Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterised by β cell dysfunction and insulin resistance, which is increasingly acknowledged as one of the most prevalent public health issues, particularly in low-income and middle-income nations(Reference Khan, Hashim and King1). T2DM is expected to affect 4·63 billion individuals worldwide (9·3 %), and its toll on public health is growing(Reference Safiri, Karamzad and Kaufman2). Disability-adjusted life year and mortality rates due to T2DM were reported to be 801·5 and 18·5, respectively, per 100 000 people in age-standardised terms in 2019(Reference Safiri, Karamzad and Kaufman2).
Oxidative stress plays an important role in the onset and progression of T2DM by failing β cells and promoting insulin resistance(Reference Luc, Schramm-Luc and Guzik3). Antioxidant deficits prior to the initiation of T2DM may be a sign of its progression(Reference Harding, Wareham and Bingham4–Reference Mason, Parker and van der Pligt6), which has led to extensive research into the health-promoting effects of dietary antioxidants, specifically vitamin A, vitamin C, vitamin E, Mg and Zn, in the prevention of T2DM, but the findings of these studies are inconsistent(Reference Sacco, Pellegrini and Roncaglioni7–Reference Schulze, Schulz and Heidemann15). This discrepancy may be caused by wide variations in dietary antioxidant intake(Reference Costarelli, Muti and Malavolta12) and their source of intake (diet v. supplement) in various researches(Reference Montonen, Knekt and Järvinen8,Reference Bjelakovic, Nikolova and Gluud16) , as well as characteristics of the study participants, such as glucose tolerance status(Reference Gunasekara, Hettiarachchi and Liyanage17). Moreover, as a well-known risk factor for oxidative stress status, obesity may alter the dietary antioxidants in preventing T2DM(Reference Harding, Wareham and Bingham4,Reference Neeland, Poirier and Després18,Reference Zhou, Na and Shan19) . Obesity increases the requirements of dietary antioxidants; according to some studies, in the association between dietary antioxidants and incident T2DM, the obesity status must be considered(Reference Harding, Wareham and Bingham4,Reference Sacks, Bray and Carey20) . However, few studies have investigated the effect of modifying obesity on the association between dietary antioxidants and T2DM(Reference Dong, Xun and He21–Reference Zhao, Zeng and Zhao25). In a meta-analysis, the beneficial effects of Mg on the risk of T2DM were more evident among individuals with a BMI ≥ 25 kg/m2; however, the interaction test was not significant(Reference Dong, Xun and He21). In addition, BMI status was not found to be a potential modifier for these associations in another meta-analysis(Reference Zhao, Zeng and Zhao25). However, the findings of the Women’s Health Study(Reference Song, Manson and Buring22), a large cohort of American women, revealed that an inverse association between Mg intake and T2DM was significantly more pronounced among subjects with BMI ≥ 25 kg/m2. However, no evidence was reported regarding the effect of modifying BMI status on the association among dietary Zn, vitamin C and non-communicable chronic diseases(Reference Canoy, Wareham and Welch9,Reference Joo, Kim and Lee24) .
In order to fill the gap mentioned above, undertaking the Tehran Lipid and Glucose Study (TLGS), a population-based cohort study, we aimed to prospectively investigate the association between dietary antioxidants and incident T2DM and to determine whether BMI status might affect these associations.
Methods
Study design and population
This prospective study is designed within the TLGS framework; a longitudinal population-based cohort study initiated in 1999 to inquire about the risk factors of non-communicable diseases and implement a healthy lifestyle in an urban population of Tehran, with follow-up visits at approximately 3-year intervals. The study’s examination cycles after the baseline examination (1999–2002) were as follows: Phase 2 (2002–2005), Phase 3 (2005–2008), Phase 4 (2009–2011), Phase 5 (2012–2015) and Phase 6 (2015–2018). Details of the study design and methodology are available elsewhere(Reference Azizi, Zadeh-Vakili and Takyar26). In the present study, Phase 3 was considered the baseline, including 12 519 participants aged ≥ 3 years, and was followed-up by Phase 6 of the TLGS (2016–2018).
