approved by the Hamilton Health Science Research Ethics Board in Hamilton, Ontario.
Results
Fifty-one patients met the inclusion criteria; however, five patients declined participation, which left 46 patients that were ultimately included in the study. The median age was 61 years (range 34-89 years). Twenty patients were male (43.5%) and 26 were female (56.5%). Terson syndrome was found in 10 patients, giving an incidence of 21.7%. There was an equal proportion of male to female patients among the non-Terson syndrome group; however, females outnumbered males four to one among the Terson syndrome group (Table 1).
Table 1 Characteristics of patients in the Terson syndrome and non-Terson syndrome groups
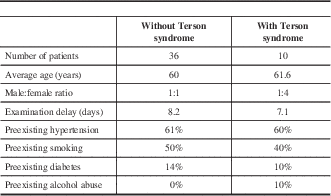
The majority of patients were examined within 14 days of their SAH (89%). Five patients were examined 15–30 days after their SAH because of medical instability (11%) (Table 2). The average examination delay was 7.1 days among the Terson syndrome group and 8.2 days among the non-Terson syndrome group. Preexisting risk factors for SAH (i.e., hypertension, smoking, diabetes, alcohol abuse) were similar between patients in the Terson and non-Terson syndrome groups (Table 1). Figure 1 summarizes the locations of patient’s aneurysms, based on proportions within each group. Anterior communicating artery aneurysms represented the most common location in both the Terson syndrome and non-Terson syndrome groups (50% and 35%, respectively). The second most common location was the posterior communicating artery in the Terson syndrome group and middle cerebral artery in the non-Terson syndrome group. There was, however, an equal number of patients in the non-Terson syndrome group with no definitive aneurysm as those with middle cerebral artery aneurysms (Figure 1).

Figure 1 Summary of patient’s aneurysms based on proportions within the Terson syndrome and non-Terson syndrome groups. ACOM, anterior communicating artery; MCA, middle cerebral artery; ICA, internal carotid artery; PCOM, posterior communicating artery.
Table 2 Distribution of time from patient subarachnoid hemorrhage to funduscopy examination

Table 3 summarizes the secondary outcomes between groups. There was a statistically significant difference in the median GCS between the patients with Terson syndrome (6.5, interquartile range [IQR] 4-11) and patients without Terson syndrome (14, IQR 8-15) upon admission to the hospital (p=0.0052). Logistic regression analysis revealed that for every increase in GCS by 1 point, there was a 0.81 times (therefore decrease) in the chance of Terson syndrome (95% confidence interval [CI] 0.69-0.96, p=0.013). There was also a statistically significant difference in the H&H scores in patients with Terson syndrome (4, IQR 3-5) compared with patients without Terson syndrome (2, IQR 1-3) upon admission to the hospital (p=0.0032). Logistic regression analysis revealed that for every increase in H&H by 1 point, there was a 2.84 times increase in the chance of Terson syndrome (95% CI 1.34-6.05, p=0.007).
Table 3 Summary of secondary outcomes between groups

* =statistical significance;
GCS=Glasgow Coma Scale; H&H=Hunt and Hess scale; IQR=interquartile range.
There was no significant difference found in the size of the SAH based on the median Fisher score between patients with Terson syndrome (4, IQR 4-4) and patients without Terson syndrome (3, IQR 3-4) (p=0.013). The modified Rankin score upon discharge from the hospital was statistically different in patients with Terson syndrome (6, IQR 4-6) versus those without Terson syndrome (3.5, IQR 2-4.5; p=0.0019). The odds of all-cause mortality with Terson syndrome versus no Terson syndrome was 12:1 (95% CI 2.33-61.7), p=0.003. Only four of the 10 patients with Terson syndrome survived.
