Worldwide, gastrointestinal nematodes (GIN) remain the main challenge associated with small ruminant production since these infections impose a serious problem on health, welfare, productivity, and reproduction (Oliveira Santos et al., Reference Oliveira Santos, MoraisnCerqueira, Branco, Moreira Batatinha and Borhes Botura2019; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022; Nolinda et al., Reference Nolinda, Ikusika, Akinmoladun and Mpendulo2024). Goats and sheep are infected with the same GIN species, mainly Haemonchus contortus, Trichostrongylus axei, Teladorsagia circumcincta (abomasum), T. colubriformis, Strongyloides papillosus, Nematodirus spp. (small intestine), Oesophagostomumco lumbianum and O. venulosum (large intestine: Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022). Since the mid-1960s, GIN control in livestock has relied heavily on anthelmintics (Froehlich et al., Reference Froehlich, McAnulty and Greer2022; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022), however, over-dependency and misuse of these drugs has led to anthelmintic resistance that has now become a serious problem on small ruminant farms in many countries (Oliveira Santos et al., Reference Oliveira Santos, MoraisnCerqueira, Branco, Moreira Batatinha and Borhes Botura2019; Froehlich et al., Reference Froehlich, McAnulty and Greer2022; Nolinda et al., Reference Nolinda, Ikusika, Akinmoladun and Mpendulo2024). Today, not only the efficient control of GIN infections is essential to improve livestock health and production (Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022), but also widespread resistance to synthetic anthelminthic has inspired research for alternative strategies of parasite control, including the use of plant extracts (Oliveira Santos et al., Reference Oliveira Santos, MoraisnCerqueira, Branco, Moreira Batatinha and Borhes Botura2019; Nolinda et al., Reference Nolinda, Ikusika, Akinmoladun and Mpendulo2024).
It is well documented that natural-derived feedstuffs can contain a wide array of plant secondary metabolites (PSM) arranged in a variety of chemical classes (Castagna et al., Reference Castagna, Piras, Palma, Musolino, Lupia, Bosco and Britti2021) that may provide an alternative, cost-effective treatment to gastrointestinal parasites, limiting both drug use and development of anthelmintic resistance (Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021; Froehlich et al., Reference Froehlich, McAnulty and Greer2022). It has been suggested (Oliveira Santos et al., Reference Oliveira Santos, MoraisnCerqueira, Branco, Moreira Batatinha and Borhes Botura2019; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022) that feeding small ruminants with tannin-rich plants (30–40 g of condensed tannins per kg DM) promotes anthelminthic effect. However, condensed tannins, at higher concentrations (7–8% DM), can depress feed intake, decrease nutrient digestibility and overall production (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016; Castagna et al., Reference Castagna, Piras, Palma, Musolino, Lupia, Bosco and Britti2021).
Garlic (Allium sativum) has been reported to be a parasiticide, amebicide, larvicide and immune-stimulant, so garlic essential oil can be used as an alternative or supplement to the current anthelmintic therapies (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011; Zhong et al., Reference Zhong, Xiang, Cheng, Zhao, Wang, Zhao and Fang2019). Garlic powder has been shown to increase rumen propionate, increase N retention and absorption in cattle (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011). Garlic oil has been shown to exhibit a wide spectrum of antibacterial activity against Gram-negative and Gram-positive bacteria (Zhong et al., Reference Zhong, Xiang, Cheng, Zhao, Wang, Zhao and Fang2019) which have been related to several small terpenoid and phenolic compounds (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011).
Little is known about the nutritive value of willow (Salix babylonica; Salix) in goat diets, however, extracts of Salix have been evaluated as feed additives in ruminant diets due to their anti-microbial effects and ability to modulate ruminal fermentation and improve nutrient utilization (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016). In addition, Salix extract enhanced digestibility and average daily gain of lambs (Salem et al., Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a), and improved milk production of cows (Salem et al., Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014b). It is also well established that Salix has natural anthelmintic activity (Cedillo et al., Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015; Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016; Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021).
Until now, the effects of using rumen bypass (for example, calcium soaps) plant extracts have not been analyzed in dairy goat nutrition and parasite loads. Therefore, the aim of this study was to determine the effect of using dietary calcium soaps with garlic and Salix extracts on nutrient intake and digestibility, nitrogen balance and rumen fermentation kinetics in dairy goats, as well as studying their effect on gastrointestinal nematode load. The hypothesis of this study is that calcium soaps of plant extracts will not affect intake. Results from this study will improve our knowledge on plant material alternatives to the use of conventional anthelmintic drugs.