As shown in Fig. 1, the exclusion criteria for the total of 9645 participants aged ≥ 21 years (male = 4160) included individuals with T2DM at baseline (n 1341) and missing data of age, sex, BMI and variables of diabetes risk score(Reference Bozorgmanesh, Hadaegh and Ghaffari27) during follow-up (n 923), leaving us with 7381 subjects.
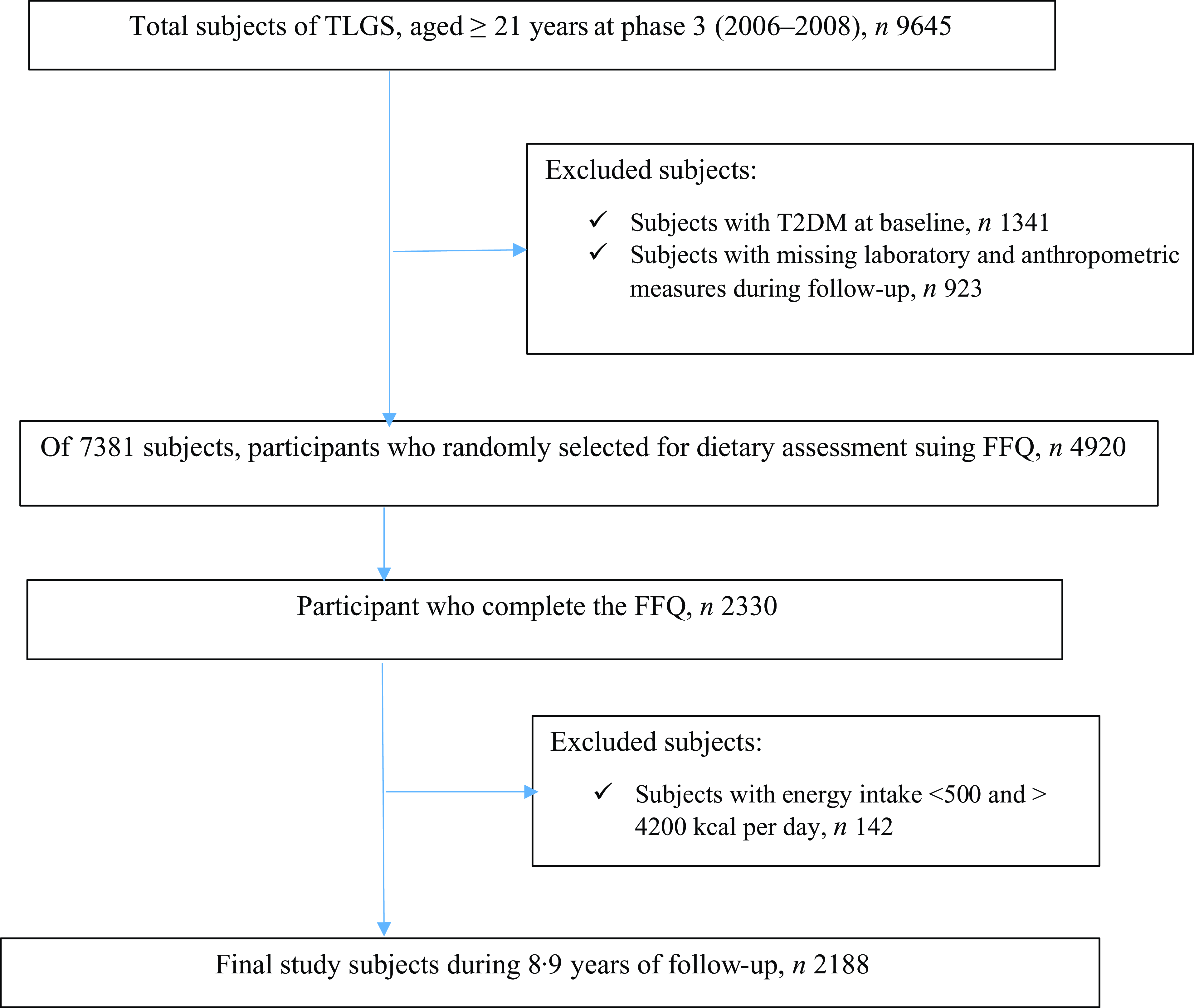
Fig. 1. Flow diagram of the study population, the Tehran Lipid and Glucose Study (2006–2008 to 2016–2018). TLGS, Tehran Lipid and Glucose Study; T2DM, type 2 diabetes.
