In contrast to schizophrenia, little work has been done on the neurohistology of mood disorders, indicating that this kind of research strategy is still in its infancy, and the results obtained from these studies provide only a fragmentary pattern. Most of the studies include subjects who had committed suicide, and a retrospective diagnosis of a mood disorder was not established in all cases. Some investigations did not focus on affective disorders, but used subjects with mood disorders as a control group. Since this article emphasises regional neurohistology, biochemical studies performed on homogenate of brain tissue are not considered in the following survey.
POST-MORTEM STUDIES
Studies on brain structure
One early post-mortem study found a greater brain weight, a thicker parahippocampal cortex and smaller temporal horns in patients with affective disorder compared with those with schizophrenia (Reference Brown, Colter and CorsellisBrown et al, 1986), suggesting that temporal lobe structure differs in these disorders. Unfortunately, no control group without neuropsychiatric disorder was included in this study. Therefore, it is difficult to compare these data with magnetic resonance imaging (MRI) studies that report smaller entorhinal cortex sizes in schizophrenia, but not in bipolar disorder (Reference Pearlson, Barta and PowersPearlson et al, 1997). It is conceivable, however, that a structural disturbance of the parahippocampal cortex is present in mood disorders, since one post-mortem study showed a reduction in size of this cortex in suicide victims compared with non-psychiatric controls (Reference Altshuler, Casanova and GoldbergAltshuler et al, 1990). Further evidence for a disturbed morphological integrity of this region comes from cyto-architectural studies reporting that malformations in the entorhinal lamination were observed in patients with bipolar disorder or with major depression (Reference Beckmann and JakobBeckmann & Jakob, 1991; Reference Bernstein, Krell and BaumannBernstein et al, 1998). In addition, one case report described a temporo-occipital cortical dysplasia associated with rapid-cycling bipolar disorder and learning disability (Reference Raghavan, Day and PerryRaghavan et al, 1995). These studies suggest that both bipolar and major depressive mood disorder might be associated with circumscribed neurodevelopmental disturbances in temporal regions.
Another alteration in the temporal lobe was reported by Benes and co-workers who showed a selective reduction of non-pyramidal neurons in the CA2 region on the hippocampus of patients with bipolar disorder and schizophrenia (Reference Benes, Kwok and VincentBenes et al, 1998). These studies confirm and extend recent MRI findings suggesting a temporal pathology not only in schizophrenia but also in bipolar disorder (Reference Altshuler, Bartzokis and GriederAltshuler et al, 1998; Reference Roy, Zipursky and Saint-CyrRoy et al, 1998; Reference Strakowski, DelBello and SaxStrakowski et al, 1999).
The prefrontal lobe is another focus of interest in mood disorders (Reference GoodwinGoodwin, 1997). Decreased cortical and laminar thickness but unchanged overall neuronal density and laminar density were demonstrated in the dorsolateral prefrontal cortex in both bipolar disorder and major depressive disorder. In contrast to patients with schizophrenia, who also showed smaller cortical thickness but an increased neuronal density in this area, patients with mood disorders appear to have unchanged neuronal densities in this part of the prefrontal cortex (Reference RajkowskaRajkowska, 1997). Also relevant to mood disorders, theoretically speaking, are the medial prefrontal cortex and the anterior cingulate cortex. Drevets and colleagues found a volume reduction and fewer glial (but no neuronal) cells in the subgenual prefrontal cortex — which is part of the anterior cingulate cortex — in familial mood disorders (Reference Drevets, Ongur and PriceDrevets et al, 1998). No differences were described between bipolar disorder and unipolar depression. In another post-mortem study a layer-specific reduction of interneurons in the anterior cingulate cortex was observed in subjects with bipolar disorder (Reference Vincent, Todtenkopf and BenesVincent et al, 1997). This is consistent with the finding of a deficit in a neuronal subpopulation detected by immunoreactivity for the calcium-binding protein calretinin (Reference Diekmann, Baumann and SchmidtDiekmann et al, 1998). These results confirm and extend previous neuroimaging work revealing structural and functional abnormalities of certain parts of the prefrontal cortex in mood disorders (Reference GoodwinGoodwin, 1997).
