Nano-oxides and the biological world
Metal oxide nanostructures can be broadly grouped into two categories, namely industrial and medicinal nanoparticles. Industrially engineered metal oxides are heavily used in various fields ranging from chemical industries, the automobile sector, environmental remediation, and food and cosmetic industries. The medicinal use of metal oxide nanoparticles is in rapid expansion and deals with highly reactive oxides (e.g., TiO2, FeO2, CeO2) that deeply interfere with the metabolic network of cells and organisms. In addition to the nanoscale, which provides peculiar features to materials, nano-oxides develop interactions with the complex chemistry of living matter due to their surface reactivity, which is enhanced by their extremely high surface area to volume ratio. Moreover, their ability to shed ions that can alter the surrounding bioenvironment makes them especially reactive. Such reactivity on the one hand is a biohazard, but on the other, if correctly managed, can be turned into invaluable pharmaceutical tools.
Oxide nanoparticles used for medicinal studies have, in general, well-defined surface chemistry due to the co-synthetic or post-synthetic conjugation of linker molecules—generally of organic nature—that prevents/modulates the release of metal ions in cellular environments and imparts colloidal stability, a parameter of paramount importance when nanoparticles travel within living organisms. In addition, surface functionalization with bifunctional linkers allows selective conjugation with biomolecules, and this imparts tailored properties, such as control of delivery and cellular uptake.Reference Bloemen, Van Stappen, Willot, Lammertyn, Koeckelberghs, Geukens, Gils and Verbiest1
Great emphasis has been placed recently on the spontaneous adsorption of proteins and lipids to nano-oxide surfaces during their passage through biological fluids and tissues, forming the so-called protein or lipid corona. The binding of different proteins or lipids, which may depend, in part, on the chemical reactivity of the nanoparticle surface, theoretically could change the nanoparticle/organism interface and consequently the behavior of the nanoparticle.Reference Faunce, White and Matthaei2 In practice, the formation of a protein corona can be viewed as an example of spontaneous functionalization, based on weak, reversible molecular interactions that buffer the nanoparticles’ surface charge, ameliorating colloidal stability and reducing ion release. The weak interactions may cause a continuous binding-and-detaching cycle with different proteins “dressing” the nanoparticle surface, conferring an ever-changing interface with the biological environment.
In general, the kinetics of interaction of nanoparticles with organisms consists of crossing of the epithelia (skin, lungs, intestine) via occasional discontinuities or active passage, which is favored by the nanoparticle’s small size; once internalized, nano-oxides can cross the endothelial barrier, entering the blood flow; they can escape via endothelia fenestration, small discontinuities in vessel walls occurring in inflammatory sites including tumor areas, and localize into internal organs. Here, static conditions may allow crossing the plasma membrane of the cells, reaching the cell cytoplasm or even membrane-bound cellular organelles, where they exert their characteristic actions, possibly altering cell behavior. Alternatively, the blood flow can push them into filter organs to be excreted. Clearance (the elimination of exogenous molecules/particles from the body) is very important for non-biodegradable structures, both for accidental or medicinal exposure, and is the object of many toxicological studies (Figure 1). Each of these passages requires interactions between the simple chemical composition of the nano-oxide surface and the highly sophisticated chemistry of biological structures. The ever-changing assets of the protein corona may influence this interaction; therefore, the route of entry, “dressing” the transiting nano-oxides with macromolecules characteristic of the specific environment, determines the type of biocorona and the subsequent fate of the internalized nano-oxides.Reference Pietroiusti, Campagnolo and Fadeel3

Figure 1. Biokinetics of nano-oxides. Nano-oxides can enter the body following independent routes (inhalation, skin penetration, or ingestion), which cause different fates for the internalized particles. Accumulation in specific organs and slow excretion rates can possibly cause hazards.