In phase 3, regarding characteristics such as being costly, time-consuming and complexity of dietary data collection in large populations, a representative sample of 4920 participants was randomly selected based on their age and sex to collect dietary data. The FFQ was completed by 3462 of the 4920 individuals. The characteristics of participants who completed the FFQ were similar to those of the total population in phase 3 of TLGS(Reference Hosseinpour-Niazi, Aghayan and Mirmiran28).
Therefore, of the remaining 7381 subjects, 2330 completed nutritional information. Subjects with energy intake < 500 and > 4200 kcal per day (n 142) were excluded. The final analysis was performed on 2188 subjects during an 8·9-year follow-up period (interquartile range: 8·9–9·6).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Institutional Review Board of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.MSP.REC.1401·462). Written informed consent was obtained from all subjects/patients.
Dietary assessment
During face-to-face interviews with expert dietitians, the valid and reliable semi-quantitative FFQ was used to estimate dietary intake. The frequency of consumption of each food item was documented based on portion size over the previous year. After that, the portion sizes were converted to grams. The Iranian Food Composition Table was used to determine the macronutrient and micronutrient consumption amounts(Reference Smaeili and Hushiarrad29). In the current study, dietary antioxidants included vitamin E, vitamin C, vitamin A, Zn, Mg, Se, Mn, α-carotene, β-carotene and β-cryptoxanthin.
Of the 2188 participants at baseline, 675 completed all four FFQ, 738 completed three, 520 completed two and 255 declined to complete the FFQ during the follow-up period. The missing values were imputed using the last observation carried forward method(Reference Hu, Stampfer and Rimm30). Regarding the crucial effect of recent dietary intakes on chronic disease events, consumption of dietary variables was estimated based on an alternative approach suggested by Hu et al.(Reference Hu, Stampfer and Rimm30). The mentioned approach, which is more important than the baseline measures, adds more weight to the recent diet, reduces within-subject variability and evaluates the long-term diet.
Intake of dietary variables, collected using the FFQ, reported a valid estimate against multiple twenty-four recalls and between two FFQ(Reference Mirmiran, Esfahani and Mehrabi31,Reference Esfahani, Asghari and Mirmiran32) . Moreover, the reliability, validity and stability of the dietary patterns were reasonable based on the data collected from the FFQ over 8 years(Reference Asghari, Rezazadeh and Hosseini-Esfahani33).
Clinical and laboratory measurements
As the TLGS design, a standard questionnaire, including demographics information, age, education, physical activity, smoking status, marital status, medication and a family history of T2DM, was used and completed by a skilled interviewer.
A digital scale with a sensitivity of 0·1 kg (Seca 707: range 0/0–150/0 kg) was used to measure weight with light clothing and without shoes. Height was measured standing with a tape meter without shoes. BMI (kg/m2) was determined by dividing weight by the square of height. Waist circumference was measured at the umbilical level by an upstretched tape meter. Physical activity was assessed using the Modifiable Activity Questionnaire, and the frequency and amount of time spent per week on physical activity over the last year were recorded(Reference Kriska, Knowler and LaPorte34). The physical activity levels were expressed as metabolic-equivalent (MET) minutes per week (MET-min/week)(Reference Ainsworth, Haskell and Whitt35). The reliability and convergent validity of the Persian version of the Modifiable Activity Questionnaire have been reported elsewhere(Reference Momenan, Delshad and Sarbazi36).
