It is now well known that the composition of the colonic microbiota can be modified by changes in diet, for instance, by supplementation with prebiotics aiming to improve or maintain host health( Reference Macfarlane, Macfarlane, Fuller and Perdigon 1 ). Dietary prebiotics are defined as ‘selectively fermented ingredients that result in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health’( Reference Gibson, Scott and Rastall 2 ).
To date, the majority of studies on prebiotics have focused on inulin, fructo-oligosaccharides and galacto-oligosaccharides due to their selective fermentation and their history of safe commercial use. However, there are some candidate prebiotic oligosaccharides under investigation, including α-glucan( Reference Sarbini, Kolida and Gibson 3 ). Dextran is a complex α-1,6 glucan with additional α-1,2, α-1,3 and α-1,4 branching produced by lactic acid bacteria of the genera Leuconostoc, Streptococcus, Lactococcus and Lactobacillus ( Reference Van Geel-Schutten, Faber and Smit 4 ). The α-1,2 glycosidic linkages present are able to resist hydrolysis by digestive enzymes in both humans and animals( Reference Valette, Pelenc and Djouzi 5 ). In addition, these gluco-oligosaccharides are selectively metabolised by bifidobacteria, lactobacilli and bacteroides, but are poorly metabolised by potentially pathogenic bacteria such as enterobacteria and clostridia( Reference Djouzi, Andrieux and Pelenc 6 ). A recent study has demonstrated the prebiotic potential of dextrans using in vitro culture experiments with healthy, lean human faecal microbiota( Reference Sarbini, Kolida and Naeye 7 , Reference Sarbini, Kolida and Naeye 8 ). Low-molecular-weight dextran (linear and α-1,2 branched) has been found to selectively increase Bifidobacterium populations.
The human gut microbiota is dominated by two major phyla: Bacteroidetes and Firmicutes, while bacteria belonging to the Actinobacteria, Proteobacteria, Verrucomicrobia, Fusobacteria and Cyanobacteria phyla are present in lower numbers( Reference Eckburg, Bik and Bernstein 9 ). Over the past 5 years, some animal studies have focused on the role of gut microbiota in energy utilisation, host metabolism and adiposity. It is thought that the gut microbiota of obese rodents may be more efficient at salvaging energy from the diet than the microbiota of lean animals( Reference Turnbaugh, Ley and Mahowald 10 , Reference Bäckhed, Manchester and Semenkovich 11 ). An increased ratio of Firmicutes:Bacteroidetes has been hypothesised to be relevant to obesity. A study has found that genetically obese mice have a higher proportion of Firmicutes relative to Bacteroidetes compared with lean mice( Reference Ley, Bäckhed and Turnbaugh 12 ). A further in vivo study investigating the faecal microbiota in healthy adults has suggested that obese individuals have a higher Firmicutes:Bacteroidetes ratio than lean individuals( Reference Ley, Turnbaugh and Klein 13 ). However, this study only determined changes in the bacterial groups of the Bacteroidetes and Firmicutes phyla each containing various genera (twenty genera in Bacteroidetes and 250 genera in Firmicutes) that have diverse metabolic capabilities. Therefore, identifying changes through analysis at the phylum level rather than at the genus level could be misleading. Some more recent studies( Reference Collado, Isolauri and Laitinen 14 , Reference Duncan, Lobley and Holtrop 15 ) have contradicted these findings as they have failed to find any differences in the composition of Bacteroidetes populations between lean and obese individuals. Therefore, the role of the gut microbiota in obesity remains unclear.
Some in vivo animal studies( Reference Hildebrandt, Hoffmann and Sherrill-Mix 16 , Reference Murphy, Cotter and Healy 17 ) have demonstrated that diet composition, but not the obese state, causes changes in the composition of the gut microbiota. This raises the possibility of developing functional foods such as prebiotics that may influence microbiota composition and could be consumed as part of a weight management diet.
The main aim of the present study was to investigate the response of the obese human faecal microbiota to novel dextrans. These substrates of various molecular weights and degrees of branching could reveal some structure-to-function information. Another aim was to evaluate the fermentation profiles of various dextrans incubated over time (0, 10, 24 and 36 h). In addition, the fermentation rate of the substrates via gas production measurement was determined. Slower-fermenting nutrients may be advantageous for obese individuals, as energy would be made available more gradually. Fermentation parameters were compared with those obtained in our previous dextran fermentation experiments using lean human faecal microbiota( Reference Sarbini, Kolida and Naeye 7 ). Direct statistical comparison can be made as a similar experimental design was used in both studies.