Materials and methods
Goats, experimental design, and dietary treatments
All animal studies were conducted according to the Animal Care and Use Committee of Veterinary and Animal Science from Universidad Autonoma del Estado de México (Toluca, Mexico) (Project ID UAEMex4335/2017). Nine adult non-lactating Saanen does were grouped into a complete randomized block design with 3 treatments (n = 3). At the onset of the study, all goats were adapted to the diets for 7 d, and then were weighed. Individual fecal samples were taken, and the fecal egg count and parasite identification were determined. At the onset of the study, average body weight was 36.7 ± 6.2 kg (mean ± sd) while body condition score (scored on a 5-point scale) was 2.0 ± 0.1. For 28 d, animals were fed ad libitum a basal diet consisting of 60% forage (alfalfa hay) and 40% concentrate (containing crushed rapeseed, sorghum grain, soybean meal, wheat grain, and corn stover) supplemented with calcium soaps of safflower, garlic, or Salix extract as potential anthelmintic (Tables S1 and S2), with free access to water. Garlic and Salix extracts were prepared weekly, and the process of fat saponification was performed following the double decomposition method of Jenkins and Palmquist (Reference Jenkins and Palmquist1984), using safflower oil as control. The concentrate was supplemented with 65 g/kg DM of calcium soaps from safflower (control), garlic, or Salix extract and treatments were manually mixed. Animals were housed in individual roofed pens with concrete flooring and straw bedding. Individual body weights were recorded on days 0, 7, 14, 21 and 28.
Experimental measurements, samplings, and analyses
All goats were naturally infected with gastrointestinal parasites. The animals were grazing a pasture where they are naturally infested with parasites and these animals had not been dewormed in the last 6 months prior to the study. Animals were divided into three groups. Animals were monitored for parasite loads of Haemonchus contortus, Monienzia spp., Ostertagia spp., Chabertia spp., and Trychostrongilus spp. Fecal samples (4 g/animal) were taken from the rectum on days 0, 14, 21 and 28. Fecal samples were removed using examination gloves with lubricant, and then samples were individually bagged, labeled, and kept refrigerated at 4°C until further analysis. Fecal samples were analyzed on the same day of the collection, where the number of eggs per gram of feces (FEC) as fresh matter were quantified using the modified McMaster technique (Boareki et al., Reference Boareki, Schenkel, Willoughby, Suarez-Vega, Kennedy and Cánovas2021). Briefly, fecal samples of 4 g were mixed well with 56 mL of flotation solution. The mixture was then strained and put in the two-chamber McMaster slide and allowed to sit for 5 min before counting the eggs under microscope. The fecal egg count reduction per gram of feces was determined according to the method described by Coles et al. (Reference Coles, Bauer, Borsteede, Geerts, Klei, Taylor and Waller1992).
After 28 d of the nutritional intervention, for 5 d, all animals were housed in a roofed pen with individual metabolic cages (1.20 × 0.80 m) with slatted floor and had free access to water. Each cage had individual drinkers and feeders and the goats were fed with their individual diet. During those days, daily records of feed intake, and amount of feces and urine were collected. Samples of feed, feces and urine were collected at 0800 h and were frozen at −20°C until analyzed. For feces and urine, only 10% of the total sample collected was used for analysis. Feces and urine samples were used to determine N intake and excretion. Nutrient digestibility was determined as [(nutrient intake, g/d– nutrient excreted, g/d)/(nutrient intake, g/d)] × 1000.
In vitro gas production (GP) test was performed according to Theodorou et al. (Reference Theodorou, Williams, Dhanoa, McAllan and France1994). Briefly, 800 mg of DM of each diet was placed in 125 ml glass flasks and 90 ml of buffer solution and 10 ml of rumen fluid were added to them. Three incubation runs were carried out as replicates. In each incubation run, three flasks per treatment were used and a set of appropriate blanks were included. Rumen fluid (300 ml per animal) was collected from the three-rumen cannulated lactating goats into a pre-warmed thermos flask, and then filtered and flushed with CO2. GP was measured using a pressure transducer (Delta Model HD 8804, Italy) at 6, 12, 24, 48, 72, and 96 h and a set of blanks were included.