After a 15-minute sitting rest, the systolic and diastolic blood pressures were taken twice on the right arm with a standard mercury sphygmomanometer to determine blood pressure. Finally, the individuals’ blood pressure was determined using the mean scores of two measurements.
After fasting for 12 to 14 h overnight, all participants were examined for venous blood samples to measure fasting plasma glucose (FPG) and lipid measurements. A Selectra 2 auto-analyser (Vital Scientific, Spankeren, Netherlands) and commercial kits (Pars Azmoon, Tehran, Iran) were used for all laboratory assays, which were completed at the lab on the day of collecting blood samples. Plasma glucose, TAG levels and HDL-cholesterol were all measured using an enzymatic colorimetric method. Glucose oxidase was employed to measure glucose, and lipoprotein lipase and glycerol phosphate oxidase were utilised to measure TAG levels. HDL-cholesterol was measured after precipitation of the apolipoprotein B-containing lipoproteins with phosphotungstic acid. An oral glucose tolerance test was performed for all subjects who were not using glucose-lowering medications by consuming 82·5 g of glucose monohydrate (equivalent to 75 g of anhydrous glucose). Intra- and inter-assay coefficients of variation were both less than 2·2 % for FPG, 1·9 % for TAG and 2·9 % for HDL-cholesterol.
Definition of terms
T2DM was defined as the presence of at least one of the following criteria based on the American Diabetes Association: FPG level of ≥ 126 mg/dl or 2-h post-challenge plasma glucose (2-h-PCPG) level of ≥ 200 mg/dl or a history of using glucose-lowering medications(37). Moreover, BMI status was defined as ≤ 24·9, 25·0–29·9 and ≥ 30·0 kg/m2.
Statistical analysis
Dietary antioxidant intakes were adjusted for energy by the residual method(Reference Willett, Howe and Kushi38) and were modeled as tertiles. Baseline characteristics were expressed as mean (standard deviation (s e)) or number (percentage) across tertiles of dietary vitamin E, using ANOVA and the χ 2 test. Cox proportional hazard regression analyses were used to estimate the hazard ratios (HR) and 95 % CI for the incidence of T2DM across tertiles of dietary antioxidant intakes in the total population. Two models were fit. The first model was adjusted for age, sex, total energy intake, physical activity and dietary cholesterol. The second model additionally adjusted for diabetes risk score. The diabetes risk score was estimated based on five variables, including systolic blood pressure (< 120 mmHg = score 0; 120–140 mmHg = score 3; ≥ 140 mmHg = score 7), a family history of diabetes (score 5), waist:height ratio (< 0·54 = score 0; 0·54–0·59 = score 6; ≥ 0·59 = score 11), TG/HDL-cholesterol (< 3·5 = score 0; ≥ 3·5 = score 3) and FPG (< 5·0 = score 0; 5·0–5·5 = score 12; 5·6–6·9 = score 33)(Reference Bozorgmanesh, Hadaegh and Ghaffari27). This score could better identify the development of T2DM in a Middle Eastern adult population.
Furthermore, we evaluated the effect of modification by BMI status. Since our research was an exploration study, no correction was performed for the multiple tests. SPSS software version 18 (SPSS) was used for data analysis.