Materials and methods
Materials
Unless stated otherwise, all reagents and chemicals used were purchased from Sigma Laboratories. The following tentative prebiotics, synthesised as described previously( Reference Sarbini, Kolida and Naeye 7 ), were evaluated: dextran 1 kDa (molecular weight: 1000 Da); dextran 1 kDa with 16 % α-1,2 linkages (molecular weight: 1200 Da); dextran 1 kDa with 32 % α-1,2 linkages (molecular weight: 1500 Da); dextran 6 kDa (molecular weight: 6000 Da); dextran 6 kDa with 33 % α-1,2 linkages (molecular weight: 9000 Da); dextran 70 kDa (molecular weight: 70 000 Da); dextran 70 kDa with 15 % α-1,2 linkages (molecular weight: 80 000 Da); dextran 70 kDa with 37 % α-1,2 linkages (molecular weight: 11 000 Da). Their main features including degree of purity, total dietary fibre percentage, theoretical molecular weights and degree of branching have been reported previously( Reference Sarbini, Kolida and Naeye 7 ). Inulin Frutafit TEX (Sensus) was used as a positive prebiotic control. All test substrates were supplied by Tate & Lyle Innovation Centre.
Faecal inocula
Faecal samples were obtained from four male, apparently healthy obese volunteers (BMI: 35–40 kg/m2; age: 30–36 years) who were free of known metabolic and gastrointestinal diseases (e.g. diabetes, ulcerative colitis, Crohn's disease, irritable bowel syndrome, peptic ulcers and cancer) and had not taken antibiotics for 6 months before participation in the study. The samples were collected on site, kept in an anaerobic cabinet (10 % H2, 10 % CO2 and 80 % N2) and used within a maximum of 15 min after collection. The samples were diluted 1/10 (w/w) in anaerobic PBS (0·1 m-phosphate buffer solution, pH 7·4) and homogenised (Stomacher 400; Seward) for 2 min at ‘normal’ speed.
In vitro fermentations
Sterile, stirred batch culture fermentation systems (50 ml working volume) were set up and aseptically filled with 45 ml of sterile, pre-reduced, basal medium (2 g/l peptone water (Oxoid), 2 g/l yeast extract (Oxoid), 0·1 g/l NaCl, 0·04 g/l K2HPO4, 0·04 g/l KH2PO4, 0·01 g/l MgSO4.7H2O, 0·01 CaCl2.6H2O, 2 g/l NaHCO3, 2 ml Tween 80 (BDH), 0·05 g/l haemin, 10 μl vitamin K1, 0·5 g/l cysteine.HCl, and 0·5 g/l bile salts, pH 7·0) and gassed overnight with oxygen-free N2 (15 ml/min).
The carbohydrates (eight dextrans and inulin, 1/100, w/w) were added to the respective fermentation vessels just before the addition of the faecal slurry. Culture temperature was kept at 37°C and the pH was controlled between 6·7 and 6·9 using an automated pH controller (Fermac 260; Electrolab), and vessels were continually supplied with oxygen-free N2 (15 ml/min). Each vessel was inoculated with 5 ml of fresh faecal slurry (1/10, w/w). Cultures were run over a period of 36 h, and the samples (5 ml) were removed from each vessel at 0, 10, 24 and 36 h for fluorescent in situ hybridisation and HPLC analysis. A total of four replicate batch culture fermentations were set up, each inoculated with one of the four different obese human faecal slurries.
Bacterial enumeration
Synthetic oligonucleotide probes targeting specific regions of 16S rRNA labelled with the fluorescent dye Cy3 were utilised for the enumeration of bacterial groups (Table 1) according to the previous procedure( Reference Sarbini, Kolida and Naeye 7 ). Labelled cells were visualised using fluorescent microscopy.
