The metabolic syndrome (MetS) is considered to be a clustering of metabolic alterations conferring a high risk of developing type 2 diabetes and CVD, and a higher risk of all-cause mortality. The prevalence of the MetS differs according to the criteria used to define it. In developed countries, the prevalence of the MetS reaches about 25 % of the population(Reference Ford, Giles and Mokdad1–Reference Delavari, Forouzanfar and Alikhani7), and its incidence has been increasing over the last years.
However, there is a heterogeneous distribution of the MetS across different countries and races studied(Reference Alkerwi, Donneau and Sauvageot5–Reference Delavari, Forouzanfar and Alikhani7). In Spain, the prevalence of the MetS has apparently increased in the past years from 10·2 % in 2005(Reference Alegria, Cordero and Laclaustra8) to 31 % in 2012(Reference Fernández-Bergés, Cabrera de León and Sanz9). These differences in prevalence cannot be explained by population genetics alone. Environmental influences may play an important role.
Low physical activity, smoking, low social class and low educational level have been associated with an increased risk of the MetS(Reference Alegria, Cordero and Laclaustra8, Reference Brouwer, Visseren and van der Graaf10). Food habits may also be one of the most important factors determining the MetS. Although solid food is an important source of nutrients in the diet, drinks can be a major source of carbohydrates as well. Some of them contain sugar, sweeteners, alcohol or caffeine(Reference Lustig, Schmidt and Brindis11) with the potential to increase the risk of the MetS.
Several reports have indicated an increasing consumption of soft drinks among children, adolescents and adults over the past three decades(Reference Popkin12, Reference Barquera, Hernandez-Barrera and Tolentino13). Even though, subsequently, in recent years, a mild decrease in sugar-sweetened beverages (SSB) has been described(Reference Welsh, Sharma and Grellinger14). Many clinical studies have linked the rising consumption of soft drinks to an increase in obesity and diabetes mellitus among children and adolescents(Reference Malik, Popkin and Bray15–Reference Malik, Popkin and Bray18), and to the development of hypertension(Reference Winkelmayer, Stampfer and Willett19) and CVD(Reference de Koning, Malik and Kellogg20) in adults. There is no consensus in the literature on the association between soft drink consumption and TAG levels(Reference Parks and Hellerstein21–Reference Jurgens, Haass and Castaneda25); however, some studies have found a negative association between soft drink consumption and serum HDL-cholesterol(Reference de Koning, Malik and Kellogg20, Reference Dhingra, Sullivan and Jacques26, Reference Høstmark and Tomten27).
The Oslo Health Study found that soft drink consumption was positively associated with the prevalence of the MetS, and this cross-sectional association was independent of the presence or absence of sugar in soft drinks and a large number of possible confounders(Reference Høstmark28). Prospective studies about this topic are scarce. There are only three prospective studies that have shown a link between soft drink consumption and the risk of developing the complete MetS(Reference Dhingra, Sullivan and Jacques26, Reference Nettleton, Lutsey and Wang29, Reference Lutsey, Steffen and Stevens30). All of them were summarised in a meta-analysis(Reference Malik, Popkin and Bray31). However, none of these studies has been conducted in the context of a Mediterranean dietary pattern where the average consumption of SSB is very low in comparison with the USA or North European countries. Additionally, all previous studies only assessed baseline consumption, but not the changes in the consumption of soft drinks during follow-up.
The aim of the present study was to assess the association between changes in SSB consumption and the incidence of the MetS and its components in a cohort of Spanish university graduates.