Results
The mean (s d) age and BMI of the total population were 40·6 (13·3) years and 27·0 (4·7) kg/m2, respectively. The median (interquartile range) dietary antioxidant intake was as follows: 10·8 (8·6–13·4) mg/d for vitamin E, 144 (99–203) mg/d for vitamin C, 541 (403–737) µg/d for vitamin A, 11·6 (9·6–14·6) mg/d for Zn, 422 (335–524) mg/d for Mg, 8·3 (6·4–10·9) mg/d for Mn, 114 (90–146) µg/d for Se, 864 (559–1332) µg/d for α-carotene, 3339 (2261–4910) µg/d for β-carotene and 238 (148–364) µg/d β-cryptoxanthin. Generally, individuals with diabetes were less educated, more likely to be married and more likely to have a family history of T2DM. Additionally, this group consumed more dietary cholesterol and protein while considerably consuming less energy (online Supplementary Table 1).
The baseline participant’s characteristics across tertiles of dietary vitamin E are shown in Table 1. Subjects with higher dietary vitamin E were younger, more likely to be female, more likely to be active, had higher BMI and consumed more energy and dietary fat, fibre, cholesterol, vegetables, fruits, poultry and fish, nuts, legumes and dairy products. However, the intake of carbohydrate, protein, whole grains and refined grains decreased across the tertile of vitamin E.
Table 1. Baseline characteristics of participants across tertiles of dietary vitamin E
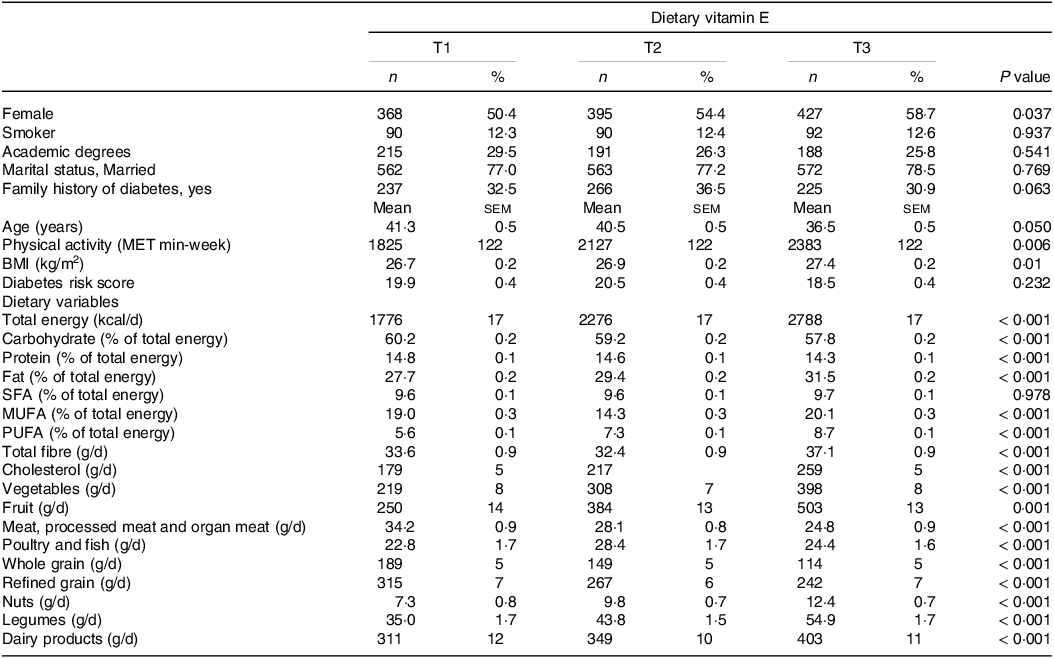
MET, metabolic equivalent; BMI, body mass index; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Dietary variables were adjusted for energy intakes.