Table 1 16S rRNA oligonucleotide probes used in the present study

Samples (375 μl) obtained from each vessel at each sampling time point were fixed for 4 h (4°C) in 1125 μl of 4 % (w/v) paraformaldehyde. The fixed cells were centrifuged at 13 000 g for 5 min and washed twice with 1 ml of filtered sterilised PBS. The washed cells were resuspended in 150 μl of filtered PBS and stored in 150 μl of ethanol (99 %) at − 20°C for at least 1 h before further processing. The samples (10 μl) were diluted in a suitable volume of PBS to obtain 20–100 fluorescent cells in each field of view, and 20 μl of the above solution were added to each well of a six-well polytetrafluoroethylene/poly-l-lysine-coated slide (Tekdon, Inc.). The slides were dried for 15 min in a drying chamber (46°C). They were then dehydrated, using an alcohol series (50, 80 and 96 % (v/v) ethanol) for 3 min in each solution. The slides were again placed in the drying chamber for 2 min to evaporate excess ethanol before adding a hybridisation mixture. This mixture (50 μl), consisting of 5 μl of probe and 45 μl of hybridisation buffer, was added to each well and left to hybridise for 4 h in a microarray hybridisation incubator (Grant-Boekel). After hybridisation, the slides were washed with 50 ml of washing buffer for 15 min. They were then dipped in cold water for a few seconds and dried with compressed air. Then, 5 μl of polyvinyl alcohol mounting medium with 1,4-diazabicyclo(2.2.2)octane were added into each well and a cover slip was placed on each slide (20 mm, thickness no. 1; VWR). The slides were examined under an epifluorescence microscope (Eclipse 400; Nikon) using the Fluor × 100 lens. For each well, fifteen random different fields of view were enumerated.
Organic acid analysis
Organic acid analysis was carried out using an ion-exclusion HPLC system (LaChrom Merck Hitachi) equipped with a pump (L-7100), a refractive index detector (L-7490) and an autosampler (L-7200). Data were collected using Jones Chromatography Limited for Windows 2.0 software. The column used was an ion-exclusion Rezex ROA-Organic Acid H+ (8 %), 300 × 7·80 mm (Phenomenex). Guard columns used were SecurityGuard™ Carbo-H+ 4 × 3·0 mm cartridges (Phenomenex). The eluent used was 0·0025 mm-H2SO4 in HPLC-grade water.
Samples (1 ml) collected at each fermentation time point (1 ml) were centrifuged at 13 000 g for 10 min. The supernatants were filtered through a 0·22 μm filter unit (Millipore) and 20 μl were injected into the HPLC system, operating at a flow rate of 0·5 ml/min with a heated column at 84·2°C. The sample run time was 35 min. Sample quantification was carried out using calibration curves of external standard for lactate, formate, acetate, propionate, isobutyrate, butyrate, isovalerate and valerate at concentrations of 12·5, 25, 50, 75 and 100 mm. An internal standard of 20 mm-2-ethylbutyric acid was included in the samples and external standards.
Determination of gas production rate
Sterile glass Balch tubes (18 × 150 mm; Bellco) containing 13·5 ml of pre-reduced basal medium were kept overnight in an anaerobic cabinet. Substrates (1/100, w/v) were added to the fermentation tubes just before the addition of the faecal slurry (1/10, v/v). The tubes were then sealed with a gas-impermeable butyl rubber septum (Bellco) and an Al crimp (Sigma Aldrich). The tubes were incubated at 37°C with constant agitation.
The head space pressure (pounds per square inch) generated by faecal bacteria gas production from each substrate was measured every 3 h up to 36 h of fermentation by inserting a sterile needle (23G × 1”) attached to a transducer (Gems Sensors) into the butyl rubber septum of each tube. After each measurement, the tubes were allowed to equilibrate with the atmosphere. The gas production experiments were performed in four replicates for each substrate. Quantification of gas volume (ml) was carried out using calibration curves of air pressure (pounds per square inch) by injecting known volumes of air into the culture tubes (0·5–7 ml).
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 22.0; SPSS, Inc.). The repeated-measures ANOVA was used to determine significant changes in the bacterial populations and SCFA concentrations over time. The one-way ANOVA and post hoc Tukey's test were used to determine differences in the rate of total gas production among the substrates fermented. Differences were deemed significant when P< 0·05.
