Introduction
Invasive species, particularly invasive vertebrates, have contributed to c. 60% of historical extinctions (Bellard et al. Reference Bellard, Cassey and Blackburn2016, Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016) and are the primary driver of extinctions on islands (Tershy et al. Reference Tershy, Shen, Newton, Holmes and Croll2015, Bellard et al. Reference Bellard, Cassey and Blackburn2016). They remain one of the main drivers of biodiversity loss, with well-demonstrated negative impacts on terrestrial and marine biodiversity (Littnan et al. Reference Littnan, Stewart, Yochem and Braun2006, Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016, Graham et al. Reference Graham, Wilson, Carr, Hoey, Jennings and MacNeil2018) and indirect impacts on ecosystem function (Peltzer et al. Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010, Beltran et al. Reference Beltran, Kreidler, Van Vuren, Morrison, Zavaleta and Newton2014). The control and eradication of invasive species is a powerful conservation tool for biodiversity, particularly on islands where the eradication of invasive vertebrates has resulted in significant biodiversity benefits (Tershy et al. Reference Tershy, Croll and Newton2012, Jones et al. Reference Jones, Holmes, Butchart, Tershy, Kappes and Corkery2016, Brooke et al. Reference Brooke, Bonnaud, Dilley, Flint, Holmes and Jones2018), yet understanding of how this conservation tool benefits people and the sustainability of economies is limited. It is well established that biodiversity and human well-being are linked (Díaz et al. Reference Díaz, Pascual, Stenseke, Martín-López, Watson and Molnár2018), including evidence that invasive vertebrates impact local economies and food security through crop damage, erosion and biodiversity losses, and some of these invasive vertebrates are also known to transmit zoonotic pathogens to island human residents (Stenseth et al. Reference Stenseth, Leirs, Skonhoft, Davis, Pech and Andreassen2003, Doherty et al. Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016, de Wit et al. Reference de Wit, Croll, Tershy, Correa, Luna-Pasten, Quadri and Kilpatrick2019). An analysis of these benefits is especially relevant for small island developing states (SIDS) and other islands with developing economies, many of which host some of the most globally important, threatened biodiversity (Kier et al. Reference Kier, Kreft, Lee, Jetz, Ibisch and Nowick2009) and isolated human populations with limited economic development opportunities (Pelling & Uitto Reference Pelling and Uitto2001, Scheyvens & Momsen Reference Scheyvens and Momsen2008). There are over 400 000 islands globally, of which at least 1400 maintain highly threatened species (Critically Endangered or Endangered) as listed by the International Union for Conservation of Nature (IUCN). Of these islands, 1113 have at least one invasive vertebrate species and 706 are inhabited by people (Threatened Island Biodiversity Database Partners 2019). A total of 55% of the 706 inhabited islands have developing economies, and 38% are SIDS (Threatened Island Biodiversity Database Partners 2019, United Nations 2019). A comprehensive review of the impacts of invasive vertebrates on biodiversity, ecosystem processes, human well-being and economic development can identify opportunities where eradication can benefit biodiversity, ecosystems and human communities.
Invasive predators such as rodents (Mus musculus, Rattus spp.), cats (Felis catus) and dogs (Canis familiaris) can directly decimate populations of seabird species (Towns et al. Reference Towns, Vernon Byrd, Jones, Rauzon, Russell, Wilcox, Mulder, Anderson, Towns and Bellingham2011) and indirectly reduce the input of nutrients (e.g., guano) into ecosystems (Graham et al. Reference Graham, Wilson, Carr, Hoey, Jennings and MacNeil2018). These nutrients are often important for terrestrial ecosystems, as well as for adjacent reefs and fish nurseries (Polis & Hurd Reference Polis and Hurd1996, Honig and Mahoney Reference Honig and Mahoney2016, Graham et al. Reference Graham, Wilson, Carr, Hoey, Jennings and MacNeil2018). Through compaction, rooting and grazing, invasive ungulates such as goats (Capra hircus), sheep (Ovis aries), cows (Bos taurus) and pigs (Sus scrofa) alter soil structure and nutrient cycling dynamics, causing sediment and nutrient runoff from land to sea and subsequent terrestrial erosion and eutrophication and sedimentation of coastal marine ecosystems (Peltzer et al. Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010, Dunkell et al. Reference Dunkell, Bruland, Evensen and Litton2011, Beltran et al. Reference Beltran, Kreidler, Van Vuren, Morrison, Zavaleta and Newton2014). Similarly, invasive herbivores can reduce rates of carbon sequestration through the consumption of woody plants and seeds (Peltzer et al. Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010, Beltran et al. Reference Beltran, Kreidler, Van Vuren, Morrison, Zavaleta and Newton2014), whereas invasive rodents can indirectly increase rates of aboveground carbon sequestration in islands with seabird populations (e.g., Wardle et al. Reference Wardle, Bellingham, Fukami and Mulder2007).