The good
In the field of nanomedicine, nano-sized materials promise impressive developments in diagnostic and therapeutic procedures. Their particulate nature allows binding of multiple molecular determinants, allowing building platforms containing delivery molecules such as tissue or tumor-specific antibodies, active drug(s), sensitizing agents, or diagnostic molecules for imaging at the same time. These platforms facilitate the simultaneous localization to the pathological tissue of the different determinants, combining therapeutic and diagnostic procedures in novel “theranostic” approaches. Nanomedicine is expected to overcome problems of body retention, solubility, stability, and selectivity that impair the use of many potentially useful pharmaceutical drugs.Reference Mishra, Hubenak and Mathur4
Many oxides on the nanoscale possess intrinsic activities displaying pharmacological potential that promise important advances in therapy against oxidative pathologies (Figure 2) on one side, and against cancer on the other. The two approaches are depicted in Figure 3. It must be stressed that if antioxidant therapies are aimed at protecting cells and preventing damage, thereby necessitating the use of cell-protective agents, anticancer therapies are aimed instead at killing tumor cells, thus necessitating the use of toxic agents, though in a tightly controlled fashion.
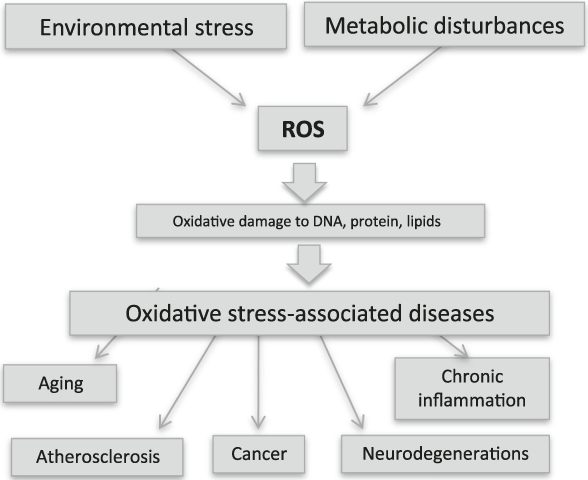
Figure 2. Environmental stress (UV or x-rays, pollution, etc.) and metabolic disturbances (including inflammation, aging, and infections) produce reactive oxygen species (ROS). This phenomenon is known as oxidative stress and damages cellular constituents, thereby altering cell functions and promoting pathological conditions. Almost all human pathologies are caused or accelerated by oxidative stress, and researchers are searching for antioxidant therapies to prevent their onset.

Figure 3. Clinical exploitations of nano-oxides. The reactivity of the nano-oxide surface allows anticancer actions, such as FeOx particles exerting strong cytotoxic effects against cancer cells in hyperthermic therapies (thermoablation), or CeO2, which, as nanoparticles, exert an extraordinary antioxidant effect, freeing damaged cells of the most noxious reactive oxygen species.
Nano-oxides such as FeO2 or TiO2 can perform peculiar trans-excitation reactions (up-conversion) that transform radio-frequency (RF), ultraviolet (UV), visible, or infrared (IR) radiation into cell-killing physicochemical agents such as reactive oxygen species or heat.Reference Laurent, Dutz, Häfeli and Mahmoudi5 This allows highly localized thermo-ablationReference Hilger and Kaiser6 and proficient photodynamic therapies even in internal tissues due to the transformation of body-penetrating infrared radiation into visible light.Reference Chatterjee, Fong and Zhang7 These approaches, some of which are already at the stage of clinical trials, increase the efficacy of anticancer therapies, focusing toxic actions against tumor masses and sparing healthy tissues.