Results
Bacterial enumeration
The bacterial concentrations of the Firmicutes, Bacteroidetes and Actinobacteria phyla during obese human faecal fermentations are given in Tables 2–4, respectively. The average total cell concentrations are given in Table 5. The following significant changes were observed: a decrease in Erec482 groups in response to the fermentation of the highly branched 70 kDa dextran; a decrease in Prop853 groups in response to that of the highly branched 6 kDa dextran, but an increase in response to that of the highly branched 70 kDa dextran; a significant decrease in Fpra655 groups in response to that of the highly branched 6 kDa dextran, linear 70 kDa dextran and inulin; a decrease in Rbro730/Rfla729 groups in response to that of the linear 70 kDa dextran; an increase in Bac303 groups in response to that of the linear 1 kDa dextran and highly branched 70 kDa dextran, but a decrease in response to that of the linear 6 kDa dextran.
Table 2 Average bacterial concentrations† (log10 cells/ml batch culture fluid) of the Firmicutes phylum at 0, 10, 24 and 36 h during pH-controlled batch culture fermentations using obese human faecal microbiota inocula (Mean values with their standard errors, n 4)

1 kDa: dextran 1 kDa; 1 kDa+16 % α-1,2: dextran 1 kDa with 16 % α-1,2 linkages; 1 kDa+32 % α-1,2: dextran 1 kDa with 32 % α-1,2 linkages; 6 kDa: dextran 6 kDa; 6 kDa+33 % α-1,2: dextran 6 kDa with 33 % α-1,2 linkages; 70 kDa: dextran 70 kDa; 70 kDa+15 % α-1,2: dextran 70 kDa with 15 % α-1,2 linkages; 70 kDa+37 % α-1,2: dextran 70 kDa with 37 % α-1,2 linkages.
* Mean value was significantly higher than the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
† Starting concentrations of the test substrates were 1 % of 50 ml batch culture fluid (w/v).
‡ P value (from repeated-measures analysis at 0, 10, 24 and 36 h) for every substrate is given below the bacterial group enumerated.
Table 3 Average bacterial concentrations‡ (log10 cells/ml batch culture fluid) of the Bacteroidetes phylum at 0, 10, 24 and 36 h during pH-controlled batch culture fermentations using obese human faecal microbiota inocula (Mean values with their standard errors, n 4)
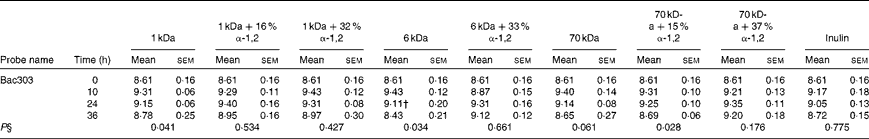
1 kDa: dextran 1 kDa; 1 kDa+16 % α-1,2: dextran 1 kDa with 16 % α-1,2 linkages; 1 kDa+32 % α-1,2: dextran 1 kDa with 32 % α-1,2 linkages; 6 kDa: dextran 6 kDa; 6 kDa+33 % α-1,2: dextran 6 kDa with 33 % α-1,2 linkages; 70 kDa: dextran 70 kDa; 70 kDa+15 % α-1,2: dextran 70 kDa with 15 % α-1,2 linkages; 70 kDa+37 % α-1,2: dextran 70 kDa with 37 % α-1,2 linkages.
† Mean value was significantly lower than the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
‡ Starting concentrations of the test substrates were 1 % of 50 ml batch culture fluid (w/v).
§ P value (from repeated-measures analysis at 0, 10, 24 and 36 h) for every substrate is given below the bacterial group enumerated.
Table 4 Average bacterial concentrations‡ (log10 cells/ml batch culture fluid) of the Actinobacteria phylum at 0, 10, 24 and 36 h during pH-controlled batch culture fermentations using obese human faecal microbiota inocula (Mean values with their standard errors, n 4)

1 kDa: dextran 1 kDa; 1 kDa+16 % α-1,2: dextran 1 kDa with 16 % α-1,2 linkages; 1 kDa+32 % α-1,2: dextran 1 kDa with 32 % α-1,2 linkages; 6 kDa: dextran 6 kDa; 6 kDa+33 % α-1,2: dextran 6 kDa with 33 % α-1,2 linkages; 70 kDa: dextran 70 kDa; 70 kDa+15 % α-1,2: dextran 70 kDa with 15 % α-1,2 linkages; 70 kDa+37 % α-1,2: dextran 70 kDa with 37 % α-1,2 linkages.