Experimental methods
Study sample
The Seguimiento Universidad de Navarra (SUN) Project is a dynamic prospective cohort study conducted in Spain with permanently open recruitment and whose participants are all university graduates who are contacted and followed up using mailed or Web-based questionnaires. A detailed description of study methods has been published previously(Reference Martinez-Gonzalez, Sanchez-Villegas and De Irala32, Reference Segui-Gomez, de la Fuente and Vazquez33). Briefly, beginning in December 1999, all graduates of the University of Navarra, registered nurses from some Spanish provinces and university graduates from other colleges and associations received a mailed questionnaire and a letter of invitation to participate in the SUN Project.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving participants were approved by the Institutional Review Board of the University of Navarra. A response to the initial questionnaire was assumed to be a surrogate of detailed informed consent that it implied the willingness to participate in the study. For this purpose, this informed consent was obtained from all participants.
After baseline assessment, participants received follow-up questionnaires every 2 years that contained a wide variety of questions on diet, lifestyle, risk factors and medical conditions.
For the present MetS study, only a subsample of the cohort was selected. To warrant a minimum follow-up of 6 years, we included only those participants who had completed questionnaires up to 6 years of follow-up. A total of 14 716 SUN participants responded to the baseline questionnaire before September 2004. Among them, 3728 participants were excluded because they had one or more criteria for the MetS. Another 1139 participants with extreme energy intake ( < 3349 kJ/d ( < 800 kcal/d) for men or < 2093 kJ/d ( < 500 kcal/d) for women, >16 747 kJ/d (>4000 kcal/d) for men or >14 654 kJ/d (>3500 kcal/d) for women) were also excluded. Among the remaining, 1136 participants answered some follow-up questionnaire but not the 6-year or 8-year follow-up questionnaire, and 556 did not answer any follow-up questionnaires. Therefore, 8157 participants were included in the final analyses.
Definition and components of the metabolic syndrome
We defined the MetS according to the International Diabetes Federation and American Heart Association/National Heart, Lung, and Blood Institute's new definition of the MetS that has harmonised all previous definitions(Reference Alberti, Eckel and Grundy34). According to this definition, the diagnosis of the MetS needs the presence of three of any of five risk factor components. These five components are as follows: (1) elevated waist circumference according to the population and country-specific definition (in the present study: ≥ 94 cm in males and ≥ 80 cm in females); (2) elevated TAG ( ≥ 1500 mg/l) or presence of drug treatment for elevated TAG; (3) reduced HDL-cholesterol ( < 400 mg/l in males and < 500 mg/l in females) or presence of drug treatment for reduced HDL-cholesterol; (4) elevated blood pressure (systolic ≥ 130 and/or diastolic ≥ 85 mmHg) or presence of antihypertensive drug treatment in a patient with a history of hypertension; (5) elevated fasting glucose ( ≥ 1000 mg/l) or drug treatment for elevated glucose.
In the 6- and 8-year follow-up questionnaires, self-reported data about these specific MetS criteria were collected. A measuring tape was sent via ordinary mail to all participants with the 6- or 8-year follow-up questionnaire including an explanation about how to measure their own waist. The validation of the self-reported data of the criteria needed to classify participants into the MetS was assessed in a subsample of the cohort, finding significant intraclass correlation coefficients between 0·5 and 0·9 (P< 0·001) depending on the criteria between each self-reported MetS component and their direct assessments by an experienced physician(Reference Fernandez-Montero, Beunza and Bes-Rastrollo35). In another validation study also conducted in a subsample of this cohort on the complete MetS itself, we found a proportion of confirmed MetS of 91·2 % (95 % CI 80·7, 97·1) and non-confirmed MetS of 92·2 % (95 % CI 85·1, 96·4) between the self-reported diagnosis of the MetS and the MetS diagnosed by the medical records of our participants(Reference Barrio-Lopez, Bes-Rastrollo and Beunza36).
Incident cases of the MetS were defined as those participants who did not have the MetS at baseline, and they reported criteria of the MetS in either the 6- or 8-year follow-up questionnaire.