Detailed information on in vitro experimental procedures and calculation as well as preparation of the extracts and calcium soaps are given in the online Supplementary File.
Statistical analysis
A MIXED model of repeated measurements was implemented to analyze the effect of garlic and Salix administration in goat on in vivo variables using R statistical program. The model included the fixed effects of treatment, time of measurement, the random effect of goat nested within treatment and the residual error. Interactions between treatments and sampling times were included in the model if they were significant. The results are presented as least squares means (LSM). The differences in LSM were tested using the Tukey–Kramer procedure. For all data, significance was declared at P ≤ 0.05. Each variable analyzed was subjected to three covariance structures including autoregressive order, ante-dependence and unstructured and the one resulting in the lowest Akaike information criterion was chosen. For the dependent variables body weight (BW), metabolic BW and BW change, BW at baseline was used as a covariate.
The data were tested for normality using the Shapiro–Wilk test and since most of the FEC variables were not normally distributed they were transformed prior to analysis. Fecal egg counts were transformed as ln (FEC + 100) prior to analysis to improve normality. The resulting LSM were presented on either of the transformed scales and after back-transformation of LSM as (eLSM −25) and standard error (se) of back-transformed means were estimated by multiplying them by se of LSM. The percentages of FEC for each parasite genus to be derived from the frequency of counts were subjected to an angular or arcsine transformation. However, as the arcsine transformation only works on values between 0 and 1, we first converted each value using the following formula: y = x/max(x) where y is the transformed value, x is the original value and max is the highest value in the data set.
Results
Intake of dry matter, organic matter (OM), neutral detergent fiber (NDF), and acid detergent fiber (ADF) were similar between treatments (P > 0.05, Table 1). The highest digestibility of DM (P = 0.02) and OM (P < 0.01) was observed in Salix supplemented goats. No significant differences were observed in N intake, fecal N excretion, urinary N excretion, and N balance between diets (Table 1).
Table 1. Nutrient intake, digestibility and nitrogen balance of goats fed with calcium soaps of garlic and Salix extracts

sem, Standard error of the mean.
a–cMeans with different superscript letters within a row are different (P ≤ 0.05).
1 Diets supplemented with 65 g/kg DM of calcium soaps from either safflower (control), garlic extract (garlic), or Salix babylonica extract (salix), respectively.
Total FEC reduced (P = 0.001) with ingestion of both calcium soaps. The goats fed garlic and Salix had approximately 6.9 and 5.6% lower total FEC compared to the control group (Table 2). There were no interaction effects for any of the variables. The resistance was higher (P < 0.001; Table 2) for the control group compared to salix and Garlic groups. The count of fecal parasite eggs (%) of Haemonchus spp. (P < 0.05) and Trychostrongylus spp. (P < 0.001) was lower for garlic and Salix than control (Fig. S1). No significant differences were found between treatments for Chabertia spp., Moniezia spp. and Ostertagia spp. The highest and lowest concentration of Haemonchus spp. was at day zero and day 28, respectively and the effect of time was significant (P = 0.001). The rest of the parasite eggs were similar in time and there were no treatment and time interactions.