† Mean value was significantly lower than that of the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
‡ Starting concentrations of the test substrates were 1 % of 50 ml batch culture fluid (w/v).
§ P value (from repeated-measures analysis at 0, 10, 24 and 36 h) for every substrate is given below the bacterial group enumerated.
Table 5 Average total cell concentrations† (log10 cells/ml batch culture fluid) at 0, 10, 24 and 36 h during pH-controlled batch culture fermentations using obese human faecal microbiota inocula (Mean values with their standard errors, n 4)
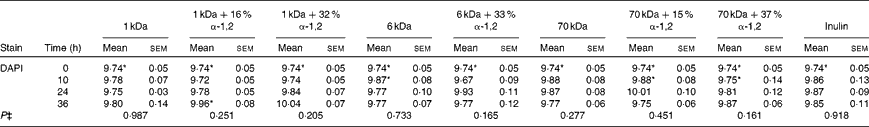
1 kDa: dextran 1 kDa; 1 kDa+16 % α-1,2: dextran 1 kDa with 16 % α-1,2 linkages; 1 kDa+32 % α-1,2: dextran 1 kDa with 32 % α-1,2 linkages; 6 kDa: dextran 6 kDa; 6 kDa+33 % α-1,2: dextran 6 kDa with 33 % α-1,2 linkages; 70 kDa: dextran 70 kDa; 70 kDa+15 % α-1,2: dextran 70 kDa with 15 % α-1,2 linkages; 70 kDa+37 % α-1,2: dextran 70 kDa with 37 % α-1,2 linkages; DAPI, 4′,6-diamidino-2-phenylindole.
* Mean value was significantly higher than that of the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
† Starting concentrations of the test substrates were 1 % of 50 ml batch culture fluid (w/v).
‡ P value (from repeated-measures analysis at 0, 10, 24 and 36 h) for every substrate is given below the bacterial group enumerated.
Organic acid analysis
Organic acid concentrations during obese human faecal fermentations are summarised in Table 6. Total SCFA concentrations increased significantly during the fermentation of most substrates tested. Acetate was the most prevalent SCFA produced during the fermentation of all substrates followed by propionate and butyrate. The following significant changes were observed: an increase in lactate concentrations in response to the linear 1 kDa dextran; an increase in acetate concentrations in response to the highly branched 1 kDa dextran; an increase in propionate concentrations in response to the linear 6 kDa dextran and branched 70 kDa dextran; an increase in butyrate concentrations in response to the linear 70 kDa dextran; an increase in the acetate:propionate ratio in the medium and in response to the highly branched 1 kDa dextran. A trend of higher acetate:propionate ratios (P= 0·068) was observed during the fermentation of the unbranched 1 kDa dextran than during that of the other dextrans. A decrease in acetate:propionate ratios was also observed with an increase in the degree of branching.
Table 6 Mean lactic acid and SCFA concentrations‡ (mm) at 0, 10, 24 and 36 h in pH-controlled batch culture fermentations using obese human faecal microbiota inocula (Mean values with their standard errors, n 4)

1 kDa: dextran 1 kDa; 1 kDa+16 % α-1,2: dextran 1 kDa with 16 % α-1,2 linkages; 1 kDa+32 % α-1,2: dextran 1 kDa with 32 % α-1,2 linkages; 6 kDa: dextran 6 kDa; 6 kDa+33 % α-1,2: dextran 6 kDa with 33 % α-1,2 linkages; 70 kDa: dextran 70 kDa; 70 kDa+15 % α-1,2: dextran 70 kDa with 15 % α-1,2 linkages; 70 kDa+37 % α-1,2: dextran 70 kDa with 37 % α-1,2 linkages; Lac: lactate; Ace: acetate; Prop: propionate; But: butyrate; Total: total SCFA; Ace/Prop: acetate:propionate ratio.
* Mean value was significantly higher than the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
† Mean value was significantly lower than the lean human faecal fermentation value (P< 0·05)( Reference Sarbini, Kolida and Naeye 7 ).
‡ Starting concentrations of the test substrates were 1 % of 50 ml batch culture fluid (w/v).
§ P value (from repeated-measures analysis at 0, 10, 24 and 36 h) for every substrate is given below the organic acid quantified.