During 8·9 (8·1–9·6) years of follow-up among 2188 participants, 234 new cases of T2DM were diagnosed. Table 2 presents the multivariable-adjusted HR for T2DM across categories of dietary antioxidants. In model 1, dietary vitamin E was not associated with incident T2DM. However, in model 2, vitamin E significantly reduced the incidence of T2DM after adjusting for DRS (HR: 0·81, 95 % CI: 0·61–1·09 in the second tertile; 0·68, 0·51–0·93 in the third tertile; P for trend = 0·039). Furthermore, in model 1, there was no association between dietary Mg intake and incident T2DM. Further adjustments for confounding factors in model 2 resulted in a nearly significant inverse association with incident T2DM (1·09, 0·81–1·49 in the second tertile; 0·76, 0·53–1·03 in the third tertile; P for trend = 0·051). No significant association was observed between other dietary antioxidant intakes and the incident T2DM (Table 2 and online Supplementary Table 2).
Table 2. Multivariable adjusted hazard ratio (95 % CI) for type 2 diabetes across tertiles of dietary antioxidants: Tehran Lipid and Glucose Study

n/N, number of MetS/number of subjects.
Model 1 adjusted for age, sex, total energy intake, physical activity and dietary cholesterol.
Model 2 further adjusted for diabetes risk score.
The statistically significant data is bold.
In different strata of BMI, it was shown that dietary vitamin E, vitamin C, Zn and Mg had different association with T2DM (Fig. 2). We found that dietary vitamin E, Mg and BMI status had interactions with the risk of T2DM (P < 0·05). The risk of T2DM was significantly reduced only in subjects with normal weight who consumed more dietary vitamin E (≥ 10·8 mg/d; 0·42, 0·17–0·95) and Mg (≥ 422 mg/d; 0·33, 0·14–0·84), after adjustment for confounders. We also found that dietary vitamin C (≥ 144 mg/d; 0·75, 0·33–0·91) and Zn (≥ 12·0 mg/d; 0·48, 0·22–0·89) had inverse associations with the risk of T2DM in individuals with normal weight, but not in overweight and obese individuals; however, the interaction test tended to be significant for these dietary variables (P interaction for dietary vitamin C = 0·079; P interaction for Zn = 0·063).

Fig. 2. Multivariable hazard ratios (95 % CI) of the association between dietary antioxidants intakes (< median v. ≥ median) and incident type 2 diabetes, stratified by BMI status. Data were adjusted for age, sex, total energy intake, physical activity, dietary cholesterol and diabetes risk score. n/N, number of T2DM events /number of subjects.
Nevertheless, none of the three BMI groups showed a statistically significant association between the incident T2DM and vitamin A, Mn, Se, α-carotene, β-carotene and β-cryptoxanthin (online Supplementary Fig. 1).
Discussion
In this population-based prospective study, with 8·9 years of follow-up, we found the preventive effect of dietary vitamin E on incident T2DM, with a 35 % risk reduction in new-onset T2DM, after controlling for confounders. Additionally, we observed that the effect of dietary antioxidants on incident T2DM was modified by BMI status. The protective effects of dietary antioxidants, including vitamin E, vitamin C, Zn and Mg, were more pronounced among participants with normal weight but not among overweight/obese individuals.
Despite being a potent biological antioxidant, vitamin E has contradictory effects on chronic diseases that might be attributed to the vitamin source (diet v. supplement). Evidence from prospective randomised clinical trials failed to show the benefit of vitamin E supplementation in reducing the risk of T2DM and its complications(Reference Sacco, Pellegrini and Roncaglioni7,Reference Liu, Lee and Song39) . Furthermore, some studies found that T2DM participants receiving high-dose vitamin E supplementation had increased cardiometabolic risk factors and mortality rates(Reference Bjelakovic, Nikolova and Gluud16,Reference Ward, Wu and Clarke40,Reference Miller, Pastor-Barriuso and Dalal41) . In contrast to these results, our findings, along with previous studies, have shown that a higher intake of dietary vitamin E reduced the incidence of T2DM and CVD(Reference Montonen, Knekt and Järvinen8,Reference Yin, Zhu and Xu42) . Vitamin E that derives from dietary sources seems to protect against T2DM. Plant-based oils, nuts, seeds, fruits and vegetables are good sources of vitamin E in the diet(43), and following dietary patterns like the Mediterranean diet, which emphasises the consumption of these dietary food groups was associated with a lower risk of T2DM(Reference Sarsangi, Salehi-Abargouei and Ebrahimpour-Koujan44). In addition, a healthy diet is rich in other antioxidant vitamins and minerals in addition to vitamin E. Previous studies suggested that vitamin E intake improved glycaemic indices and lipid profiles when consumed with other vitamins and minerals, including vitamin A(Reference Said, Mousa and Fawzi45), Zn(Reference Said, Mousa and Fawzi45), chromium(Reference Imanparast, Javaheri and Kamankesh46) and Mg(Reference Afzali, Jafari Kashi and Momen-Heravi47). Therefore, for both primary and secondary prevention of T2DM, consuming a diet rich in all antioxidants, rather than focusing on just one antioxidant such as vitamin E, is recommended.