Invasive species can ultimately affect the ecosystem services or benefits that island communities derive from islands and adjacent coastal ecosystems, including fisheries, water purification and ecotourism (Pejchar & Mooney Reference Pejchar and Mooney2009, Mace et al. Reference Mace, Norris and Fitter2012). The tight links between ecosystem function, ecosystem services (Spangenberg et al. Reference Spangenberg, Görg, Truong, Tekken, Bustamante and Settele2014, Hausknost et al. Reference Hausknost, Grima and Singh2017) and human well-being (MEA 2005) demonstrate how changes in ecosystem composition can quickly lead to effects on human societies. On islands, where the system boundaries are small and firmly defined, ecosystems are often simplified and vulnerable, and alternatives to substitute depleted or damaged resources are extremely limited (Deschenes & Chertow Reference Deschenes and Chertow2004, Chertow et al. Reference Chertow, Fugate, Ashton, Singh, Haberl, Chertow, Mirtl and Schmid2013).
Invasive rodents, cats, dogs, pigs, raccoons (Procyon lotor) and macaques (Macaca spp.) are reservoirs of several zoonotic pathogens (Supplementary Information S1, available online, adapted from de Wit et al. Reference de Wit, Croll, Tershy, Newton, Spatz, Holmes and Kilpatrick2017) (Cotruvo et al. Reference Cotruvo, Dufour, Rees, Bartman, Carr and Cliver2000, Engel et al. Reference Engel, Jones-Engel, Schillaci, Suaryana, Putra, Fuentes and Henkel2002). Many of these pathogens lead to severe health implications for humans, particularly women and children (e.g., Toxoplasma gondii infection and toxocariasis), and especially people living in marginalized communities with limited access to healthcare and clean water (Torgerson & Mastroiacovo Reference Torgerson and Mastroiacovo2013, Torgerson et al. Reference Torgerson, de Silva, Fèvre, Kasuga, Rokni and Zhou2014). Such health impacts are particularly worrisome in developing economies such as SIDS and other small islands, where infectious diseases can feed into poverty traps by burdening families with treatment costs and loss of income, slowing down economic development and perpetuating unsustainable uses of ecosystem services (Bonds et al. Reference Bonds, Keenan, Rohani and Sachs2010, Ngonghala et al. Reference Ngonghala, De Leo, Pascual, Keenan, Dobson and Bonds2017).
Negative impacts of invasive vertebrates can further extend to other areas of local economies, such as food production and storage. Due to their geographical isolation, many SIDS and low-income islands rely on subsistence agriculture or on imported food products that are often housed in inadequate storage facilities, rendering critical foods vulnerable to crop damage, contamination or consumption by invasive vertebrates through crop raids and food storage contamination (Stenseth et al. Reference Stenseth, Leirs, Skonhoft, Davis, Pech and Andreassen2003, Engeman et al. Reference Engeman, Laborde, Constantin, Shwiff, Hall, Duffiney and Luciano2010, Singleton et al. Reference Singleton, Belmain, Brown, Aplin and Htwe2010). Similarly, many island economies rely on tourism activities, including visitation of natural heritage sites, native animal watching, diving, fishing and snorkelling, all of which can be directly or indirectly negatively affected by the presence or the impacts of invasive vertebrates (Beckman et al. Reference Beckman, Hill, Farnworth, Bolwell, Bridges and Acke2014).
The United Nations (UN) 2030 Agenda for Sustainable Development includes 17 Sustainable Development Goals (SDGs) with 169 specific targets. These targets address global challenges such as improving ecosystems and the biodiversity they harbour, human health, education and economic growth (United Nations 2015). Here, we examine whether invasive vertebrate eradication can help build more resilient and sustainable island ecosystems and human communities and examine the potential for eradications to be achieved through international and local collaboration. We developed a framework to assess how the potential biodiversity and socioeconomic benefits of invasive vertebrate eradication align with the UN SDGs, and we use this framework to examine: (1) the SDGs that may have been achieved through past eradications; and (2) how planned future eradications align with SDGs and associated targets. We designed this research to direct future invasive vertebrate eradications towards the sustainability of biodiversity and human communities on islands, some of Earth’s most biologically threatened and socio-ecologically vulnerable systems.