Gas production
Total gas production from each substrate incubated with obese human faecal slurries (n 4) after 36 h of non-pH-controlled fermentations is shown in Fig. 1. The highest amount of gas was produced during the fermentation of inulin. Similar volumes of cumulative gases were produced during the fermentation of all dextrans. In Fig. 1, the gas production patterns for each substrate when incubated with obese human faecal slurries are also shown. Gases were produced at a lower rate during the fermentation of all dextrans than during that of inulin. Higher rates of gas production and higher total gas production were observed during the fermentation of inulin. A more gradual increase and a subsequent decrease in gas production were observed during the fermentation of dextrans. Higher rates of gas production were observed during the fermentation of the unbranched 1 kDa dextran, which ended earlier (approximately at 24 h), than during the fermentation of the other dextrans. Peak gas production rates were observed at 3 h for all the dextrans tested, except for the unbranched 6 kDa and branched 70 kDa dextran 15 % α-1,2, for which peak gas production rates were observed later at 9 h.

Fig. 1 Rate of gas production (ml/h) and total volume of gas produced in 36 h (ml). Gas production in the non-pH-controlled batch culture fermentations of obese human faecal microbiota with dextran 1 kDa (A), dextran 1 kDa with 16 % α-1,2 linkages (B), dextran 1 kDa with 32 % α-1,2 linkages (C), dextran 6 kDa (D), dextran 6 kDa with 33 % α-1,2 linkages (E), dextran 70 kDa (F), dextran 70 kDa with 15 % α-1,2 linkages (G), dextran 70 kDa with 37 % α-1,2 linkages (H), inulin (I) and no substrate (J). a,b,c,d,eValues with unlike letters were significantly different among the fermentations (P< 0·05; (a) lowest gas production rate/lowest total volume of gas produced to (e) highest gas production rate/highest total volume of gas produced) (n 4).
Discussion
Numerous studies have reported a possible link between human gut microbiota and obesity. Some of these studies have only investigated changes at the phylum level, e.g. Firmicutes and Bacteroidetes( Reference Turnbaugh, Ley and Mahowald 10 , Reference Ley, Turnbaugh and Klein 13 ). However, phylum-level investigations may mask changes in the constituent groups as specific genera within a phylum may have diverse metabolic capabilities. Therefore, in the present study, a panel of probes was selected to account for the majority of bacteria in the Firmicutes (Chis150, Lab158, Erec482, Prop853, Fpra655, Rbro730 and Rfla729), Bacteroidetes (Bac303) and Actinobacteria (Bif164 and Ato291) phyla.
In general, the present findings were similar to observations made in previous experiments carried out using lean human faecal microbiota under the same experimental conditions( Reference Sarbini, Kolida and Naeye 7 ). There was no difference in the enumerated bacterial populations in obese and lean human faecal fermentations during the baseline (0 h). However, the total bacterial count (4',6-diamidino-2-phenylindole; DAPI) was significantly higher in obese human faecal fermentations than in lean human faecal fermentations. These were probably due to other uncounted bacterial groups. Another similarity was the significant increase in Bac303 groups in response to the fermentation of 1 kDa dextrans as in the lean human faecal fermentation experiments. In addition, an increase in Bif164 groups (P= 0·124) in response to the fermentation of the highly branched 1 kDa dextran was also observed, which was similar to that observed in the lean human faecal fermentation experiments. However, significantly lower counts of bifidobacteria were recorded during the obese human faecal fermentation of 1 kDa dextrans than during the lean human faecal fermentation at 36 h. Bifidobacteria selectivity was found to be more pronounced and maintained for longer periods during lean human faecal fermentations than during obese human faecal fermentations. Furthermore, a study has reported the presence of lower proportions of Bifidobacterium and higher proportions of Clostridium histolyticum in obese and overweight women than in lean women( Reference Collado, Isolauri and Laitinen 14 ). Nevertheless, bifidobacteria have been known to normalise inflammatory status, which could reduce excessive hepatic and adipose tissue lipid storage, thus prevent weight gain( Reference Cani, Neyrinck and Fava 18 ).
