CASE REPORT
A 54-year-old male was found in his car near his house and was admitted in the emergency department. The patient was seen by his son shortly before he left and he seemed to be fine at that moment. The patient had a history of alcohol abuse. Except for a laminectomy and arterial hypertension, the patient had no medical history. His home medication was losartan and a pain killer when necessary. He had no known allergies.
At the moment that he was found, he was unresponsive to verbal command and painful stimuli with a Glasgow Coma Scale of 3/15. His heart rate and blood pressure were normal, and clinical examination did not reveal major injury. To protect the airway, the trachea was immediately intubated on the spot, and the patient was ventilated. To rule out severe traumatic brain injury, a CT scan was performed, which did not reveal posttraumatic injury. A 12-lead electrocardiogram showed no signs of ischemia or rhythm disturbances.
Peripheral blood count and liver biochemistry were within normal limits. The patient had an estimated glomerular filtration rate above 1.50 ml/s/m2, which was normal. Because serum prolactin levels were slightly elevated (2146.55 pmol/L, normal values [N]: 165-1010 pmol/L), an electroencephalogram was performed. There were no signs of seizure activity. Lactate was within normal range (1.67 mmol/L, N: 0.50-2.20), as well as glucose (6.16 mmol/L, N: 3.9-6.6 mmol/L). Ethanol on this sample was slightly elevated (10.2 mmol/L, N: <2.17 mmol/L). The serum osmolality was 287 mOsm/kg (N: 280-300 mSsm/kg) and serum creatinine was 83.1 micromol/L (N: 51.3-107.0 micromol/L).
An arterial blood gas analysis revealed the following: pH 7.07 (N: 7.35-7.45), bicarbonate 12.6 (N: ;22.0-26.0 mmol/L), sodium 136 (N: 136-145 mmol/L), potassium 4.11 (N: 3.50-5.10 mmol/L), chloride 95.2 (N: 98.0-107 mmol/L), and pCO2 44.2 (N: 35.0-45.0 mm Hg), indicating a HAGMA with an anion gap of 28.2 mmol/L (N: 11.0-21 mmol/L).
Carbon monoxide (CO) intoxication was unlikely because the patient’s car was parked outside before leaving and was ruled out by a normal CO hemoglobin level (3.7%) (N: <5 to 6% TIETZ) upon arrival at the hospital. An arterial blood gas analysis less than 12 hours after the first arterial blood gas analysis was normal (Table 1).
Table 1 Arterial blood gas analysis

Because ketoacidosis is a well-known cause of HAGMA, urine was tested for ketones and glycose. The test was the Roche ComburReference Heytens, Neels and Van Regenmortel 10 Test M strip, and it was negative.
Subsequent toxicological screening of serum for acetaminophen, salicylate, benzodiazepines, barbiturates, and tricyclic antidepressants was negative. Urine was screened for barbiturates, benzodiazepines, acetaminophen, tricyclic antidepressants, (meth)amphetamine, cocaine, opiates, and methadone and was found negative as well.
The analysis of (toxic) alcohols was performed by headspace gas chromatography with flame ionization detection (HS-GC-FID) and allowed simultaneous detection of methanol, ethanol, n‑propanol, isopropanol, and acetone. The analysis of glycols (ethylene glycol and propylene glycol) was performed by GC-FID. Serum concentrations of acetone, all alcohols, and glycols tested were <0.86 mmol/L at this moment (Tables 2 and 3).
Table 2 Toxicology results obtained on serum

HS-GC-FID=headspace gas chromatography with flame ionization detection; GC-FID=gas chromatography with flame ionization detection.
Table 3 Toxicology results obtained on urine
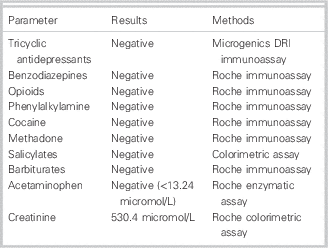
On the basis of the laboratory results of blood and urine samples and clinical examination, common causes of HAGMA (ethylene and propylene glycol, lactate, methanol, salicylate, renal failure, and ketoacidosis) were ruled out (see Tables 2 and 3).
The diagnosis of a severe GHB intoxication causing HAGMA was established after gas chromatographic-mass spectrometric analysis (GHB serum: 10.64 mmol/L, GHB urine 33.53 mmol/L). The sample tested for GHB was taken 210 minutes after the first blood gas. A plasma level of 0.048 mmol/L and a urine level of 0.096 mmol/L was used as a cut-off concentration of normal endogenous GHB concentrations.
Supportive therapy with sedation, ventilation, fluid replacement, and bicarbonate was given. The patient fully recovered after 17 hours and was discharged to the ward on the following day. Questioning of the patient and of his son confirmed the hypothesis that the patient accidentally ingested GHB. He thought the bottle that he found under the bed of his son was a bottle of gin. The bottle was not submitted for analysis.