Assessment of dietary and non-dietary exposures
Dietary habits at baseline were assessed using a semi-quantitative FFQ with 136 items, previously validated in Spain(Reference Martín-Moreno, Boyle and Gorgojo37) and recently re-evaluated(Reference Fernandez-Ballart, Piñol and Zazpe38, Reference de la Fuente-Arrillaga, Ruiz and Bes-Rastrollo39). We defined an ‘a priori’ Mediterranean dietary pattern using the 0 (minimum) to 9 (maximum) score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40). The baseline questionnaire also included different questions related to lifestyle. Sociodemographic variables (sex, age, years of university education and employment), anthropometric data, health-related habits (e.g. smoking status, alcohol consumption and physical activity) and medical history information (medication use, cholesterol level, blood pressure and family history of several diseases) were also collected. The reproducibility and validity of self-reported anthropometrics(Reference Bes-Rastrollo, Perez Valdivieso and Sanchez-Villegas41), the physical activity questionnaire(Reference Martinez-Gonzalez, Lopez-Fontana and Varo42) and the diagnosis of hypertension(Reference Alonso, Beunza and Delgado-Rodriguez43) were assessed in subsamples of the cohort.
Assessment of sugar-sweetened beverage consumption
Participants reported SSB consumption at baseline in the full-length FFQ and at the 6-year follow-up in a shorter version of this FFQ. SSB consumption was assessed by a previously validated semi-quantitative FFQ in Spain(Reference Martín-Moreno, Boyle and Gorgojo37–Reference de la Fuente-Arrillaga, Ruiz and Bes-Rastrollo39). In both the baseline assessment and the 6-year follow-up, participants reported the frequency of consumption (one drink = 200 ml) in the previous year (never, one to three drinks/month, one drink/week, two to four drinks/week, four to six drinks/week, one drink/d, two to three drinks/d, four to six drinks/d and more than six drinks/d). The wording and options of these items were exactly the same at baseline and the 6-year follow-up. We considered the following items as SSB: sugar-sweetened carbonated colas and fruit-flavoured carbonated sugar soft drinks assessed in one item of the FFQ. The FFQ also assessed in another item artificially sweetened beverages. Intraclass correlation coefficients between the FFQ and multiple dietary records were 0·67 for SSB and 0·46 for artificially sweetened beverages(Reference Fernandez-Ballart, Piñol and Zazpe38), and in the reproducibility analyses, the correlation coefficients were 0·69 for SSB and 0·64 for artificially sweetened beverages(Reference Fernandez-Ballart, Piñol and Zazpe38). In the 6-year follow-up questionnaire (Q6), we only assessed some, but not all, food items included in the baseline FFQ.
The change in SSB consumption was calculated as the difference between SSB consumption in the 6-year follow-up questionnaire and in the baseline FFQ. This difference in consumption was classified into quintiles of change (quintile 1 for those participants who decreased most of their consumption and quintile 5 for those participants who increased most of their consumption), considering the first quintile as the reference category. We did not include bottled fruit juices as SSB because we did not have the information on consumption at the 6-year follow-up, and according to the last report of the European Fruit Juice Association, almost half (45 %) of the fruit juice sold in Spain was 100 % juice content(44).
Statistical analysis
The cumulative incidence of the MetS was computed for each quintile of the change in SSB consumption. To avoid the confounding effect of other variables simultaneously associated with the outcome and the main exposure, we used unconditional logistic regression models.
We also assessed the association between the quintiles of the change in SSB consumption and each criterion for the MetS, and the association between the quintiles of the change in SSB consumption and weight change. Linear trend tests were calculated using the median change in SSB consumption of each quintile and by introducing this new variable as a continuous one in the models.
We evaluated the effect modification of some variables such as sex or age through likelihood ratio tests for the product term introduced in fully adjusted models.
As secondary analyses, we assessed the association between baseline SSB and artificially sweetened beverages and the incidence of the MetS.
The statistical software package SPSS for Windows version 14.0 (SPSS, Inc.) was used for statistical analyses. P values were based on the two-tailed test and P values less than 0·05 were considered as statistically significant.