Studies on monaminergic systems
Some neurohistological studies investigated the role of monaminergic systems in mood disorders. The serotonergic system was analysed in one study reporting increased binding of 5-HT1A autoreceptors in the midbrain of subjects with major depression who had committed suicide, suggesting reduced activity of dorsal raphe neurons (Reference Stockmeier, Shapiro and DilleyStockmeier et al, 1998). Another investigation on presynaptic markers showed unchanged serotonin transporter binding and mRNA levels in the midbrain, hippocampus or frontal cortex in cases of suicide following major depression (Reference Little, McLaughlin and RancLittle et al, 1997). Stockmeier et al (Reference Stockmeier, Dilley and Shapiro1997) found that the 5-HT1A and the 5-HT2A receptors were unchanged in the right frontopolar cortex. Thus some, but not all, post-mortem studies support the indolaminergic hypothesis of depression. This is in line with an imaging study indicating decreased density of serotonin transporter binding sites in the midbrain of patients with major depression (Reference Malison, Price and BermanMalison et al, 1998). These data on living patients contrast with the negative post-mortem findings in suicide victims with major depression (Reference Little, McLaughlin and RancLittle et al, 1997). Taken together, some post-mortem and neuroimaging studies reveal alterations in serotonergic neurons in major depression. It remains unclear whether these results, if replicable, can be generalised for bipolar disorder.
As yet, no investigation has focused on the cyto-architecture of monaminergic neuron systems, such as the raphe nuclei or the locus caeruleus, in mood disorders. An incidental note on a geriatric case of depression mentioned a considerable lack of locus caeruleus neurons (Reference Chan-Palay and AsanChan-Palay & Asan, 1989), which is in agreement with a study showing fewer neurons in this crucial noradrenergic nucleus in suicide victims (Reference Arango, Underwood and MannArango et al, 1996). This needs further confirmation by studies on larger patient samples including unipolar and bipolar affective disorder. A notable study on the noradrenergic system was performed by Klimek and co-workers who demonstrated decreased levels of the noradrenalin transporter in patients with major depressive illness (Reference Klimek, Stockmeier and OverholserKlimek et al, 1997). Another post-mortem analysis showed increased density of the α2A-adrenoreceptor in the frontal cortex of suicide victims who had depression (Reference Callado, Meana and GrijalbaCallado et al, 1998). These two results point to a presynaptic noradrenergic deficit in this disorder consistent with the noradrenergic hypothesis of depression (Reference SchildkrautSchidkraut et al, 1965). Neurohistological studies on the noradrenergic system in bipolar disorder are still required.
Despite indirect evidence that dopaminergic systems are involved in the pathogenesis of mood disorders, only one study of this transmitter system has been performed, reporting increased binding of the D1 receptor in the nucleus accumbens of suicide victims with major depression (Reference Bowden, Theodorou and CheethamBowden et al, 1997). The nucleus accumbens shows intense connectivity with the prefrontal cortex, mainly with its medial part. Since structural changes in telencephalic basal ganglia as well as in the prefrontal cortex have been shown in patients suffering from major depression, the chemo-architecture of these closely interconnected brain regions would be of interest in mood disorders.
Neuroendocrinological studies
Some histological investigations have addressed the central endocrine cell groups in the hypothalamus of patients with mood disorders. Expression and messenger mRNA transcripts of corticotrophin-releasing hormone have been shown to be upregulated in neurons of the hypothalamic paraventricular nucleus (Reference Raadsheer, van Heerikhuize and LucassenRaadsheer et al, 1995). Moreover, data from the same laboratory found an upregulation of vasopressin- and oxytocin-expressing neurons in the same nucleus (Reference Purba, Hoogendijk and HofmanPurba et al, 1996). These findings are consistent with clinical data showing a central overactivation of the hypothalamic—pituitary—adrenal axis in depressive disorders (Reference Arborelius, Owens and PlotskyArborelius et al, 1999). No differences were seen between major depression and bipolar disorder in these post-mortem studies. The investigated samples, however, were too small to allow general conclusions.