Materials and methods
We grouped the 17 SDGs into the following categories to better describe the socioeconomic, biodiversity and environmental impacts of invasive vertebrates: local economies (Economy: SDGs 1, 4, 8 and 9); peace, justice and equality (Peaceful Living: SDGs 5, 10 and 16); sustainable production and consumption (Sustainable Lifestyles: SDGs 2, 11 and 12); health and sanitation (Health: SDGs 3 and 6); climate change mitigation (Climate Action: SDGs 7 and 13); biodiversity conservation (Conservation: SDGs 14 and 15); and global partnerships (Partnerships for the Goals: SDG 17).
Literature review
We conducted a focused literature review aimed at examining how each of the 169 targets associated with the 17 SDGs could align with invasive vertebrate eradication on islands. We systematically searched Google Scholar for published literature on the socioeconomic and ecological impacts of invasive vertebrates. Our core search consisted of the terms ‘invasive’, OR ‘introduced’ AND ‘vertebrate herbivore’, OR ‘rodent’, OR ‘carnivore’, which are the most common groups of invasive vertebrate species (DIISE 2018), followed by terms associated with each of the SDGs (e.g., ‘agriculture’, ‘economic’, ‘ecotourism’, ‘health’, ‘zoonotic’, ‘neglected tropical disease’, ‘education’, ‘gender equality’, ‘gender inequality’, ‘water sanitation’, ‘water ecosystem’, ‘energy technology’, ‘job creation’, ‘income inequality’, ‘sustainable’, ‘climate change’, ‘carbon sequestration’, ‘marine pollution’, ‘coastal ecosystem’, ‘fisheries’, ‘biodiversity’, ‘conservation ecosystem’, ‘ecosystem service’, ‘peace’, ‘justice’ and ‘international collaboration’). We included articles focused on invasive vertebrates regardless of whether the invasive vertebrate had a positive or negative socioeconomic effect and/or ecological impact. For more extensive reviews on the negative impacts of invasive species on islands, see Medina et al. (Reference Medina, Bonnaud, Vidal, Tershy, Zavaleta and Josh Donlan2011), Doherty et al. (Reference Doherty, Glen, Nimmo, Ritchie and Dickman2016) and Spatz et al. (Reference Spatz, Zilliacus, Holmes, Butchart, Genovesi and Ceballos2017). We included studies regardless of whether the focus was on islands or mainland areas. This non-exhaustive search yielded 140 studies, of which we used 103 reviews and reports, as well as observational and experimental studies that suggested a negative impact of invasive vertebrates on socioeconomic or ecological factors, and which could be linked with the SDG targets (Supplementary Information S2).
Decision tree
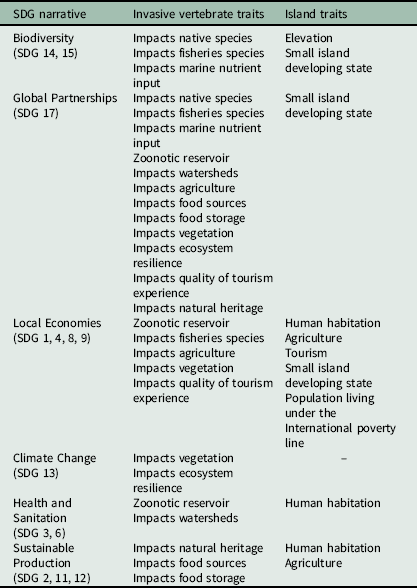
To examine how past and future eradications align with SDGs and their associated targets, we used our literature review of invasive vertebrate impacts to inform a decision tree approach to assigning potential benefits of invasive eradication for each SDG and their associated targets (Supplementary Information S3). Each decision tree contains a set of inclusion criteria based upon the presence of at least one invasive vertebrate and how eradication benefits related to specific SDGs depended upon invasive vertebrate-specific traits such as trophic level (i.e., herbivore, omnivore, carnivore) or zoonotic disease reservoir (Table 1). In addition, we included island-specific traits where necessary to evaluate a potential link to SDG targets, including presence of humans, presence of agriculture, established tourism and whether an island was classified as a SIDS or if a proportion of the population lived below the international poverty line (Table 1).