As sensitivity analyses, we estimated the full-adjusted OR for the quintiles of the change in SSB consumption after modifying several assumptions: (1) using the Adult Treatment Panel (ATP)-III criteria for the MetS; (2) using the International Diabetes Federation criteria for the MetS; (3) adopting different limits for allowable total energy intake; (4) excluding participants with diabetes, cancer or CVD at baseline. In the same line, we calculated the hazard ratio for the quintiles of the change in SSB consumption using Cox regression models instead of OR calculated by unconditional logistic regression.
According to the ATP-III criteria for the MetS, the diagnosis of the MetS needs the presence of three of the following five factors: abdominal obesity (more than 102 cm in men or more than 88 cm in women); elevated TAG (equal or more than 1500 mg/l); reduced HDL-cholesterol (less than 400 mg/l in men or less than 500 mg/l in women); elevated blood pressure (equal or more than 130/85 mmHg); elevated fasting glucose (impaired fasting glucose equal or more than 1100 mg/l or type 2 diabetes mellitus)(Reference Grundy, Cleeman and Daniels45).
According to the International Diabetes Federation criteria for the MetS, the diagnosis of the MetS needs the presence of waist circumference more than 94 cm in men and more than 80 cm in women plus any two of other criteria: raised TAG (more than 1500 mg/l or specific treatment for this lipid abnormality); reduced HDL-cholesterol (less than 40 mg/d in men or less than 500 mg/l in women or specific treatment for this lipid abnormality); raised blood pressure (systolic more than 130 mmHg or diastolic more than 85 mmHg or treatment of previously diagnosed hypertension); raised fasting plasma glucose (fasting plasma glucose more than 1000 mg/l or previously diagnosed type 2 diabetes)(Reference Alberti, Zimmet and Shaw46).
Results
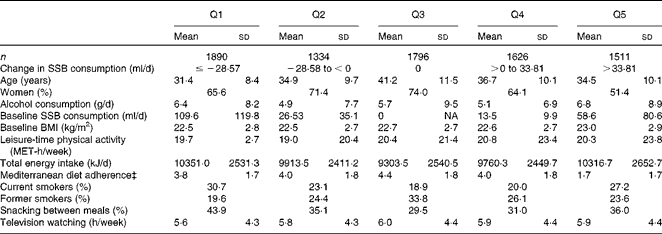
The baseline characteristics of the patients lost to follow-up did not substantially differ from those who completed the follow-up (data not shown). The characteristics of the participants according to their quintiles of the change in SSB consumption are presented in Table 1. Those participants who increased their SSB consumption the most were more likely to be men, and, on average, they presented a lower adherence to the Mediterranean dietary pattern.
Table 1 Baseline characteristics of the participants according to the quintiles (Q) of the change in sugar-sweetened beverage (SSB) consumption (Mean values and standard deviations*; percentages†)

NA, not applicable; MET, metabolic equivalent.
* For continuous variables.
† For categorical variables.
‡ Score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40) (1–9).
Incidence of the metabolic syndrome
During a median follow-up of 6 years, we identified 361 incident cases of the MetS among the 8157 young, middle-aged participants (mean age 36 years old) and initially free of the MetS.