VOLUME MEASURES IN MOOD DISORDERS
In addition to neurohistological studies, structural imaging data provide evidence for the assumption that mood disorders are not exclusively functional illnesses but also show a diversity of morphological changes (Reference Soares and MannSoares & Mann, 1997; Reference Drevets, Ongur and PriceDrevets et al, 1998; Reference Parashos, Tupler and BlitchingtonParashos et al, 1998). Despite modern computed tomography and MRI techniques, resolution is still too poor to detect subtle structural alterations of small brain nuclei such as the hypothalamus or some parts of the basal ganglia. A post-mortem study was therefore begun to investigate volumes of all telencephalic basal ganglia, of the diencephalon, and of limbic regions, to obtain data on relevant brain circuits in mood disorders using material from our new Magdeburg brain collection. Possible post-mortem artefacts arising from autolysis or tissue processing as well as effects of age and gender were excluded by careful matching of the case. Effects of medication were also controlled for.
The brains of eight patients suffering from major depression or bipolar disorder showed reduced volumes of the left nucleus accumbens, the right putamen and the right and left pallidum externum (Fig 1 & 2). No differences were seen between bipolar or unipolar depression. The nucleus accumbens was most affected. These data suggest that predominantly limbic-affiliated basal ganglia are involved in the pathology of mood disorders, irrespective of diagnostic polarity (Reference Baumann, Danos and KrellBaumann et al, 1999a ).
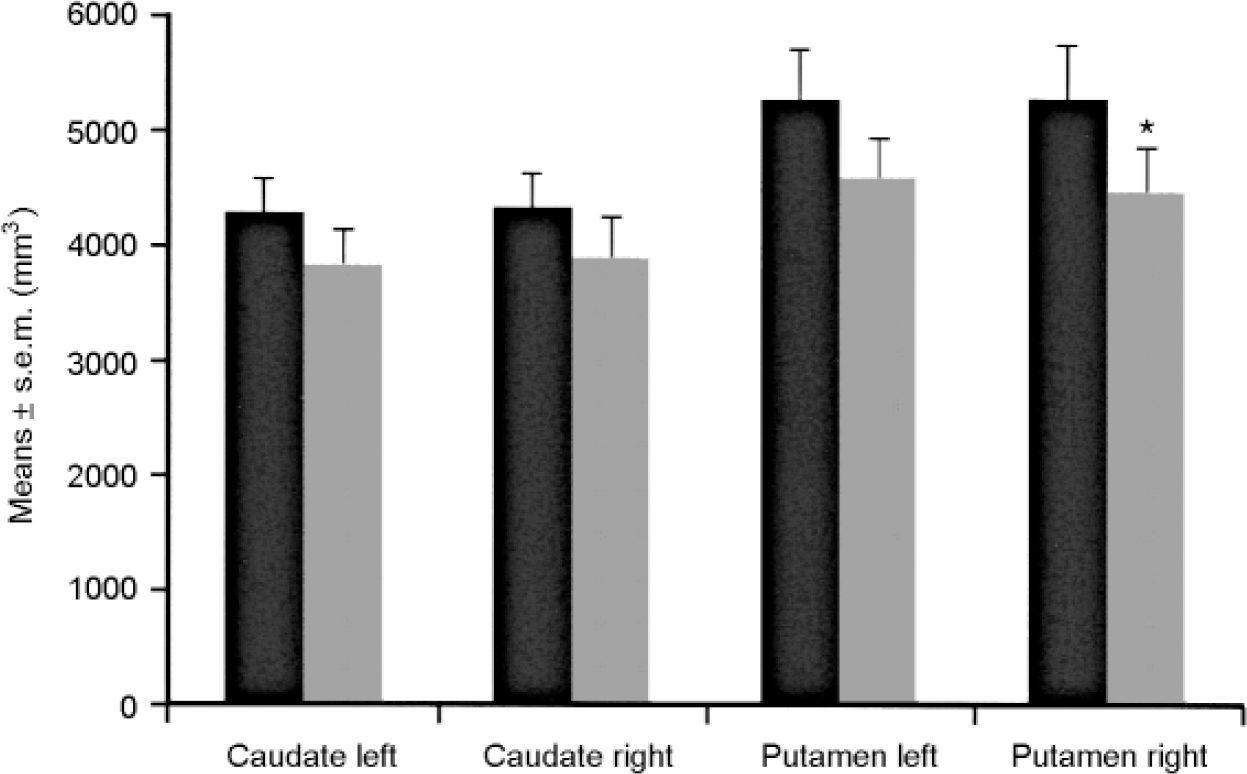
Fig. 1 Brain structure volumes of patients with affective disorder (n=8, shaded bars) and controls (n=8, black bars) — caudate nucleus and putamen. The volume of the right putamen is reduced in patients. * P ≤ 0.05. Reproduced with permission from Baumann et al (Reference Baumann, Danos and Krell1999a ).