Table 2. Anthelmintic effect of calcium soaps of garlic and salix extracts on performance and fecal parasite eggs in goats
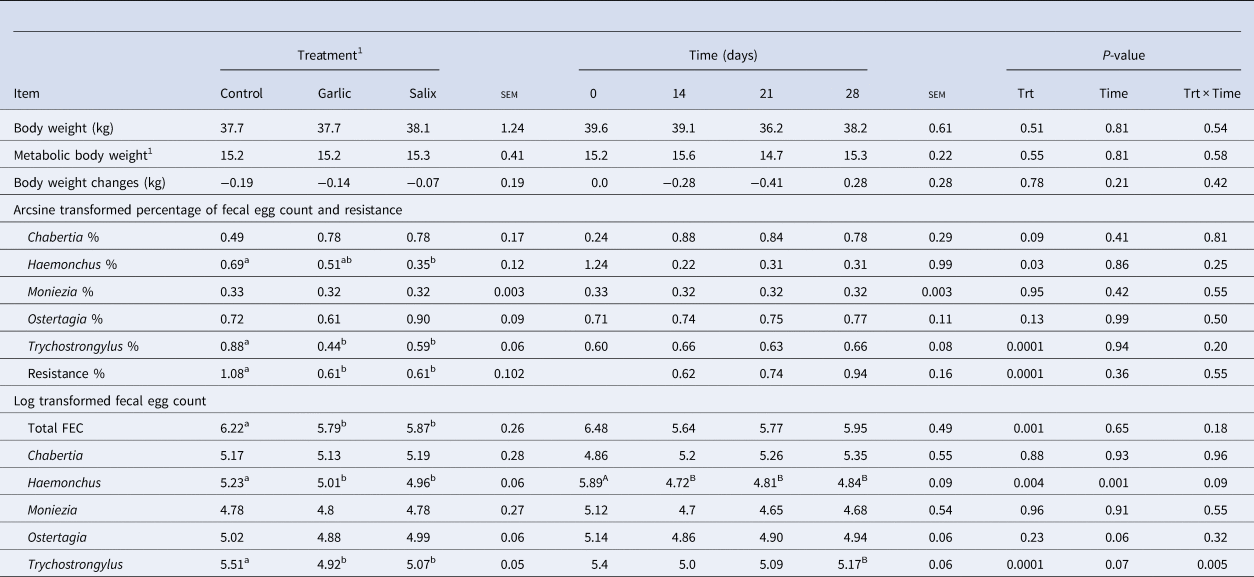
a,b,A,B,Means with different superscript letters within a row are different to treatment (lower case) and time (upper case), respectively (P ≤ 0.05). 1Metabolic live weight = (Live Weight)0.75.
Total GP, DM, gas yield at 24 h, estimated short-chain fatty acids, microbial crude protein and metabolic energy were similar between treatments (Table S3). Also, in vitro fermentation kinetics were not affected (Table S3 and Fig. S2).
Discussion
Finding alternatives to control GINs in livestock is not easy (Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021; Froehlich et al., Reference Froehlich, McAnulty and Greer2022), but natural compounds and plant extracts are a promising move in this direction. Thus, this study aimed to investigate the effect of using calcium soaps of garlic or Salix extracts on GIN control in goats as well as their impact on nutrient intake, digestibility, and N balance. The results of these field tests not only highlighted the importance of anthelmintic efficacy studies of ethnoveterinary remedies, but also pave the way for the use Salix and garlic as potential alternative and sustainable methods to reduce the use of chemicals.
GIN infection in small ruminants has been found to decrease DMI, nutrient digestion and weight gain of host animals (Froehlich et al., Reference Froehlich, McAnulty and Greer2022; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022). Our results revealed that ingestion of calcium soaps of Salix at doses of 65 g/kg DM led to higher body weight besides an enhanced digestibility of DM and OM, and we observed similar but lesser effects with garlic. However, intake of DM, OM, NDF, and ADF were not affected by dietary calcium soaps. The effects of feeding garlic extracts on nutrient digestibility and livestock growth are not consistent in the literature. Some studies (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011; Zhong et al., Reference Zhong, Xiang, Cheng, Zhao, Wang, Zhao and Fang2019) reported that the intakes and body weight of GIN-infected goats increased with garlic supplementation, whereas others (Zhong et al., Reference Zhong, Xiang, Cheng, Zhao, Wang, Zhao and Fang2019) reported that dietary garlic supplementation had no effects on DMI and weight gain of lambs with parasitic infection. However, the present improvement of nutrient digestibility in Salix fed goats may be due to positive impacts of plant secondary metabolites (PSM) on ruminal fermentation and nutrient digestibility (Salem et al., Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014b). It has been reported (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016) that 59 of these PSM (including tritetracontane, hexadecanoic acidmethyl ester, phytol and aliphatic hydrocarbons) were identified in Salix, however, none of the individual active compounds was tested for its effect on animal nutrition. In contrast, previous publications (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016) have shown that ingestion of Salix secondary metabolites increases digestibility of NDF and ADF. In agreement, Jiménez-Peralta et al. (Reference Jiménez-Peralta, Salem, Mejia-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011) suggested that extracts of Salix could positively modify rumen gas production and fermentation activities, which may improve nutrient utilization in lambs mainly due to its rich content of PSM. Moreover, it is well known (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016) that the PSM of Salix extract such as phenolic compounds, alkaloids, or saponins could improve synchronization between energy and N release and improve microbial protein synthesis that positively affects body weight. Indeed, saponins have antimicrobial properties on ciliate protozoal growth, peptidase-producing bacteria and cellulolytic bacteria (Froehlich et al., Reference Froehlich, McAnulty and Greer2022) which normally effect short-chain fatty acid production (i.e., acetate, not propionate; Salem et al., Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014b) which could improve body weight gain. In line with our results, Cedillo et al. (Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015), in a 72-day trial, showed that Salix extract (at doses 20 and 40 ml/lamb/d) increased BW of lambs by about 5 and 8.2%, respectively.