In the present study, bifidobacteria were found to show a preference for the low-molecular-weight (1 kDa) dextrans during obese human faecal fermentations. Our current understanding of prebiotic carbohydrates is that the lower-molecular-weight oligosaccharides are more rapidly fermented than the higher-molecular-weight oligosaccharides. This may be because the low molecular mass leads to more non-reducing ends per unit mass, which favours attack by enzymes produced by Bifidobacterium spp.( Reference Gibson, Probert and Loo 19 , Reference Sarbini and Rastall 20 ). Bifidobacteria possess various genes involved in carbohydrate catabolism that enable them to grow on several short-chain oligosaccharides. They have also been shown to be more efficient at metabolising gluco-oligosaccharides than other genera such as Lactobacillus, Lactococcus, Pediococcus and Streptococcus ( Reference Grimoud, Durand and Courtin 21 ).
Some bacterial groups reacted differently in obese human faecal environments compared with lean human faecal environments. A significant decrease in Rbro730/Rfla729 groups was observed during the obese human faecal fermentation of the linear 1 kDa dextran, which was not the case during the lean human faecal fermentation. This indicates that the effect of some dextrans on Rbro730/Rfla729 groups may be more evident in fermentations using obese human faecal inocula than in those using lean human faecal inocula. Ruminococcus sp. is known to be able to ferment complex carbohydrates such as cellulose, pectin and starch.
A significant decrease in Fpra655 groups was observed in response to the fermentation of the substrates tested. A study has shown that a reduction in Faecalibacterium prausnitzii counts is associated with a higher risk of ileal Crohn's disease and that it has anti-inflammatory effects( Reference Sokol, Pigneur and Watterlot 22 ). However, one contradicting study has demonstrated that a significant decrease in F. prausnitzii counts is correlated with clinical improvement of Crohn's disease( Reference Jia, Whitehead and Griffiths 23 ). Therefore, this remains speculative.
A significant increase in total SCFA concentrations was observed during the fermentation of all substrates, with acetate being the predominant SCFA produced followed by propionate and butyrate, similar to what happens in vivo during the degradation of carbohydrates( Reference Macfarlane, Gibson and Cummings 24 ). A recent study has proposed that the acetate produced improves intestinal defence mediated by epithelial cells, protecting the host against enteropathogenic infection( Reference Fukuda, Toh and Hase 25 ). Higher concentrations of acetate were observed during the fermentation of the unbranched 1 kDa dextran. Highest concentrations of propionate were observed during the fermentation of the 70 kDa dextran with 15 % α-1,2 branching. It is postulated that propionate may have anti-obesity properties through the reduction of fatty acid concentrations in the plasma( Reference Al-Lahham, Peppelenbosch and Roelofsen 26 ). High concentrations of plasma fatty acids are known to cause inflammation, leading to insulin resistance( Reference Kennedy, Martinez and Chuang 27 ). The reduction of fatty acid concentrations by propionate has been linked to the reduction of body weight( Reference Boden 28 ) and has been demonstrated to increase satiety( Reference Ruijschop, Boelrijk and te Giffel 29 ).
A significant increase in butyrate concentrations was observed during the fermentation of the linear 70 kDa dextran in the present study. Butyrate has been shown to be involved in the prevention and treatment of diet-induced obesity in a mouse model. After 5 weeks of butyrate administration (by supplementing the diet with sodium butyrate), obese mice were found to lose 10 % of their body weight and fat content( Reference Gao, Yin and Zhang 30 ). Butyrate is thought to act by increasing energy expenditure and by inducing mitochondrial function, which improve insulin sensitivity and reduce adiposity( Reference Fleischman, Kron and Systrom 31 ).
An in vivo human study has reported faecal acetate concentrations to increase, butyrate concentrations to decrease and propionate concentrations to remain unaffected when carbohydrate intake is low. It has also been reported that leaner individuals have a higher ratio of acetate:butyrate and/or propionate compared with obese individuals( Reference Duncan, Belenguer and Holtrop 32 ). However, this finding remains speculative, as this depends only on SCFA concentrations in faeces after most of the SCFA has been absorbed in the colonic epithelium( Reference Cummings, Gibson and Macfarlane 33 ). Nevertheless, through our in vitro study, we also found that the acetate:propionate ratio is generally lower during obese human faecal fermentations than during lean human faecal fermentations( Reference Sarbini, Kolida and Naeye 7 ). Acetate may act as a precursor for cholesterol synthesis, while propionate might inhibit this process. Therefore, a low acetate:propionate ratio may be of interest for regulating serum cholesterol concentrations( Reference Delzenne and Kok 34 , Reference Wolever, Spadafora and Cunnane 35 ). In the present study, the lowest acetate:propionate ratio was observed during the fermentation of the complex 70 kDa dextran with 37 % α-1,2 linkages.