From the perspective of cell-protective agents, catalytic materials such as cerium oxide nanoparticles can accomplish energy-free antioxidant cycles that scavenge the most noxious reactive oxygen species, thereby mimicking antioxidant enzymes such as superoxide dismutase (SOD) and catalase;Reference Heckert, Karakoti, Seal and Self8 this reduces the damage induced by environmental stress, and ameliorates an impressive series of clinically relevant oxidation-related pathologies.Reference Celardo, Pedersen, Traversa and Ghibelli9
Another fascinating challenge is the use of metal oxide nanoparticles to selectively isolate biomolecules of interest via well-designed synthetic protocols for surface functionalization (e.g., click-functionalized nanobeads) that can open new avenues for vectorization.Reference Ilyas, Ilyas, van der Hoorn and Mathur10
The evil
The leakage of metal oxide nanoparticles into the human environment and work sites is strictly linked to their increased production and use in many different fields, and underscores the need to analyze the routes of exposure and the possible effects or after-effects of nanoparticles internalized in cells and organs.Reference Sarkar, Ghosh and Sil11
The biohazard of nanoparticles is mainly attributed to their “unpredictable” and unique physical, chemical, and biological behavior in biological environments.Reference Sharifi, Behzadi, Laurent, Forrest, Stroeve and Mahmoudi12 A large number of studies have reported data obtained from in vitro and in vivo experiments, correlating the physicochemical characteristics of metal oxide nanoparticles with body reactions, including biokinetics, effects on organs and tissues, accumulation in filter organs, excretion rates, and the ability to elicit an immune response.Reference Johnston, Pojana, Zuin, Jacobsen, Møller, Loft, Semmler-Behnke, McGuiness, Balharry, Marcomini, Wallin, Kreyling, Donaldson, Tran and Stone13
The ultra-small metal oxide structures allow their translocation across body membranes such as the placenta; endothelia; including the blood-brain barrier; and the intestinal and respiratory epithelia. This allows passage through body organs and even internalization into cells. The extraordinarily high surface area enhances chemical activities such as agglomeration and/or dissolution (Figure 3). Agglomeration is a general feature of nano-oxides and a great hazard for the body, due to the risk of forming clumps that may obstruct small vessels. In order to circumvent this problem, researchers functionalize the nanoparticles with biocompatible molecules that increase colloidal stability. Dissolution is the release of ions from the nanoparticle surface with consequent surface changes; the biological impact of such free ions may differ from the same atom when it is present in the nanoparticle crystalline structure, with possible toxic effects. For example, CeO2 is toxic when present as a free ion, but not when it is within the CeO2 nanoparticle lattice (Figure 4). The role of released metal ions upon dissolution of metal oxide nanoparticles needs further attention because the ions may complex with, and sequester, biological ions and ligands, thereby reducing their bioavailability and increasing the toxicological hazard.Reference Sabella, Carney, Brunetti, Malvindi, Al-Juffali, Vecchio, Janes, Bakr, Cingolani, Stellacci and Pompa14

Figure 4. Mechanisms of nano-oxide biohazard. Among the possible negative effects from the body internalization of nano-oxides, the main concerns deal with ion leaching from the nano-oxide surfaces, possibly shedding toxic ions; aggregation, which may create clumps obstructing small vessels; and protein corona formation, which may change the biological identity of the nanoparticles in an unpredictable way.
Consequently, the different physicochemical characteristics of metal oxide nanoparticles after synthesis and their gradual alteration during permanence in the biological environment make qualitative and quantitative risk assessment a challenging task. Toxicological analyses report contradictory effects, which is a necessary consequence of the large number of variables that researchers have to take into account, a fact that renders it very difficult to compare different studies. Nanotoxicologists need to confront unfamiliar types of biomaterial interactions that require novel paradigms to be correctly addressed, including shape, size, surface area, and agglomeration in addition to dose and chemical composition. Therefore, the use of nano-oxides for pharmacological purposes poses unprecedented safety issues due to their particulate nature and lack of biodegradability. Therefore, the biocompatibility of metal oxide nanoparticles, in spite of intense studies, is still a controversial issue showing some effects common to nanoparticles in general and others unique to specific types of nano-oxides.Reference Nel, Xia, Mädler and Li15
The Janus effect
Janus was the Roman god of transitions, depicted with two opposite faces to stress his contradictory roles (e.g., peace and war). Many scientific themes offer contradictory faces, and Janus is often called to represent these contradictions. This is especially true for pharmaceutical drugs. Indeed, drugs in the human body must interfere with metabolism to be effective; the “safe” drug does not exist. Consequently, pharmaceutical compounds may be beneficial or detrimental depending on the circumstances. As an example, cytotoxic effects, which, in principle, are negative for the organism, become highly appreciated when tumor cells need to be killed. Moreover, it is important to balance risk/benefit, and a certain degree of risk may be acceptable when treating invalidating or life-threatening pathologies. Therefore, when nanoparticles are used as drugs, the evaluation of biocompatibility must be conditional to the situation, and the notion of toxicity must be carefully re-evaluated on a case-by-case mode.