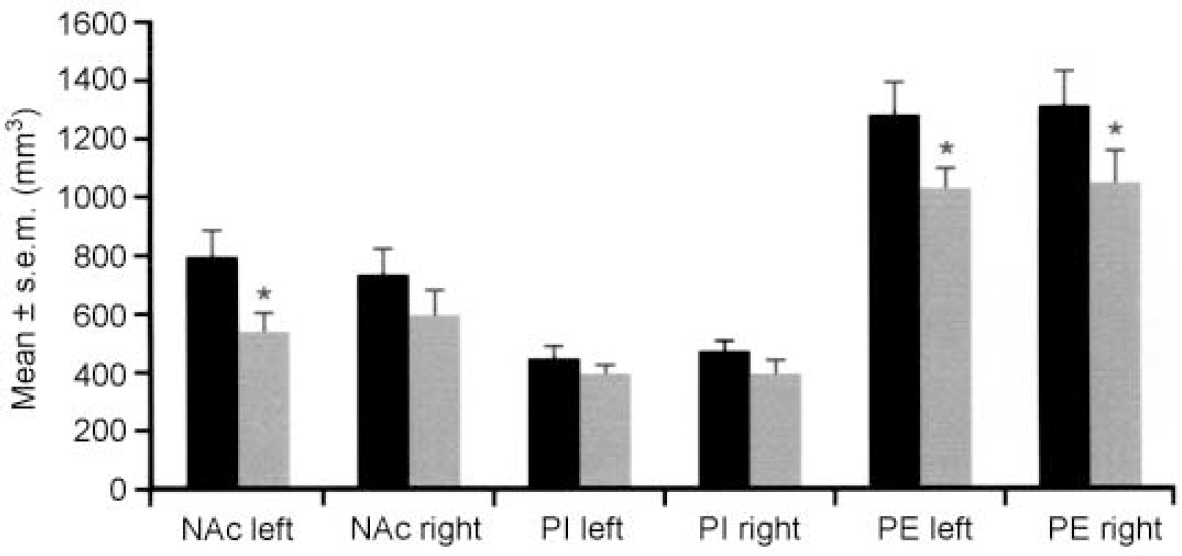
Fig. 2 Brain structure volumes of patients with affective disorder (n=8, shaded bars) and controls (n=8, black bars) — nucleus accumbens (NAc), pallidum externum (PE) and pallidum internum (PI). Reduced volumes of the left NAc and both pallida external are seen in patients. * P ≤ 0.05. Reproduced with permission from Baumann et al (Reference Baumann, Danos and Krell1999a ).
The brains of patients with major depressive illness showed a trend towards volume reduction for the left amygdala that was not seen in the bipolar disorder patients. Taking into account MRI findings of enlargement of the amygdala in bipolar disorder (Reference Altshuler, Bartzokis and GriederAltshuler et al, 1998) and reduced volumes of amygdala core nuclei (Reference Sheline, Gado and PriceSheline et al, 1998), these data suggest structural differences in the amygdala between major depression and bipolar disorder. The volumes of other limbic regions such as the hippocampus, the temporal horn, the stria terminalis and the basic limbic fore-brain were unchanged in our sample.