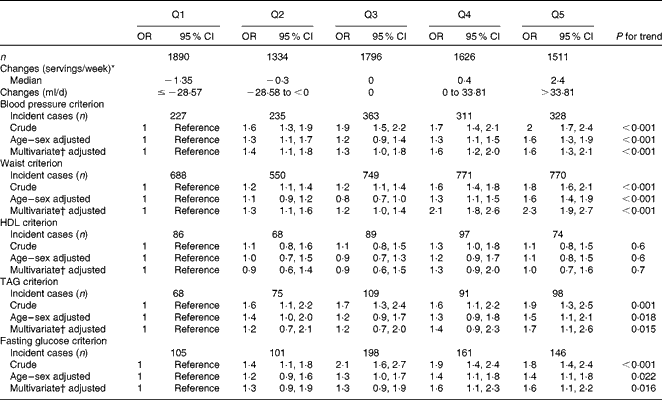
Table 2 shows the incidence of the MetS according to the quintiles of the change in SSB consumption. After adjusting for age and sex, participants in the highest quintile of the increase in SSB consumption presented a significantly greater risk of developing the MetS (OR 2·2, 95 % CI 1·4, 3·4; P for trend = 0·003) compared with those in the lowest quintile. This association persisted (OR 2·2, 95 % CI 1·4, 3·5; P for trend = 0·003) even after the adjustment for potential dietary and non-dietary confounding factors (baseline BMI, smoking, physical activity, alcohol intake, total energy intake, baseline SSB consumption, red meat, French fries, fast food and adherence to the Mediterranean dietary pattern) and even after the adjustment for the change in weight during the follow-up period (OR 2·0, 95 % CI 1·3, 3·1; P for trend = 0·038). When we repeated the analyses using servings (one can = 330 ml) of change, those participants who increased their consumption of SSB during the follow-up in more than one serving per week increased their risk of developing the MetS by 101 % (adjusted OR 2·01, 95 % CI 1·14, 3·55) in comparison with those participants who decreased their consumption to one serving per week or more.
Table 2 Incident metabolic syndrome (MetS) according to the quintiles (Q) of the change in sugar-sweetened beverage (SSB) consumption (Median values; numbers and percentages; odds ratios and 95 % confidence intervals)

* One serving: 330 ml.
† Adjusted for age, sex, baseline BMI, smoking, physical activity, alcohol intake, soft drink intake at baseline, total energy intake, consumption of red meat, French fries, fast food and adherence to the Mediterranean dietary pattern (score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40)).
‡ Also adjusted for weight change between the 6 years of follow-up and baseline.
We did not observe any significant interaction between the changes in SSB consumption and sex (P for interaction = 0·45) or age (P for interaction = 0·86).
In the SUN cohort, the consumption of SSB was very low. Only 405 participants included in the analyses (4·9 %) consumed one or more servings per d at baseline.
In a secondary analysis, we found no significant association between baseline SSB consumption and incident MetS (data not shown). The consumption of artificially sweetened soft drinks was even lower among the SUN participants. Of these study participants, 75 % never consumed artificially soft drinks, and only 2·8 % consumed one or more servings per d. Similarly, we did not find any association between baseline artificially soft drink consumption and the incidence of the MetS.
As a sensitivity analysis, we assessed the association between the changes in SSB consumption between the 6 years of follow-up and baseline (Q6–Q0) and the incidence of the MetS in the 8-year follow-up. We only identified 107 incident cases. We did not observe any significant association.
The direct association between an increase in SSB consumption and the risk of the MetS compared with a decrease in SSB consumption was robust and remained statistically significant in all but one sensitivity analysis (Table 5). The only exception was using the more strict ATP-III criteria of the MetS. Using this definition of the MetS, the changes in SSB intake were not significantly associated with incident MetS. By contrast, when we conducted Cox regression models, the hazard ratio for the fifth quintile of the change in SSB consumption was 1·9 (95 % CI 1·2, 3·0; P for trend 0·032) in comparison with the first quintile of the change in SSB consumption, therefore the association remained statistically significant.
Incidence of individual components of the metabolic syndrome
Table 3 shows the incidence of the MetS criteria during the follow-up according to the quintiles of the change in SSB consumption. The increases in SSB consumption during the follow-up were associated with a higher risk of developing high blood pressure, central obesity, hypertriacylglycerolaemia and impaired fasting glucose. After adjusting for potential confounders, participants in the highest quintile of the increase in SSB consumption presented a significantly higher risk of developing the blood pressure criterion (OR 1·6, 95 % CI 1·3, 2·1; P for trend < 0·001), the waist circumference criterion (OR 2·3, 95 % CI 1·9, 2·7; P for trend < 0·001), the TAG criterion (OR 1·7, 95 % CI 1·1, 2·6; P for trend = 0·015) and the fasting glucose criterion (OR 1·6, 95 % CI 1·1, 2·1; P for trend = 0·016) in comparison with those who decreased their consumption. An increase in SSB consumption was not associated with a higher risk of developing the HDL criterion (OR 1·0, 95 % CI 0·7, 1·6; P for trend = 0·7).