Discussion
In 1900, Terson originally reported the incidence of Terson syndrome as being 2.5%.Reference Terson 2 This is understandable given the limitations in equipment available at that time as well as the fact that he defined the condition as being exclusive to those with vitreous hemorrhage. The incidence found in this study was 21%, which parallels more recent studies that used the updated definition of Terson syndrome that includes subhyaloid, retinal, and subretinal hemorrhages. Stienen et al. (2012) found an incidence of 18.3% among patients examined in Europe.Reference Stienen, Lücke, Gautschi and Harders 9 Sung et al. (2011), reported an incidence 29% in South American patients.Reference Sung, Arnaldo, Sergio, Juliana and Michel 10 He at al. (2011) reported 14.8% in China.Reference He, Wu, Chen and Xing 11 Frizell et al. (1997) examined patients in the United States using the older definition of Terson syndrome and reported an incidence of 8%.Reference Frizzell, Kuhn, Morris, Quinn and Fisher 12 This also parallels the present study in that three of the 10 Terson syndrome patients had bleeding into the vitreous, which represents an incidence of 6.5%. Frizell et al. (1997) also reported the incidence of other intraocular hemorrhages as being 17%.Reference Frizzell, Kuhn, Morris, Quinn and Fisher 12 Thus the present study is consistent with others performed in other parts of the world.
Given that not all subjects agreed to participate in the study, the calculated incidence may be inaccurate if all those that declined belonged to one group. Hypothetically, if all the patients that declined participation did not have Terson syndrome, the overall incidence of Terson syndrome in this study would have been 19.6%. Conversely, the incidence reported in this study maybe underestimating the true incidence in the community given that between 3% and 21% of patients with SAH expire before reaching medical attention.Reference Huang and van Gelder 13 These patients likely would have had much lower GCS and H&H scores and thus a higher likelihood of having Terson syndrome.
Table 1 summarizes the patient characteristics. The average age was 60 years in both groups. Risk factors for development of SAH, such as hypertension, smoking, and diabetes were also similar in both groups. It is important to note that all diabetic patients that were included did not have a preexisting diagnosis of proliferative diabetic retinopathy before developing their SAH, and thus were allowed inclusion into the study. One of these patients was found to have retinal hemorrhages, in keeping with Terson syndrome. It is possible that this patient may have developed an undiagnosed proliferative disease before suffering her SAH; however, we did not appreciate any features of neovascularization that would suggest that her retinal hemorrhages were related to her diabetes.
Interestingly, there were four times as many female patients compared with male patients with Terson syndrome. There was no gender predilection in patients without Terson syndrome. Previous studies have shown that female gender is a risk factor for aneurysmal SAH.Reference Korja, Silventoinen and Laatikainen 14 - Reference Park, Kim, Kim, Cheong, Bak and Kim 16 Women tend to be older and have a greater likelihood of having multiple aneurysms.Reference Ghods, Lopes and Chen 17 The reasons for these differences is not fully known; however, it has been previously postulated there is an intrinsic difference in the weakness of vessel walls between men and women, and that collagen and elastin interference factors as well as hormonal changes may influence aneurysm formation in women.Reference Park, Kim, Kim, Cheong, Bak and Kim 16 How this would translate into an increased incidence of Terson syndrome is not clear.
This study supports the notion that a diagnosis of Terson syndrome does carry a worse prognosis. The modified Rankin score, which is measure of neurologic morbidly, was significantly higher in patients with Terson syndrome. This is consistent with previous studies, which looked at other neurologic outcome scores, such as the Glasgow Outcome score.Reference Stienen, Lücke, Gautschi and Harders 9 Perhaps the most telling statistic in regard to prognosis was the odds of death being 12 times more in patients with Terson syndrome compared with patients without Terson syndrome. Of the 10 patients with Terson syndrome, only four survived. Given our current understanding of the pathophysiology of Terson syndrome, it makes sense that patients with this condition may have a worse neurologic status. An accelerated increase in the intracranial pressure from an SAH would likely increase the chances of developing intraocular hemorrhages. Such a rapid increase may also lead to an increased risk of cerebral damage. Given that the Fisher scores were not significantly different in patients with and without Terson syndrome, this suggests that the rate of the rise in intracranial pressure is perhaps more important than the actual quantity of blood that is released within the intracranial space. However, this question needs to be examined further in a larger study.