Volumes of the thalamus and the hypothalamus also showed no differences between the whole patient sample group compared with controls. In major depression, however, the right hypothalamus was smaller than in controls (P=0.02) and the left hypothalamus showed a non-significant trend with 13% smaller volumes (P=0.07).
These post-mortem volume studies confirm and extend previous neuroanatomical models of mood regulation, suggesting a crucial role for ventral parts of telencephalic-basal ganglia and the involvement of the amygdala and hypothalamus in these circuits (for review, see Reference Soares and MannSoares & Mann, 1997). The preliminary results also show structural differences between major depressive and bipolar disorder for the amygdala.
BRAIN-STEM STUDIES ON MOOD DISORDERS
Monaminergic (in particular serotonergic and noradrenergic) systems are assumed to be essentially involved in the pathology of mood disorders. However, to date there is no information on the possible pathomorphology of the brain-stem nuclei that produce these transmitters. A neurohistological investigation of the dorsal raphe and the locus caeruleus — the main sources of serotonergic and noradrenergic forebrain innervation — was therefore performed.
Locus caeruleus
Twelve post-mortem brains of patients with mood disorders as well as 12 brains of non-psychiatric control subjects were included in the study. Six patients had major depressive disorder, the other six bipolar disorder. Based on the topographic organisation of projections from the locus caeruleus (LC), we performed neuron counts separated for the dorsal, medial and ventral portions, as well as the rostral, medial and caudal portions of this nucleus (Reference Baumann, Danos and KrellBaumann et al, 1999b ). Surprisingly, total neuron numbers in the whole nucleus were higher in bipolar than unipolar disorder, with this phenomenon being observed on both sides of the LC (Fig. 3). The finding of an enlarged LC in bipolar disorder is consistent with a ‘hypernormal’ pattern of bipolar disorder morphology comprising larger thalamic volumes (Reference Dupont, Jernigan and HeindelDupont et al, 1995), greater grey/white ratios (Reference Strakowski, Wilson and TohenStrakowski et al, 1993), increased gyral complexity (Reference Bullmore, Brammer and HarveyBullmore et al, 1994) and enlarged amygdala (Reference Altshuler, Bartzokis and GriederAltshuler et al, 1998; Reference Strakowski, DelBello and SaxStrakowski et al, 1999).
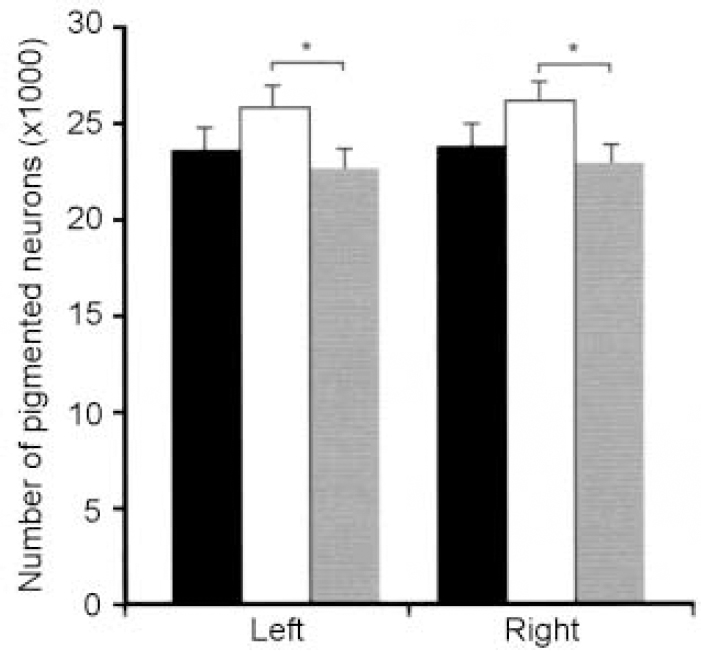
Fig. 3 Pigmented neurons in the locus caeruleus of patients with bipolar disorder (n=6, white bars), major depressive disorder (n=6, shaded bars) and controls (black bars). Reduced neuron numbers are seen in patients. * P ≤ 0.05. Reproduced with permission from Baumann et al (Reference Baumann, Danos and Krell1999b ).