Overall, our results demonstrated that the calcium soap of Salix is expected to be beneficial to digestibility of DM and OM based on a stimulating effect on fermentation. This will increase microbial protein production, which will positively affect body weight changes (Jiménez-Peralta et al., Reference Jiménez-Peralta, Salem, Mejia-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Cedillo et al., Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015). Nitrogen balance is considered as an important index of protein status in ruminants (Jenkins and Palmquist, Reference Jenkins and Palmquist1984). N intake and N balance were not affected by dietary garlic and Salix supplementation. However, numerically (but non-significant), higher N balance in both garlic and Salix diets was found and this was probably due to bioactive components being available in those extracts. It has been shown (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011) that garlic bioactive components might have a positive impact on N balance by influencing microbial proteolytic activities of rumen fluid in sheep fed with garlic silage diet. Previously, the favorable impact of Salix on N balance has been reported (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016), since its PSM prevented microbial degradation of plant proteins, causing an increased amino acid flow to the duodenum from the rumen (Salem et al., Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a). Besides having a protective effect on protein in the rumen, Salix extract can promote duodenal absorption of amino acids and enhance the N balance (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016). However, the effect of PSM on N balance is still not clear and there are inconsistencies (Kamruzzaman et al., Reference Kamruzzaman, Torita, Sako, Al-Mamun and Sano2011; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022). Future studies should consider the use of Salix and garlic extracts focusing on rumen function and N retention. Likewise, the in vitro ruminal gas kinetics and fermentation profile of goats fed calcium soaps of garlic and Salix extracts were not affected. It is well known (Theodorou et al., Reference Theodorou, Williams, Dhanoa, McAllan and France1994) that gas production is generally an indicator of nutrient digestibility and fermentability, as well as rumen microbial protein production. Salix extract was expected to be beneficial to rumen function based on a stimulating effect on nutrient digestibility and microbial protein production (Salem et al., Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014b). It has also been reported that moderate doses of Salix extract (0.6 and 1.2 ml/g DM) but not high (1.8 ml/g DM) had positive impact on ruminal GP and fermentation parameters. This could be explained by the ability of rumen microorganisms to degrade low and moderate levels of PSM in plant extracts (Salem et al., Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014b; Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021). Previous studies have shown (Jiménez-Peralta et al., Reference Jiménez-Peralta, Salem, Mejia-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011; Castagna et al., Reference Castagna, Piras, Palma, Musolino, Lupia, Bosco and Britti2021) that the effect of PSM and plant extracts on ruminal fermention parameters are often influenced by the physicochemical composition of the diet, and the structure of the forage as well as the level of PSM use. In our study, since it is hard to separate the effects of all these factors, possibly either or none of these factors affect GP or some of these factors neutralized the other effects.