Lactate was detected in the early fermentation stage, but its concentrations diminished later. This correlates with the increase in Bifidobacterium populations. It is known that Bifidobacterium spp. and lactic acid bacteria such as Lactobacillus and Enterococcus spp. produce lactate as a major product( Reference Cummings, Gibson and Macfarlane 33 ). Accumulation of lactate occurs when it is not converted at the same rate as its production during faster fermentations( Reference Cummings, Gibson and Macfarlane 33 ). Previous studies have suggested that carbohydrates that are rapidly fermented produce higher amounts of lactate compared with slowly fermented ingredients( Reference Kudoh, Shimizu and Ishiyama 36 ). This was in accordance with what we had observed in the gas production experiments where the unbranched dextran was more rapidly fermented than the other dextrans.
Gas production in the large intestine is part of a normal digestive process caused by the fermentation of carbohydrates by the gut microbiota. These gases include H2, CO2, CH4 and H2S( Reference Levitt, Gibson, Christl, Gibson and Macfarlane 37 ). Some of the gas compounds such as NH3 and substances such as indole and skatole are toxic to the gastrointestinal environment. However, the colonic H2 produced by organic acid fermentation may have beneficial effects by suppressing the metabolic syndrome via its antioxidant properties( Reference Nishimura, Tanabe and Adachi 38 ). Nevertheless, gas produced can be a clinical disincentive of prebiotic consumption due to unwanted symptoms such as bloating and discomfort( Reference Tuohy, Kolida and Lustenberger 39 , Reference Hartemink, Rombouts and Hartemink 40 ). Gas production is probably influenced by the chemical structure of carbohydrates, such as differing chain length and monosaccharide composition, as well as the composition of the colonic microbiota. Unlike fructo-oligosaccharides and galacto-oligosaccharides, α-gluco-oligosaccharides generated less gas, as has been observed in an in vitro fermentation experiment with swine faecal microbiota( Reference Smiricky-Tjardes, Flickinger and Grieshop 41 ). In the present study, all substrates produced some gas after 3 h of fermentation. The lower-molecular-weight dextrans, particularly the linear 1 kDa dextran, were rapidly fermented with shorter time to attain maximal rate of gas production (3–6 h). This may be due to the simpler structure of the linear 1 kDa dextran rendering it more accessible to enzymes compared with higher-molecular-weight and branched carbohydrates.
In conclusion, the novel dextran gluco-oligosaccharides demonstrated prebiotic potential, i.e. increasing Bifidobacterium spp. and SCFA concentrations, even in obese subjects. Interestingly, many recent studies have reported that an increase in the counts of bifidobacteria may reduce the chance of developing obesity( Reference Collado, Isolauri and Laitinen 14 , Reference Cani, Neyrinck and Fava 18 ). No differences in the selected bacterial populations, organic acid concentrations and gas production rates were observed between the obese and lean human faecal fermentations of dextrans. Therefore, we suggest that the substrate type (molecular weight and/or structure), probably not the obese state per se, modulates the composition of the microbiota.
Acknowledgements
The authors thank Thierry Naeye and Alexandra W. Einerhand from Tate & Lyle for providing the substrates and Adele Costabile and Dimitris Tzimorotas from the Department of Food and Nutritional Sciences, University of Reading, for their technical assistance in the fermentation works and the organic acid profile analysis.
The Sarawak Foundation and Universiti Putra Malaysia of the Malaysian government provided a scholarship to S. R. S. The present study was funded by Tate & Lyle. The research and all publications arising from or referable to it are considered proprietary data to which Tate & Lyle claims exclusive right of reference in accordance with Regulation (European Commission) no. 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods.
The authors’ contributions are as follows: S. R. S. carried out the study, collected and interpreted the data, and wrote the manuscript; S. K. designed and supervised all the experiments, interpreted the data and edited the manuscript; E. R. D. recruited the human volunteers and edited the manuscript; G. R. G. edited the manuscript and provided intellectual input for drafting the manuscript; R. A. R. designed, planned and supervised all the experiments and manuscript preparation. All authors approved the final content of the manuscript.
None of the authors has any conflicts of interest to declare.