DISCUSSION
GHB is endogenous to the mammalian CNS, but normal endogenous blood and urine concentrations are low (<0.096 mmol/L).Reference Haller, Thai and Jacob 1 , Reference Busardo and Jones 2 Interpretation of blood levels can be false due to collection tube additives and storage temperature, which can affect the GHB levels. The optimal specimen for testing for the presence of GHB is a urine sample. It should be taken in consideration that urine concentrations after ingestion of GHB vary widely.Reference Haller, Thai and Jacob 1 Gas chromatographic-mass spectrometric analysis is the best way to confirm the presence of GHB in blood or urine. GHB is rapidly absorbed and eliminated from the body. The elimination of biological half-life is 30-50 minutes. Because of its fast elimination, GHB can usually not be detected in plasma more than 250 minutes after ingestion.Reference Busardo and Jones 2
The concentration of GHB in urine is comparable with the concentration in the blood during the time that urine was produced in the kidney and stored in the bladder. This can explain why toxicology for GHB can be positive in urine and negative in blood.Reference Busardo and Jones 2
Gamma-hydroxybutyric aciduria is the only known metabolic disease with high endogenous GHB concentrations. Synthetic GHB was initially used as an anesthetic but is now only licensed for medical use in a limited number of indications such as the treatment of narcolepsy.Reference Corkery, Loi and Claridge 3 Because of its euphoric effects, it became popular for recreational use under the street name, Liquid Ecstasy. Other popular names for GHB are cherry meth, growth hormone booster, scoop, Liquid X, Liquid G, and Georgia Home Boy. GHB as such is a white crystalline powder but is most frequently vended as a solution. These liquid forms have widely variable GHB concentrations. It is also used as a sleep aid and is ingested chronically to increase lean body mass by body builders.Reference Benzer, Cameron and Scott Russi 4
The presentation of a GHB intoxication is well described in the literature. Low doses have a relaxant effect similar to that of alcohol, and higher doses will lead to severe CNS depression. The drug has a steep dose-response curve with a small therapeutic window leading to an abrupt decrease in the level of consciousness. The absorption of GHB is fast, and the time to reach the maximum plasma level depends on the dose that is administered. It takes approximately 30 to 60 minutes to reach peak plasma levels after ingestion of 50 mg/kg.Reference Benzer, Cameron and Scott Russi 4
The clinical presentation of a GHB intoxication can be obscured due to concomitant drug use. In a case series study, Galicia et al. demonstrated that 76% of the patients combined GHB with other drugs such as alcohol, cocaine, ketamine, cannabis, and so forth. This multiple drug abuse leads to a more severe clinical presentation and will increase the need of more invasive therapy.Reference Galicia, Nogue and Mir 5
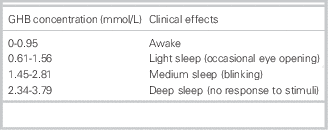
Not only CNS symptoms can be present in a GHB intoxication, but also common other symptoms, including vomiting, urinary and fecal incontinence, bradycardia, hypoventilation, miosis, mydriasis, and hypothermia. The symptoms depend on the ingested dose (Tables 4 and 5).
Table 4 Dose-related symptoms of GHB
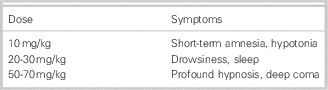
Table 5 GHB peak plasma concentration and its clinical effects
GHB=gamma-hydroxybutyrate.
GHB is a physiological metabolite of gamma-aminobutyric acid, which plays a role in the control mechanism of the pituitary hormones. The release of dopamine, which is a prolactin-release inhibiting factor, is blocked by GHB. As a consequence, GHB induces a release of prolactin.Reference Takahara, Yunoki and Yakushiji 6 Prolactin can also be raised after a generalized tonic-clonic seizure and after a non-epileptic seizure as well as after a syncope, indicating that increased prolactin levels cannot be used to differentiate between seizures, syncope, and GHB intoxication.Reference Vukmir 7 , Reference Chen, So and Fisher 8 Patients who suffered from a generalized tonic-clonic insult have a marked elevation of lactate and often suffer from lactate acidosis.Reference Baba and Zwaal 9
The treatment of GHB intoxications is supportive because no antidote is available. In our patient, the pH was corrected with bicarbonate infusions. In one other case report, dialysis was used to correct the pH because bicarbonate therapy was unsuccessful.Reference Heytens, Neels and Van Regenmortel 10
HAGMA could also be caused by the presence of methanol or ethylene glycol metabolites, while the parent compound was fully metabolized. However, given the toxicity of methanol and ethylene glycol, full recovery of the patient without sequelae would be unlikely. An intoxication with propylene glycol, a solvent for intravenous drugs, is very rare and is unlikely to provoke a coma.Reference Kraut and Kurtz 11
HAGMA after GHB intoxication is rarely reported in the literature. To our knowledge, this is only the second case described in the literature. The first case was a gamma-butyrolactone (GBL) intoxication with a high anion gap and osmolal gap. However, because GBL is readily converted to GHB in the body, only GHB was detected, in this case.Reference Heytens, Neels and Van Regenmortel 10
The pKa of GHB is 4.7, meaning that in physiological conditions 99% of GHB is ionized, leading to excess anions causing HAGMA.Reference Heytens, Neels and Van Regenmortel 10 Textbooks and clinical teaching do not incorporate GHB as a possible cause of HAGMA. Mehta et al. suggested a 21st century mnemonic for HAGMA: GOLDMARK (glycols, oxoproline, L-lactate, D-lactate, methanol, aspirin, renal failure, ketoacidosis).Reference Mehta, Emmett and Emmett 12 We suggest that “G” should stand not only for glycols but also for GHB.
CONCLUSION
In the case of HAGMA and severe neurological depression in the absence of other causes of HAGMA, clinicians should consider an intoxication with GHB as a possible cause.
Competing interests: None declared.