Table 1. Summary of invasive vertebrate and island-specific traits used to define the inclusion criteria for aligning eradication benefits with the United Nations Sustainable Development Goals (SDGs).
Past and potential future eradication islands
Using the Database of Island Invasive Species Eradications (DIISE 2018), we applied each decision tree to successful, whole-island invasive vertebrate eradications without reinvasion and with good or satisfactory data quality (as defined by DIISE 2018) (794 islands), accessing data in July 2019 and following recommendations for data use in Holmes et al. (Reference Holmes, Keitt, Spatz, Will, Hein, Russell, Russel and West2019a). In order to evaluate the potential benefits from future invasive vertebrate eradications, we applied our decision trees to globally important islands identified by Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b), where the eradication of invasive vertebrates could significantly reduce the risk of extinction to the world’s most threatened biodiversity (species classified as Critically Endangered or Endangered on the IUCN Red List), by concentrating eradication efforts on a small number of islands. Specifically, Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b) used threatened species extinction risk and irreplaceability, severity of impact from invasive species and technical and socio-political feasibility of eradication to identify 169 islands where invasive mammal eradication planning or operation could be initiated by 2020 (107 islands), or 2030 (62 islands) to increase survival prospects of globally threatened vertebrates. In addition, the study identified 49 islands where eradication was not feasible in the foreseeable future, as well as 74 additional islands where the authors did not receive expert opinion on the socio-political feasibility of eradication (Holmes et al. Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b). In total, we included all 292 islands identified by Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b) in our analysis. We included islands in which eradication is currently not feasible to describe the potential sustainable development benefits on islands where future technology is needed to achieve eradication. We designated the eradication of an invasive vertebrate as being in alignment with meeting an SDG if at least one of the associated targets benefitted from this conservation action.
Given the limited socioeconomic information available for most of the identified islands, we made some assumptions for all islands for several inclusion criteria (Supplementary Information S3). We used the DIISE (DIISE 2018) and the Threatened Island Biodiversity Database (TIB; Threatened Island Biodiversity Database Partners 2019) to obtain information on the presence of humans as described in Spatz et al. (Reference Spatz, Zilliacus, Holmes, Butchart, Genovesi and Ceballos2017), and we considered an island inhabited if at least one person was present. The TIB and DIISE also described the type of human habitation present on the island as either permanent community, seasonal community, military, research station, multiple, none or unknown based on available information. Using the Poverty and Equity Data Portal of the World Bank and the international poverty threshold of US$1.90 day–1 (2011 purchasing power parity) established by this organization (World Bank Group 2019), we classified islands as having some proportion of the population living below the international poverty line if any percentage of the population of a country an island was part of lived at or at less than this threshold. However, this list does not include poverty data for developed countries; thus, we assumed developed countries have 0% living below the absolute poverty threshold. We also excluded islands from developing countries for which country-level poverty data were unavailable. In addition, we excluded islands that exclusively held research or military stations if the island belonged to a country that crossed the poverty threshold. We classified islands as SIDS based on the UN classification of the island’s home country (United Nations 2019), regardless of whether the island was a UN Member or Associate Member. To establish whether agriculture was present on human-inhabited islands, we used Google Earth imagery to survey entire islands for visible agricultural plots at an eye altitude of c. 8 km. We defined agricultural plots as human-made and geometrically distinguishable areas with barren soil or vegetation growing in defined rows (e.g., uniform lines of vegetation or sharp boundaries between native vegetation and crops), and we confirmed them at an eye elevation of c. 1.5 km. We assumed an invasive vertebrate impacted human health if the invasive vertebrate species is an identified zoonotic disease reservoir (i.e., rodents, cats, dogs, pigs, raccoons and macaques) (Supplementary Information S1). To establish whether an island has tourism, we performed a Google search (in English) using each island’s name or archipelago followed by the terms ‘tourism’, ‘tours’, ‘visits’, ‘dive’, ‘diving’, ‘snorkelling’, ‘fishing’ and ‘bird watching’. We determined tourism to be present if at least one search indicated that any of the above forms of tourism were present. The tourism activities identified in our search may exist on some islands, but not all, and may underestimate the outcome of whether tourism does occur on the island. We also assumed that all invasive vertebrate eradications are associated with an improvement in the quality of education through the involvement of island and/or country residents in implementation or monitoring efforts (Cromarty et al. Reference Cromarty, Broome, Cox, Empson, Hutchinson, McFadden, Veitch and Clout2002, Varnham et al. Reference Varnham, Glass, Stringer, Veitch, Clout and Towns2011, Glen et al. Reference Glen, Atkinson, Campbell, Hagen, Holmes and Keitt2013, Santo et al. Reference Santo, Sorice, Donlan, Franck and Anderson2015). Overall, our assumptions are intentionally broad in scale and may need further refinement (especially regarding human habitation, ownership and resource use) at the specific island scale. See Supplementary Information S3 for a detailed list of assumptions made for each inclusion criterion.