Table 3 Incident metabolic syndrome criteria according to the quintiles (Q) of the change in sugar-sweetened beverage (SSB) consumption (Odds ratios and 95 % confidence intervals)

* One serving: 330 ml.
† Adjusted for age, sex, baseline BMI, smoking, physical activity, alcohol intake, soft drink intake at baseline, total energy intake, consumption of red meat, French fries, fast food consumption and adherence to the Mediterranean dietary pattern (score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40)).
In addition, the changes in SSB consumption were positively associated with weight changes. Participants in the group with the highest increase in SSB consumption presented an average 1·3 (95 % CI 1·1, 1·6) kg greater weight gain than the group with the highest decrease in SSB consumption after the 6 years of follow-up (Table 4).
Table 4 Estimates for subsequent weight change (kg) according to the quintiles (Q) of the change in sugar-sweetened beverage (SSB) consumption (β Regression coefficients and 95 % confidence intervals; mean values and standard deviations)

* One serving: 330 ml.
† Adjusted for age, sex, baseline BMI, smoking, physical activity, alcohol intake, soft drink intake at baseline, total energy intake, consumption of red meat, French fries, fast food consumption and adherence to the Mediterranean dietary pattern (score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40)).
Table 5 Sensitivity analyses: multivariate-adjusted OR and 95 % CI of the metabolic syndrome (MetS) associated with the extreme quintile of the change (Q5) in sugar-sweetened beverage (SSB) consumption, taking as the reference category the first quintile (Q1) of the change in SSB consumption (Numbers and percentages; odds ratios and 95 % confidence intervals)

ATP, Adult Treatment Panel; IDF, International Diabetes Federation.
* Adjusted for age, sex, baseline BMI, smoking, physical activity, alcohol intake, soft drink intake at baseline, total energy intake, consumption of red meat, French fries, fast food and adherence to the Mediterranean dietary pattern (score proposed by Trichopoulou et al. (Reference Trichopoulou, Costacou and Bamia40)).
As secondary analyses, we assessed the association between baseline quintiles of SSB consumption and the incidence of each criterion of the MetS. We found that a higher baseline consumption of SSB was significantly associated with a higher risk of developing the waist circumference criterion during the follow-up (adjusted OR 1·18, 95 % CI 1·01, 1·37). We did not find any other significant association between SSB consumption and the rest of the criteria.
Discussion
In the present prospective study of young, middle-aged, free-living university graduates from a Mediterranean country, an increase in SSB consumption during the follow-up (when compared with reduced consumption) was associated not only with a higher risk of developing the MetS but also with the risk of developing four of the five defining criteria for the MetS: high blood pressure; central obesity; hypertriacylglycerolaemia; and impaired fasting glucose.
Among the 8157 participants included in the present study, we observed 361 incident cases of the MetS. This incidence proportion of the MetS is lower than those described in the general population of Spain(Reference Alegria, Cordero and Laclaustra8, Reference Fernández-Bergés, Cabrera de León and Sanz9) as it is to be expected in a cohort of young, middle-aged, active adults, with a low baseline BMI and a high educational level. Some studies have shown that a low social class and a low educational level are associated with a higher prevalence of the MetS(Reference Alegria, Cordero and Laclaustra8, Reference Brouwer, Visseren and van der Graaf10).