Our calcium soaps of Salix extract showed promising results for controlling Trychostrongylus spp. and Haemonchus, spp. probably due to the presence of tannins (Cedillo et al., Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015). Consistently, in vitro studies have shown that Salix extracts have significant activity against ruminant nematodes (Jiménez-Peralta et al., Reference Jiménez-Peralta, Salem, Mejia-Hernández, González-Ronquillo, Albarrán-Portillo, Rojo-Rubio and Tinoco-Jaramillo2011). The anthelmintic efficacy of Salix extract was confirmed by Cedillo et al. (Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015). In addition, Castagna et al. (Reference Castagna, Piras, Palma, Musolino, Lupia, Bosco and Britti2021) indicated that the weekly administration of an aqueous extract of Salix (at a dose of 20 ml/animal) reduced the fecal egg and oocyst counts of many parasitic species in both sheep and goats. Hence, it would be reasonable to suggest that the observed anthelmintic activity of the Salix extracts against different nematode species could be attributed to the presence of PSM. It is well established that condensed tannins have shown anthelmintic activity against gastrointestinal nematodes of sheep and goats (Oliveira Santos et al., Reference Oliveira Santos, MoraisnCerqueira, Branco, Moreira Batatinha and Borhes Botura2019). A recent paper (Nolinda et al., Reference Nolinda, Ikusika, Akinmoladun and Mpendulo2024) has demonstrated that the anthelmintic effects of PSM are associated with the deactivation of several key enzymes within the parasite. In addition, condensed tannins that can be found in Salix, can impair parasite feeding because tannins bind free proteins for larvae nutrition causing larval starvation and death, and tannins can disrupt the integrity of parasite cuticles (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016). Moreover, Salix's tannins can affect parasites in the gastrointestinal tract directly through the inhibition of oxidative phosphorylation, causing larval death (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016). Cedillo et al. (Reference Cedillo, Kholif, Salem, Elghandour, Vazquez, Alonso, Barbabosa, Chagoyan and Reyna2015) reported that Salix extract contains considerable amounts of PSM (namely saponins, alkaloids, tannins, other polyphenols, nonprotein amino acids, lignin, and glycoside) all of which demonstrated antiparasitic effects. Salem et al. (Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016) suggested that tannins are not solely responsible for the anthelmintic activity of Salix extract, but it is likely that alkaloids in the plant extract may also contribute to the paralysis and consequent worm death. In confirmation, extracts of several tannins and alkaloids have been shown to cause a delay or total inhibition of the ensheathment process of H. contortus and T. colubriformis larvae (Froehlich et al., Reference Froehlich, McAnulty and Greer2022; Nolinda et al., Reference Nolinda, Ikusika, Akinmoladun and Mpendulo2024). In this study, tannins were not quantified, and neither was larval ensheathment, however, those factors should be explored further. Previously, Zhong et al. (Reference Zhong, Xiang, Cheng, Zhao, Wang, Zhao and Fang2019) have reported garlic parasiticidal properties against Ostertagia spp. and Trychostrongylus spp. However, the present study used calcium soaps of garlic extract as a nematode control strategy as nematodes have become resistant to commercial drugs (Dehuri et al., Reference Dehuri, Palai, Mohanty and Malangmei2021; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022). The presence of drug-resistant helminths represents an important restriction for the sustainability of the current helminth control strategies in sheep and goats. These types of anti-helminthic chemicals have negative impact on agroecosystems (Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022). Our results showed that both garlic and Salix calcium soaps possess anthelmintic activities due to their rich PSM profile. However, it is very important to point out that higher concentrations of the active compounds may cause antinutritional effects, in some cases, such as a reduction in feed intake and performance (Salem et al., Reference Salem, Kholif, Olivares, Elghandour, Mellado and Arece2014a). It is suggested to carry out further assays to assess the isolation, development, and validation of the effects of these herbal extracts to provide evidence that might support their wider acceptance (Salem et al., Reference Salem, Elghandour, Kholif, López, Pliego, Cipriano-Salazar, Chagoyán, de Oca-Jiménez and Alonso2016; Hoste et al., Reference Hoste, Meza-OCampos, Marchand, Sotiraki, Sarasti, Blomstrand and Morgan2022).
In conclusion, we investigated the effects of adding 65 g/kg DM of calcium soaps from garlic or Salix extracts in goat diets, and the findings revealed that both treatments not only enhanced digestibility of DM and OM but also reduced total FEC and fecal egg resistance, as well as reducing fecal parasite eggs of Haemonchus spp. and Trychostrongylus spp. Results from this study suggest that calcium soaps from garlic or Salix extracts can be used for nematode control without deleterious effects on performance, N balance and rumen fermentation kinetics of goats.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029924000141
Acknowledgments
This study was funded by the Autonomous University of the State of Mexico, with project UAEM 4335/2017. During the study, Dr Einar Vargas-Bello-Pérez was a visiting scholar also supported by project number 4974/2020CIB from Universidad Autónoma del Estado de Mexico.