Results
Of the 17 UN SDGs and 169 associated targets, we found the benefits of invasive vertebrate eradication to align with 13 SDGs and 42 associated targets (Supplementary Information S2). Aligned SDGs include: Goal 1, No Poverty; Goal 2, Zero Hunger; Goal 3, Good Health and Well-Being; Goal 4, Quality Education; Goal 6, Clean Water and Sanitation; Goal 8, Decent Work and Economic Growth; Goal 9, Technology and Innovation; Goal 11, Sustainable Cities and Communities; Goal 12, Responsible Consumption and Production; Goal 13, Climate Action; Goal 14, Life below Water; Goal 15, Life on Land; and Goal 17, Partnerships for the Goals. We found no clear connection between invasive vertebrate eradications and Goals 5 (Gender Equality), 7 (Affordable and Clean Energy), 10 (Reduced Inequalities) and 16 (Peace, Justice and Strong Institutions). Eradications aligned with the following categories: biodiversity conservation (Conservation: SDGs 14 and 15), global partnerships (Partnerships for the Goals: SDG 17), climate change mitigation (Climate Action: SDG 13), local economies (Economy: SDGs 1, 4, 8 and 9), health and sanitation (Health: SDGs 3 and 6) and sustainable production and consumption (Sustainable Lifestyles: SDGs 2, 11 and 12).
We analysed 794 islands (Supplementary Information S4) where invasive vertebrates have been eradicated and 292 islands (Supplementary Information S5) where future invasive vertebrate eradication is a priority for biodiversity conservation based on Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b). We identified 58 unique invasive vertebrate species targeted for eradication (Supplementary Information S6). Forty-four of these species are mammals, including 14 ungulate species, 11 carnivores, 9 rodents, 4 marsupials, 3 lagomorphs, 2 procyonids and 1 primate; the remaining 12 species are non-carnivorous birds, reptiles and amphibians (Supplementary Information S6). Of the 794 islands with past eradications, 121 had confirmed human habitation. From these 121 islands, 18% are classified as SIDS, 21% are classified as having populations living at or below the international poverty line and agriculture was identified on 11% of islands (Table 2). In addition, tourism is present on 76% (602) of the 794 islands. For future eradications on islands identified by Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b), 146 have confirmed human habitation, of which 57% are SIDS, 40% are classified as having populations living at or below the international poverty line and agriculture is present on 50% of islands (Table 2). In addition, tourism is present on 89% (259) of the 292 islands identified by Holmes et al. (Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b).
Table 2. Socioeconomic characteristics of past and future human-inhabited eradication islands.

a Based on any percentage of the population living at equal to or less than US$1.90 per day as established by the World Bank; islands with no data were excluded from analyses, as well as islands with research or military stations (14 past eradication islands and 25 future eradication islands).
Islands with past eradications aligned with a median of 17 SDG targets (range 13–38) (Supplementary Information S4), and islands where future eradication is a biodiversity priority aligned with a median of 25 SDG targets (range 15–39) (Supplementary Information S5). Of the 19 islands where past and future eradications aligned with most SDG targets, rodents were the invasive vertebrate most commonly targeted for eradication (84%), followed by domestic cats (79%) (Table 3). Based on the categories described above, local economies were associated with invasive vertebrate eradications through country and island residents being trained in invasive vertebrate management and monitoring techniques on all islands with past and future eradications, and these were associated with 75% and 88% of islands, respectively, through improvements in tourism opportunities (Fig. 1 & Table 4). Invasive vertebrate eradications aligned with SDGs and associated targets that promote sustainable lifestyles on 15% of past eradication islands and 50% of future eradication islands through the elimination of impacts to local food sources and food storage and through harmony with nature (Table 4). Human health and clean water sanitation were associated with invasive vertebrate eradication on 13.5% and 50.0% of islands with past and future eradications, respectively, through elimination of zoonotic disease reservoirs and through reduced impacts of herbivores on watersheds (Table 4). A total of 83% of past and 90% of future invasive vertebrate eradications were associated with climate change through the elimination of the impacts of invasive herbivores on vegetation and potential net-positive effects on rates of carbon sequestration (Table 4). All invasive vertebrate eradications (past and future) were associated with biodiversity conservation through elimination of invasive vertebrate impacts on terrestrial ecosystems and native species and through elimination of invasive vertebrate impacts on coastal ecosystems (Table 4). All past and future eradications enhance international partnerships, mostly through capacity building and the transfer of invasive vertebrate eradication and monitoring technologies between developed and developing countries (Table 4).