Topographical analysis revealed that the structural difference is restricted to the rostral two-thirds and the dorsal part of the LC, in which patients with bipolar disorder showed at least a trend to higher neuron numbers compared with patients with major depression or controls. With regard to the topography of LC projections (Reference Mason and FibigerMason & Fibiger, 1979; Loughlin et al, Reference Loughlin, Foote and Fallon1982, Reference Loughlin, Foote and Bloom1986), it is conceivable that cortical, hippocampal and hypothalamic projections from the LC are part of a morphological substrate for the unipolar—bipolar dichotomy of mood disorders. Together with measurements of noradrenalin or its metabolites in mood disorders (Reference Roy, Pickar and LinnoilaRoy et al, 1985; Reference Azorin, Pupeschi and ValliAzorin et al, 1990), our data indicate that the span between minimum and maximum caerulean noradrenergic transmission in certain brain areas is greater in bipolar disorder than in major depressive disorder. Since noradrenergic tone correlates primarily with clinical symptoms of drive (Reference Carr, Edwards and PriorCarr et al, 1988; Reference Katz, Maas and FrazerKatz et al, 1994; Reference Swann, Stokes and SecundaSwann et al, 1994), this wide range of transmission could provide a predisposition for bipolar psychomotor symptoms.
In order to prove noradrenergic function of the LC we performed immunohistochemical analysis of tyrosine hydroxylase, the key enzyme in noradrenalin synthesis (Reference Baumann, Danos and DiekmannBaumann et al, 1999c ). The number of tyrosine hydroxylase-immuno-reactive (TH-ir) neurons did not differ between patients with bipolar and unipolar disorder, nor between patients and controls. Further data analysis indicated that suicide might be associated with caerulean noradrenergic function. The number of TH-ir neurons in patients with unipolar or bipolar disorder who died from natural causes was lower than in suicide victims and controls (Fig. 4). This difference remained significant after covarian analysis for mean doses of recent medication with tricyclic or tetracyclic antidepressants. Unipolar and bipolar diagnoses were equally disturbed in the suicide and non-suicide group. Numbers of TH-ir neurons correlated positively with mean doses of tricyclic or tetracyclic antidepressants. These results suggest a presynaptic noradrenergic deficit of the LC in non-suicidal patients with depression irrespective of unipolar or bipolar diagnosis; they also provide indirect evidence that suicidal behaviour may be related to normalised noradrenergic function, and that traditional antidepressants may enhance noradrenergic activity of the LC in patients with depression.
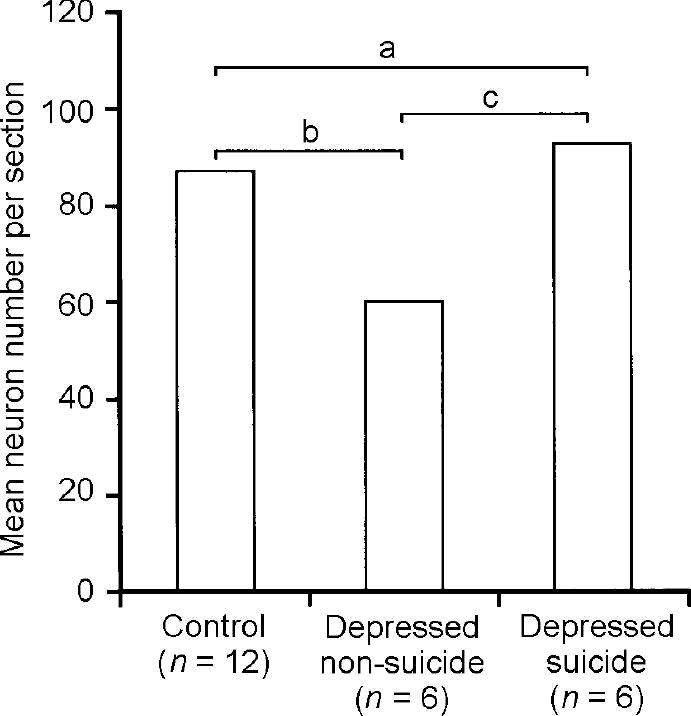
Fig. 4 Tyrosine hydroxylase-immunoreactive neurons in the locus caeruleus. There are fewer immunoreactive neurons in the depressed non-suicide group compared with the depressed suicide group and controls; a, significant group effect (F=4.63, d.f.=2, 21, P=0.02); b, significant post-hoc test; c, significant post-hoc test. Reproduced with permission from Baumann et al (Reference Baumann, Danos and Diekmann1999c ).