The dilemma is formidable. On one hand, there are a large number of reports demonstrating the potential of nano-sized particles and capsules in medicinal applications such as drug delivery and diagnostics; on the other hand, the medicinal impact awaits clinical reality due to the lack of standardized assessment procedures that will enable comparative evaluation of nano-specific pharmaco- or toxico-kinetic effects. This paradoxical Janus effect is summarized in Figure 5.

Figure 5. Janus-like bioeffects of oxide nanoparticles. Prospective use of nano-oxides in anticancer and antioxidant therapies not only promise revolutionary developments, but also pose questions of being a possible biohazard.
Metal oxide nanoparticles such as TiO2, ZnO, and Fe2O3 are earth-abundant materials, which explains their traditional large-scale production and use in numerous applications due to their unique optical, catalytic, and electronic properties. Recently, their importance in medicine, pharmacy, and cosmetics is largely increasing. Importantly, these nanoparticles can be synthesized in different nanoscopic dimensions and shapes (particles, wires/tubes, films, and bulk composites), which display different biological behavior despite similar chemical compositions.
Examples of the Janus effect include the wide use of zinc and titanium oxide nanopowders as active agents in sunscreens.Reference Rampaul, Parking and Cramer16 Their undoubted beneficial action as barriers against UV-ray penetration into skin is contrasted by their pro-oxidant photocatalytic effect, the phenomenon that transforms the energy of UV rays into reactive oxygen species. This confers toxicity on the nanoparticles that overlaps with the basic protective action; the search for agents that block UV rays without producing oxidative stress is fervently ongoing.
Given their widespread utilization, models to understand and assess the toxicological burden of nano-oxides are of critical importance. Mechanistic analyses have revealed, for instance, that the solubility of ZnO nanoparticles is a function of their size,Reference Demir, Creus and Marcos17 with ultrafine nanoparticles being more toxic than their micro- or macroscale counterparts.
Metal oxide nanoparticles made of redox-active metals such as cerium oxide nanoparticles (nanoceria) exert anti-oxidant effects at the cellular level, preserving viability after cell insult.Reference Celardo, De Nicola, Mandoli, Pedersen, Traversa and Ghibelli18 In some instances, however, cerium oxide nanoparticles increase, rather than decrease, the intracellular levels of reactive oxygen species and may become toxic due to electron transfer processes; the reason for such discrepancies is presently still unclear.Reference Sabella, Carney, Brunetti, Malvindi, Al-Juffali, Vecchio, Janes, Bakr, Cingolani, Stellacci and Pompa14 As a matter of fact, cerium oxide nanoparticles can be used as a paradigmatic model to explain the Janus-like effect, whose final result depends on many independent factors acting at multiple levels. For instance, the differential toxicity of CeO2 in their free versus nanoparticle form may turn into an invaluable anti-tumor strategy. Indeed, solubility (ion leaching) of cerium oxide nanoparticles requires low (<5) pH. In biological environments, which usually display neutral pH, this acidity is found only in specific intracellular organelles (lysosomes). It was shown that in tumor cells, cerium oxide nanoparticles localize in lysosomes, and this is linked to tumor cell killing.Reference Asati, Santra, Kaittanis and Perez19 In other non-transformed cells, nanoceria localization differs, and their presence is not only non-toxic, but even beneficial to the treated cells.Reference Caputo, De Nicola and Ghibelli20
In this issue
This issue of MRS Bulletin contains a selection of articles reflecting both the bright and dark sides (the good and the evil) of metal oxide nanoparticles present in cellular or intracellular environments.
Hemmer et al. highlight the potential of lanthanide-doped metal oxides and fluorides as viable alternatives for cellular and small animal imaging, when compared to organic dyes that suffer from photo-bleaching and photo-toxicity in bioenvironments. Since any potential biomarkers are likely to be present in very small quantities in the body, lanthanide-based nanophosphors are promising due to their high optical conversion efficiencies, which in turn can reduce the concentration of the effective dose and thus circumvent possible cytotoxicity. In addition, the use of near-infrared (NIR) light in lanthanide-based optical markers enables working in the so-called biological window (>1000 nm), exhibiting absorption maxima for human skin. Despite the unique optical properties of Ln3+-based nanoparticles as a bioimaging probe, concerns regarding the cytotoxicity and accumulation of lanthanide ions in the body need further attention and systematic studies.