Table 3. Islands where past and future feasible invasive vertebrate eradications align with the most Sustainable Development Goals and targets.


Fig. 1. Percentages of past and future eradication islands that align with the United Nations Sustainable Development Goals.
Table 4. Numbers and percentages of past and future eradication islands that align with the United Nations Sustainable Development targets.

a Islands with no data were excluded from analyses, as well as islands with research or military stations (14 past eradication islands and 25 future eradication islands).
Discussion
We used the UN SDGs to analyse potential biodiversity, socioeconomic and ecological benefits of past and potential future invasive vertebrate eradications on 1086 islands worldwide. Despite being conducted for biodiversity conservation outcomes, we found that eradication of invasive vertebrates from islands contributed directly to the SDGs, aligning with multiple goals and associated targets and encompassing categories that include biodiversity conservation, global partnerships, climate change mitigation, local economies, health and sanitation and sustainable production and consumption. Inclusion of these broader categories can improve individual project evaluation and expand funding opportunities, presenting new opportunities for cross-sector collaboration.
Overall, past eradications aligned with proportionally fewer SDGs and associated targets than potential future eradications. This is mostly because more than half of the SDG decision trees included human habitation as a requisite element of our selection criteria (Supplementary Information S3), and 85% of past eradications primarily occurred on uninhabited islands. On islands with human habitation, eradication of invasive herbivores, rodents or cats aligned with the highest number of SDGs and associated targets through the elimination of impacts on local food sources, food storage and zoonotic disease reservoirs and reduced impacts to watersheds. In most cases, these inhabited islands harboured multiple invasive species, the potential impacts of which on island communities could overlap. However, we did not seek to differentiate the benefits of removing a single versus multiple invasive species. Furthermore, our analysis did not incorporate any potential socioeconomic or ecological benefits that eradications on uninhabited islands could have on neighbouring inhabited islands, nor on the transnational effects of eradications due to the migration of protected species (i.e., seabirds). Research quantifying the direct and indirect impacts of invasive herbivores, rodents and cats on island communities could catalyse partnership opportunities for entities interested in socioeconomic and ecological sustainability development.
Although past eradications were implemented with the aim of protecting island native species (i.e., biodiversity conservation), most eradications also aligned with benefits beyond biodiversity conservation. These non-biodiversity benefits were principally concentrated in SDGs focused on local economies, global partnerships and climate change through the training and capacity building of eradication techniques and monitoring, improvements in tourism opportunities and elimination of impacts on vegetation (Box 1). However, a small number of past eradications (15%) were also associated with SDGs aligned with sustainable production and consumption and health and sanitation, suggesting that invasive vertebrate eradications probably benefited the livelihoods of island communities (Box 2). Importantly, while past eradications were focused on protecting the world’s most threatened biodiversity, future eradications aligned more consistently with SDGs related to sustainable production and consumption and health and sanitation than past eradications, despite being focused on protecting the world’s most threatened biodiversity. This implies that even with a significant biodiversity focus, invasive species eradications provide genuine opportunities to provide ecological and socioeconomic benefits (Box 3). Thus, the eradication of invasive species from islands provides opportunities for scalable cross-sector sustainability development.
Box 1. Carbon sequestration and invasive sheep eradication.