Dorsal raphe
The dorsal raphe (DR) exhibits a topographic organisation similar to that of the LC. For example, neuronal projections to the prefrontal cortex and combined projections to the nucleus accumbens and the prefrontal cortex arise from more ventral and rostral subnuclei of the DR (Reference O'Hearn and MolliverO'Hearn & Molliver, 1984; Reference Wilson and MolliverWilson & Molliver, 1991; Reference Kazakov, Kravtsov and KrakhotkinaKazakov et al, 1993; Reference Van Bockstaele, Biswas and PickelVan Bockstaele et al, 1993). Both these projection targets have been implicated in the pathology of depression. Together with the median raphe, the DR provides the ascending serotonergic raphe projections to the fore-brain. From these topographic aspects of the raphe system it has been assumed that the DR has a major role in mood regulation (Reference MolliverMolliver, 1987). The cyto-architecture of this complex nucleus was investigated in post-mortem brains of 12 patients with mood disorders and 12 subjects without any neuropsychiatric disorder. The ventral parts of the mesencephalic DR (DRvp), i.e. the interfascicular, ventral and ventrolateral subnuclei, showed fewer neurons in patients with a mood disorder (Fig. 5). No differences were seen between major depression and bipolar disorder in this respect. Morphological analysis revealed that the reduction in the number of neurons in patients with a mood disorder was restricted to the ovoid and round neurons.
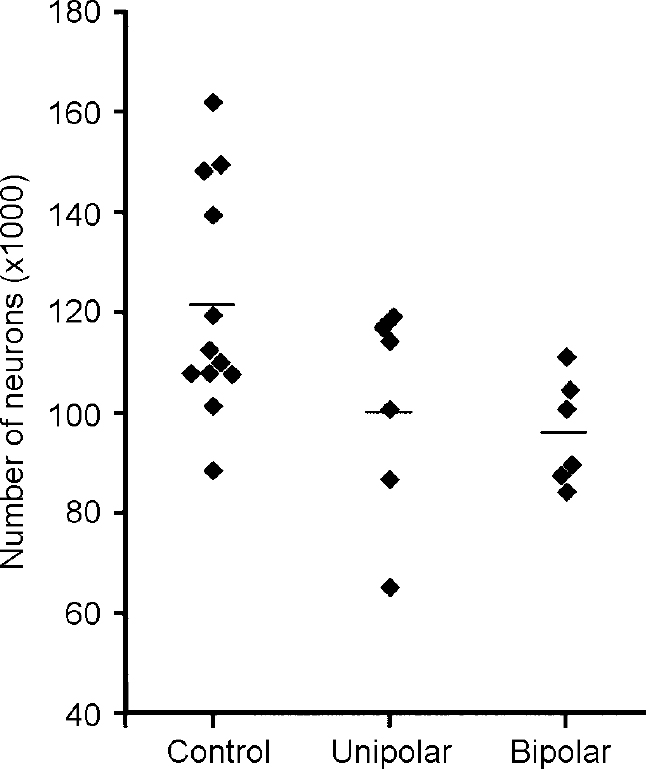
Fig. 5 Neuron numbers in ventral subnuclei of the dorsal raphe. Reduced numbers are seen in patients with major depressive disorder (unipolar) and bipolar disorder. The line indicates the mean value for each group.