For the presence of Terson syndrome to be used as a prognostic indicator, it is important that the diagnosis be made. There are, however, a number of hurdles in diagnosing Terson syndrome. First of all, patients are often unconscious during the acute phase of their hemorrhage and thus are not able to vocalize any change in their vision. Second, fundus examinations are not routinely performed in intensive care units to look for signs of intraocular hemorrhage.Reference Ashrafi, Chakrabarti and Laidlaw 18 , Reference Middleton, Esselman and Lim 19 Third, the skills and/or motivation required to perform funduscopy to identify intraocular hemorrhages are not always sufficient among nonophthalmologist.Reference Morad, Kim, Mian, Huyer, Capra and Levin 20 Last, there does appear to be a lack of awareness of the existence of Terson syndrome within the medical community. Obtaining an ophthalmologic assessment for all patients who enter the hospital with a diagnosis of SAH would conceivably ameliorate many of these issues; however, this would take a great deal of resources that many ophthalmology services are not able to provide. A better approach would be to develop guidelines for intensive care unit staff to obtain an ophthalmology consult based on the patient’s neurologic status.
Logistic regression analyses were performed on the GCS and H&H scores of patients obtained upon admission to the hospital. This analysis revealed that for every increase in GCS score by 1 point, there was a corresponding 0.8 times decrease in the risk of having Terson syndrome. Furthermore, for every increase in H&H by 1 point, there is a 2.8 times increase in the chance of Terson syndrome. If a GCS score ≤8 or a H&H score ≥3 was used to trigger an ophthalmology consult, then 27 of the 46 patients would have been screened and nine of the 10 Terson syndrome patients would have been discovered. This represents a sensitivity of 90% (95% CI 0.55-0.98) and specificity of 50% (95% CI 0.33-0.67), with a negative predictive value of 94.7% (95% CI 0.74-0.99). Using these guidelines would decrease the demands on ophthalmology services, while still capturing the majority of patients with Terson syndrome.
With regard to the limitations of this study, first, patients were recruited from a tertiary trauma center, which serves patients with a more severe spectrum of disease, and thus the results of this study may not be generalizable to all medical settings. Second, there was variability in the timing of the ophthalmologic assessments of these patients. Given that it takes at least 2 weeks for retinal hemorrhages to clear, the goal of the study was to examine patients within 2 weeks of their SAH. Most patients were evaluated within that time frame; however, permission to dilate the eyes of five patients could not be obtained in time because of their unstable medical courses. One of those five patients was found to have Terson syndrome; however, it is possible that a case of Terson syndrome may have been missed within the remaining four patients because the retinal hemorrhages could have cleared before an examination was performed. Last, this study was not able to demonstrate any major differences in patient characteristics between patients with Terson syndrome versus those without Terson syndrome except for a female preponderance among patients with Terson syndrome. It is possible that other differences between these groups may exist, but were not uncovered from a lack of power in the study to show a difference.
In conclusion, the results of this study suggest that approximately one-fifth of all patients admitted to a tertiary trauma hospital with a spontaneous SAH could have Terson syndrome. Patients with Terson syndrome have statistically significantly worse GCS and H&H scores upon admission to the hospital, lower modified Rankin scores upon discharge, and greater mortality. Thus, Terson syndrome is not rare among patients with spontaneous SAH and carries a worse prognosis. Based on these data, more awareness of this condition should be disseminated within the medical community, not only so that appropriate ophthalmic care can be provided for patients with Terson syndrome but also to allow for greater awareness of the neurological prognosis of patients with SAH.
Acknowledgments
The authors thank Charmaine Martin for assisting with patient recruitment and recording the modified Rankin scores of all the patients and Dr. Maureen Meade for promoting this study within the intensive care unit at the Hamilton General Hospital.