Invasive herbivores can reduce rates of carbon sequestration by changing the ecosystem structure through the introduction and spread of non-native plant species, by eroding the soil and by reducing plant cover (Reaser et al. Reference Reaser, Meyerson, Cronk, De Poorter, Eldrege and Green2007, Peltzer et al. Reference Peltzer, Allen, Lovett, Whitehead and Wardle2010). Twenty-eight years after invasive sheep (Ovis aries) were eradicated from Santa Cruz Island, California (Bowen & Van Vuren Reference Bowen and Van Vuren1997), overall bare ground cover decreased and woody over-story cover increased, resulting in an estimated 97% increase of above and below ground carbon storage (1.73 versus 3.41 Tg C pre- versus post-eradication, 1 Tg = 1012 g; Beltran et al. Reference Beltran, Kreidler, Van Vuren, Morrison, Zavaleta and Newton2014) (Box Fig. 1). There are 194 islands globally where invasive herbivores have been eradicated (DIISE 2018) and 32 islands with globally threatened vertebrates where future invasive herbivore eradications are feasible (Holmes et al. Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b) (Supplementary Information S4 & S5). Compared to global carbon budgets, the significance of carbon gains due to invasive herbivore eradication would probably be limited due to the small size of islands (Holdaway et al. Reference Holdaway, Burrows, Carswell and Marburg2012). Nevertheless, carbon sequestration resulting from invasive herbivore eradication could still make positive contributions towards national commitments and indirectly align with climate change mitigation United Nations Sustainable Development Goals.

Box Figure 1. Landscape vegetation changes on Santa Cruz Island, California (a) pre-eradication (March 1980) and (b) post-eradication (May 2008) of sheep. Figure adapted from Beltran et al. (Reference Beltran, Kreidler, Van Vuren, Morrison, Zavaleta and Newton2014).
Box 2. Human health benefits of cat eradication.
The majority of islands do not harbour native cats, and invasive cats (Felis catus) are the sole reservoirs of the zoonotic parasite Toxoplasma gondii (Dubey Reference Dubey1998). Infection can cause miscarriage and severe ocular and neurological lesions in new-borns (Torgerson & Mastroiacovo Reference Torgerson and Mastroiacovo2013, Maenz et al. Reference Maenz, Schlüter, Liesenfeld, Schares, Gross and Pleyer2014, Ngô et al. Reference Ngô, Zhou, Lorenzi, Wang, Kim and Zhou2017). Invasive cats were eradicated from Isla Natividad, Mexico, resulting in complete elimination of the sole reservoir of T. gondii on the island. Human health benefits of cat eradication on Natividad were assessed by measuring serological exposure (seroprevalence) to T. gondii, and the results show that seroprevalence was significantly lower on Natividad compared to five other inhabited islands in the region where invasive cats were present (de Wit et al. Reference de Wit, Croll, Tershy, Correa, Luna-Pasten, Quadri and Kilpatrick2019) (Box Fig. 2). Similarly, seroprevalence of children born after cats were eradicated was 0% and significantly lower than in children of that same age group from the three islands with cats that had comparable sample sizes. Invasive cats have been eradicated from 30 human-inhabited islands globally, and their eradication is feasible on another 40 islands (Holmes et al. Reference Holmes, Spatz, Oppel, Tershy, Croll and Keitt2019b) (Supplementary Information S4 & S5). Invasive vertebrate eradication can have tangible human health benefits, particularly if the invasive vertebrate target is a zoonotic pathogen reservoir. Island inhabitants can further benefit from these eradications if health services and infrastructure on the island are limited and if the consequences of the disease result in unsustainable costs associated with diagnosis, treatment and labour disability.

Box Figure 2. Age-adjusted seroprevalence of Toxoplasma gondii in people from Natividad Island, Mexico (white) compared to the seroprevalence in people from five islands of the same region where cats were present (grey). Figure adapted from de Wit et al. (Reference de Wit, Croll, Tershy, Correa, Luna-Pasten, Quadri and Kilpatrick2019).
Box 3. Agricultural and economic impacts of invasive macaques.
The socioeconomic impacts of non-native non-hominid primates such as macaques (Macaca fascicularis) have been documented on several islands (Engeman et al. Reference Engeman, Laborde, Constantin, Shwiff, Hall, Duffiney and Luciano2010, Jones et al. Reference Jones, Campbell, Burke, Baxter, Hanson and Mittermeier2018). Aside from the potential for macaques to transmit zoonotic pathogens (e.g., Cercopithecine herpesvirus 1-B virus) (Engel et al. Reference Engel, Jones-Engel, Schillaci, Suaryana, Putra, Fuentes and Henkel2002, Huff & Barry Reference Huff and Barry2003) and engage in violent behaviour against people (mostly children), macaques are known to take advantage of food sources form agriculture (Jones et al. Reference Jones, Campbell, Burke, Baxter, Hanson and Mittermeier2018). In Puerto Rico, the economic losses on commercial farms caused by crop raiding from non-native non-hominid primates have reached up to US$1.46 million per year (Engeman et al. Reference Engeman, Laborde, Constantin, Shwiff, Hall, Duffiney and Luciano2010). On Angaur Island, Palau, macaques raid fruit crops, causing significant economic and social impacts, as well as exacerbation of gender inequalities, as management of these crops is one of the main economic activities for women on the island (McGregor & Bishop Reference McGregor and Bishop2011). As a consequence, macaques on Angaur Island are currently a target for eradication for multiple purposes, including biodiversity conservation, food security and gender and income equality (McGregor & Bishop Reference McGregor and Bishop2011).