Since approximately 70% of the human DR neurons are serotonergic, the lack of neurons in the DRvp is mostly consistent with a regional deficit of serotonergic neurons in mood disorders. Neuronal nucleoli appeared enlarged in the DRvp of patients with mood disorders, suggesting neuronal activation in this area. While antidepressant medication showed no correlation with neuron numbers in any part of the DR, a positive correlation of mean dosage of anti-depressant agents with nucleolar size was found for the right ventrolateral subnucleus, which is part of the DRvp.
These results show that patients with a primary mood disorder have a numeric neuronal deficit in the DRvp, irrespective of depressive illness subtype. This structural change may contribute to impaired serotonergic innervation of brain regions that are involved in the pathology of mood disorder. Increased nucleolar size indicating neuronal activation in the DR may reflect an adaptive process partly induced by anti-depressant medication.
In order to gain a closer insight into serotonergic function of the DR, tryptophan hydroxylase was investigated by immunodetection in the same sample. Numbers of tryptophan hydroxylase-immunoreactive (TPH-ir) neurons did not differ between patients and controls, either in the DRvp or in the dorsal or caudal parts of the DR. In the group of patients, however, there was a significant correlation of TPH-ir neurons in the DRvp with mean dosage of antidepressants given in the last 7 days before death (r=0.69, n=12, P=0.01). On the level of single subnuclei, this association was most prominent in the right ventrolateral nucleus. Using antidepressant medication as covariate (ANCOVA), a nearly significant reduction was found for TPH-ir neurons in the DRvp, but not in the dorsal or caudal DR of patients. No difference was observed between major depressive and bipolar disorder samples.
Unchanged serotonin immunohistochemistry and a reduction in Nissl-stained neurons indicate that a neuronal deficit in the DRvp in patients with mood disorders treated by antidepressants is partly compensated for by upregulation of serotonin synthesis as an effect of antidepressant medication. Moreover, our data suggest that in mood disorders, irrespective of diagnostic polarity and apart from medication, synthesis of serotonin may be state-dependently reduced in distinct regions of the DR.
CONCLUSION
There are few post-mortem studies on bipolar disorder, and definite conclusions can hardly be drawn owing to the small and selective nature of the samples, which in particular might produce false-negative results. Moreover, problems sometimes arise from effects of medication or agonal and post-mortem changes. Data obtained from neuroimaging in mood disorders showing structural abnormalities in the frontal and temporal cortices as well as in subcortical regions are widely confirmed, and also extended and specified, by results from neurohistological investigations. Basal ganglia, preferentially those closely associated with the limbic system, have smaller volumes in patients with depressive illness irrespective of diagnostic polarity. Higher neuron numbers in the locus caeruleus of patients with bipolar disorder than in that of patients with major depressive disorder are in agreement with neuroimaging data indicating a ‘hypernormal’ structural pattern in bipolar disorder that does not appear to exist in major depression. Post-mortem studies showed no differences between the two types of disorder for noradrenalin and serotonin synthesis in the locus caeruleus and the dorsal raphe. Our data suggest a regionally reduced synthesis of these neurotransmitters, which have a major role in the pathogenesis of mood disorders. Antidepressant medication seems to increase serotonin and noradrenalin production in depressive states and may thereby contribute to normalisation of monaminergic functions.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Morphological changes in basal ganglia and monaminergic transmitter systems may be vulnerability markers for unipolar disorder.
-
▪ Brain biology differs between bipolar and unipolar disorders, suggesting the need for different treatment strategies.
-
▪ Effects of antidepressants may be mediated by changes in monaminergic brainstem neurons.
LIMITATIONS
-
▪ Neurohistological research in bipolar disorder is in its infancy and as yet provides only a preliminary assessment of morphological changes involved with this disease.
-
▪ Relatively small sample sizes and the high proportion of suicide cases in post-mortem studies on mood disorders make it difficult to generalise findings.
-
▪ It is difficult to correlate morphological findings with acute symptoms.








eLetters
No eLetters have been published for this article.