The present study is partially consistent with previous results found in three prospective cohorts in the context of a North American population(Reference Dhingra, Sullivan and Jacques26, Reference Nettleton, Lutsey and Wang29, Reference Lutsey, Steffen and Stevens30). All of these three previous longitudinal studies, whose results have been summarised in a published meta-analysis(Reference Malik, Popkin and Bray31), found that SSB consumption is a risk factor for developing the MetS. However, we did not find a direct association between baseline SSB consumption and a higher risk of developing the MetS. By contrast, we found an association between the changes in consumption during the follow-up and the risk of developing the MetS (P= 0·006). There are some differences between those studies and our cohort that may explain these differences in the results. First, the subjects included in these cohorts are different: in the SUN Project, we recruited only Spanish university graduates; in the Framingham Offspring Study, the participants were white Americans(Reference Dhingra, Sullivan and Jacques26); in the Multi-Ethnic Study of Atherosclerosis study, the participants were Caucasian, African American, Hispanic and Chinese(Reference Nettleton, Lutsey and Wang29); in the Atherosclerosis Risk in Communities (ARIC) study, the cohort was formed by white and black American men and women(Reference Lutsey, Steffen and Stevens30). Second, our cohort is younger than the other cohorts. Third, in the SUN cohort, the incidence of the MetS was only 4·4 % during the 6 years of follow-up, but it was 18·7 % after a 4-year follow-up in the Framingham Offspring Study, and 12·8 % in the Multi-Ethnic Study of Atherosclerosis study after a 5-year follow-up and 39·8 % after a 9-year follow-up in the ARIC study. Finally, the distribution of baseline SSB consumption was very different too, and while in the SUN cohort, only 1·1 % of participants consumed two or more servings of SSB per d, in the Framingham Offspring Study, this percentage was 13·8 %, in the Multi-Ethnic Study of Atherosclerosis study 13·6 % of participants and in the ARIC study 33 % of participants consumed a median of one serving per d.
Heidemann et al. (Reference Heidemann, Scheidt-Nave and Richter47) found that a dietary pattern high in refined grains, processed meat, red meat, high-sugar beverages, eggs, potatoes, beer, sweets and cakes, snacks and butter was associated with a higher prevalence of abdominal obesity, hypertension, hypertriacylglycerolaemia and the MetS in a cross-sectional study of a nationally representative sample of 4025 German adults. Interestingly, the present results are in accordance with recent results from the Coronary Artery Risk Development in Young Adults cohort(Reference Duffey, Gordon-Larsen and Steffen48) reporting that baseline SSB consumption was associated with several components of the MetS but not with the incidence of the MetS as a whole.
The consumption of SSB was very low among our participants. In spite of this, we found that an increase in the consumption of SSB was a risk factor for the MetS in a cohort of young adults. A relatively small increase in SSB consumption (more than one serving: 330 ml/week) was associated with a significantly higher risk of developing the MetS and its conditions in comparison with participants who decreased their consumption to more than one serving per week. In fact, changes from repeated measurements of dietary habits controlling for baseline exposure are useful in assessing the effects of the changes in dietary intakes over time on the development of chronic diseases such as the MetS in a cohort study(Reference Hu and Hu49). This is a methodological strength of our assessment.
There are different mechanisms that can explain the higher risk of the MetS associated with greater SSB consumption. A larger consumption of added sweeteners such as high-fructose corn syrup (the primary sweetener in SSB) can lead to weight gain, increased insulin resistance(Reference Bray, Nielsen and Popkin50, Reference Elliott, Keim and Stern51), lower HDL-cholesterol(Reference Høstmark and Tomten27, Reference Frost, Leeds and Dore52) and increased TAG levels(Reference Høstmark and Tomten27, Reference Willett, Manson and Liu53). Consumption of liquids is associated with a lesser degree of dietary compensation (the adjustment in energy intake made in subsequent meals in response to food intake)(Reference Cassady, Considine and Mattes54) because beverages hold weak satiety properties in comparison with solid foods. Some studies have suggested that this property might be due to the food form (i.e. liquid in beverage form compared with a semisolid or solid physical state) and not to the macronutrient or energy source (i.e. carbohydrate, fat or protein). In any case, both mechanisms are likely to be synergistic and responsible for the association between energy-yielding beverage consumption and positive energy balance(Reference Mattes55).