The socioeconomic consequences of invasive vertebrate eradication should be considered on an island-by-island basis, since there may be benefits derived from the presence of invasive species to island inhabitants. For example, local inhabitants on some islands hunt invasive herbivores such as goats, sheep, cows and pigs for food, or as part of their culture (Pejchar & Mooney Reference Pejchar and Mooney2009). Therefore, eradication of these species could substantially limit access to important food sources. The eradication of invasive predators (e.g., cats) can result in changes in rodent population dynamics (Rayner et al. Reference Rayner, Hauber, Imber, Stamp and Clout2007), potentially impacting food production and storage and the transmission dynamics of rodent-borne diseases. Eradication of certain invasive vertebrates such as non-hominid primates can affect the tourism industry on islands, as these species can be tourist attractions (Serio-Silva Reference Serio-Silva2006). Evaluating all of the potential positive and negative consequences of invasive vertebrate eradication and incorporating them into feasibility assessments of invasive vertebrate management will dictate the applicability of this conservation tool to additional areas of sustainable development.
Our study demonstrates that past and future invasive vertebrate eradications create multiple socioeconomic and ecological benefits and highlights the potential for invasive vertebrate eradication to be used as an effective sustainable development tool for island communities and ecosystems. Although our results are based on multiple assumptions due to the limited availability of specific and accurate socioeconomic data for most islands (e.g., poverty, agriculture, tourism, ownership, resource use), there is growing interest, beyond biodiversity conservation, in the impacts of invasive vertebrates on local communities (Pejchar & Mooney Reference Pejchar and Mooney2009, Shackleton et al. Reference Shackleton, Larson, Novoa, Richardson and Kull2019). Additional research, focused upon specific islands, is needed in order to more explicitly assign potential economic, human health and social outcomes from conservation actions such as invasive vertebrate eradication. Unlike many conservation and social interventions, invasive vertebrate eradication is usually a one-time intervention conducted over short timeframes that can simultaneously result in the implementation of sustainable food production and consumption systems, improvements in human health and water quality, generation of employment and opportunities for climate change mitigation. Thus, ongoing or long-term investment in intervention is not necessary, and benefits can be realized over relatively short timeframes (Jones et al. Reference Jones, Holmes, Butchart, Tershy, Kappes and Corkery2016). These potential outcomes of invasive vertebrate eradication are particularly important for SIDS and other developing islands that are vulnerable to economic and environmental instability. Our study enables the incorporation of economic development and human health and well-being into the narrative and rationale for invasive vertebrate management, while offering an effective tool for working towards the achievement of the UN 2030 Sustainable Development Agenda.
Invasive vertebrate eradications can have far-reaching and mutual benefits for biodiversity and the human communities that rely on islands. Eradication of invasive vertebrates can serve as a nature-based solution for countries to help meet their contributions to the UN SDGs. In addition, because islands are particularly vulnerable to the impacts of climate change, vertebrate eradications can enhance much-needed island resilience (Spatz et al. Reference Spatz, Zilliacus, Holmes, Butchart, Genovesi and Ceballos2017). By evaluating the ability of eradication projects to promote human well-being and biodiversity conservation, we hope to promote focused investment and innovation in insular vertebrate eradications, so that future eradication efforts can be adjusted in scope and scale to best support the SDGs of improved health and well-being, economic development, environmental restoration and the future of life on Earth.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0376892920000211
Acknowledgements
The authors would like to thank Jamie Bechtel, Hazel Thornton, Lauren Weatherdon and Holly Brooks for their insightful comments.
Financial support
This work was supported by a grant from the Willow Grove Foundation and Charities Aid Foundation Canada. LAdW would like to thank the Gund Institute for Environment for institutional support.
Conflict of interest
None.
Ethical standards
None.