The article by Goya et al. presents the current status of iron oxide nanoparticles in regenerative medicine, in particular the guided-growth of neural cells through an external magnetic field. This article highlights the scope for both the up- and down-side of cell-nanomaterial interactions. While localized high concentrations of magnetic nanoparticles in tissue can absorb energy from an external alternating magnetic field to induce cell death (magnetic hyperthermia), minor amounts of magnetic nanoparticles can be used as vectors to selectively deliver a pharmaceutical component to tumor cells and to induce selective uptake by appropriate surface modification. The advancements in the field clearly demonstrate the potential use of magnetic oxides as a non-invasive approach toward nerve regeneration by guiding neurite outgrowth in a preferential direction through an external magnetic field. However, the in vitro proof-of-concept studies await translation into human health applications.
The chameleon-like behavior of nanoparticles in biological environments poses the biggest challenge for the quantification of the outcome of nanotoxicological research, which is addressed in the article by Bhattacharya et al. This article points out the need to view nanoparticles present in a living system as possessing a “biological” identity, defined by the pattern of adsorbed proteins (and other biomolecules) on the surface of the nanoparticles, in addition to a “chemical” identity, determined by the nanoparticle composition, and to differentiate between the two when performing biocompatibility analyses. The work reviewed implies that any potential toxicity assessment of nanoparticles should consider the dynamics of nano-bio interactions that, in some cases, can aggravate cytotoxic response.
The article by Das et al. focuses on the possible application of cerium oxide nanoparticles (nanoceria) in regenerative medicine, a novel branch of medicine that aims to achieve functional restoration of damaged tissues via the in vitro “fabrication” of functional tissues starting from stem cells. Nanoceria has been shown to control intracellular as well as extracellular reactive oxygen and nitrogen species and enhance long-term cell survival, enable cell migration and proliferation, and promote stem cell differentiation. Moreover, the self-regenerative property of nanoceria permits a small dose to remain catalytically active for an extended time.
Wick and co-workers in their article examine a key issue, critically reviewing the reliability of current in vitro tests to assess the functional interaction between nano-oxides and biological structures. They point out that reliable, cost- and time-effective, rapid and mechanistic-based testing strategies are needed to replace current conventional phenomenological assessments. Today’s in vitro technology, providing human-based advanced cellular models representing different organ barriers such as skin, lung, placenta, or liver may cover this need. The aim of this article is to review how far in vitro models have achieved general acceptance and how these models can further be improved.
Podila et al. review spectroscopic tools to study the nature of nano-bio interactions at the cellular, subcellular, and protein levels. They describe spectroscopic tools that clearly identify the net bioavailable metal ion content and help establish relationships between ion-release from a nano-oxide and the induced toxicity. The various spectroscopic tools (e.g., Raman, photoluminescence, IR, and surface plasmon) can probe the intrinsic properties of nano-oxides (e.g., crystallinity and electronic states) and biomolecules (e.g., protein structure), and can provide a comprehensive understanding of their transport within organisms (e.g., via biodistribution) and new perspectives of biological interactions (e.g., loss of protein structure upon adsorption on nano-oxides) at the nanoscale.
Conclusion and outlook
The prospective use of metal oxide nanoparticles in nanomedicine promises great advances in anticancer and antioxidant therapies. This clearly deserves the efforts required not only for setting up clinical devices and clinical trials, but also for exploring and solving biocompatibility issues. These include the synthesis and surface functionalization of metal oxide nanoparticles to enable their controlled uptake and internalization, and the comprehension of their dynamic behavior and associated physicochemical changes, which are, unfortunately, of limited statistical value thus far. Investigations pertaining to toxicological hazards of engineered metal oxide nanostructures on human health are in the early stage of investigations, and this issue of MRS Bulletin was conceptualized to address the major gaps and need for coherent R&D and regulatory efforts. Although it is now known that toxicological properties of nanostructures differ from their bulk counterparts, recent findings on the biotransformation of nanoparticles necessitate more comprehensive studies to relate them to systemic toxicity.