Individuals with a greater intake of SSB have also a dietary pattern characterised by a greater intake of energy and saturated and trans-fats, a lower consumption of fibre(Reference Pereira, Kartashov and Ebbeling56) and dairy products(Reference Rampersaud, Bailey and Kauwell57), and a more sedentary lifestyle(Reference Hu, Li and Colditz58). Nevertheless, in the present study, we adjusted for total energy intake, smoking, physical activity, alcohol intake, French fries, red meat, fast food and adherence to a Mediterranean dietary pattern in a multivariable analysis, and still we observed a significant association between the changes in SSB consumption and the risk of developing the MetS and its component criteria.
SSB consumption has been associated with obesity(Reference Qi, Chu and Kang59). Waist circumference is a criterion of the MetS; moreover, some MetS definitions consider central obesity as the more important MetS criterion(Reference Alberti, Zimmet and Shaw46). However, when we adjusted for weight change during the follow-up, we observed that the association between SSB and the MetS still remained significant, although the magnitude of the association decreased. Therefore, other potential causal pathways may contribute to explaining the relationship between the increases in SSB consumption and the MetS in addition to weight gain.
It is conceivable, though, that because of the observational nature of the present study, we cannot completely rule out residual confounding caused by lifestyle factors not adjusted for in the present analyses.
Our participants are not representative of the general Spanish population. We restricted our cohort to highly educated participants to obtain a better quality of self-reported information, to improve the retention rate and to minimise confounding by educational level, and therefore by socio-economic status(Reference Rothman, Greenland, Lash, Rothman, Greenland and Lash60). We estimated the relative effects, comparing MetS incidence between the different categories of the change in SSB consumption within the same homogeneous educational-level population.
Inherent to nutritional epidemiology methods, we have to point out the possibility of some degree of misclassification in the dietary assessment. However, we used a FFQ previously validated in Spain(Reference Fernandez-Ballart, Piñol and Zazpe38, Reference de la Fuente-Arrillaga, Ruiz and Bes-Rastrollo39). In addition, we would expect this misclassification to be non-differential, and therefore it may drive the association towards the null value.
Other possible causes of concern could be related to the differential losses of the follow-up; however, the baseline characteristics of the participants lost to follow-up did not substantially differ from those who completed the follow-up (data not shown).
The present study has important strengths such as the repeated measurement of the dietary exposure of interest, the relatively large number of participants, the long follow-up period, the previously published validation studies assessing the validity of our methods, the control for an important number of potential confounders (including the overall dietary pattern) and the good quality of the self-reported data of our highly educated volunteers.
In summary, even a relatively small increase in SSB consumption was associated with a higher risk of developing the MetS and other metabolic disorders in a cohort of young, middle-aged Spanish university graduates. However, since the incidence of the MetS was low, further studies should be conducted to confirm these findings.
Acknowledgements
The authors thank the collaboration of the participants in the SUN Project and the assistance of Dr Joan Fernandez-Ballart for his information on specific validity data of SSB in the last validation study of the FFQ. The SUN Project has received funding from the Spanish Government (Grants PI01/0619, PI030678, PI040233, PI042241, PI050976, PI070240, PI070312, PI081943, PI080819, PI1002658, PI1002293, PND2010/87, RD06/0045 and G03/140), the Navarra Regional Government (36/2001, 43/2002, 41/2005, 36/2008 and 45/2011) and the University of Navarra. M. T. B.-L. participated in the study design, statistical analyses, data interpretation and manuscript drafting. M. A. M.-G. participated in the data interpretation, funding, concept and design of the study. A. F.-M., J. J. B. and I. Z. participated in the study design and data interpretation. M. B.-R. participated in the study design, statistical analyses, data interpretation, funding and manuscript drafting. All authors revised the manuscript for important intellectual content, and read and approved the final version of the manuscript. The authors declare that they have no competing interests.