Additionally, in the current study, the inverse relationship between dietary vitamin E and incident T2DM was only significant among normal-weight individuals. In contrast to our findings, two large prospective and trial studies found no significant interaction between vitamin E and BMI in relation to T2DM(Reference Montonen, Knekt and Järvinen8,Reference Liu, Lee and Song39) . A 23-year follow-up study found that dietary vitamin E had an inverse association with the risk of T2DM. However, there was no significant interaction between established diabetes risk factors such as BMI, smoking status, hypertension or serum cholesterol and dietary vitamin E in relation to risk of T2DM(Reference Montonen, Knekt and Järvinen8). In a 10-year follow-up trial, there was no evidence that BMI modified the effect of vitamin E supplements on the risk of type 2 diabetes(Reference Liu, Lee and Song39).
Vitamin C intake as an antioxidant has been hypothesised to reduce the risk of T2DM. Although population-based prospective studies with long-term follow-up periods have shown the protective effects of vitamin C against incident T2DM(Reference Harding, Wareham and Bingham4,Reference Canoy, Wareham and Welch9) , evidence from randomised controlled trials and Mendelian randomisation suggests no effect of vitamin C on glycaemic control and the risk of diabetes(Reference Ashor, Werner and Lara10,Reference Zheng, Luan and Sofianopoulou48) . These discrepancies may be due to variations in the underlying cause of oxidative stress, such as obesity. The effect of vitamin C intake on T2DM may be modified by oxidative stress(Reference Zhou, Na and Shan19); obesity, as a known risk factor for oxidative stress, may also affect the outcome(Reference Harding, Wareham and Bingham4). The need for vitamin C is increased with obesity, and BMI adjustment attenuated the association between vitamin C and T2DM(Reference Harding, Wareham and Bingham4). Our results are consistent with these findings, implying that the association between the risk of T2DM and vitamin C intake was dependent on BMI status; higher vitamin C intake was associated with a lower risk of incident T2DM, but only among subjects with normal weight. These findings, however, were not supported by other studies(Reference Canoy, Wareham and Welch9).
The effect of Zn, derived from supplements or foods, on glycaemic control and insulin resistance is controversial and remains to be elucidated(Reference Beletate, El Dib and Atallah11,Reference Gunasekara, Hettiarachchi and Liyanage17) . Based on previous studies, the effect of Zn on the improvement of cardiometabolic risk factors was influenced by intake levels(Reference Costarelli, Muti and Malavolta12), FPG(Reference Gunasekara, Hettiarachchi and Liyanage17) and obesity status. Inconsistent results were obtained as a result of wide variations in Zn intake between the studies(Reference Kaidar-Person, Person and Szomstein49). In our study population, dietary Zn, with a median intake of 12·0 mg/d, was not associated with the risk of T2DM. This finding contrasts with that of the Jiangsu Nutrition Study, which found a U-shaped relationship between Zn consumption and newly diagnosed T2DM, indicating the minimal risk of T2DM at a dietary Zn intake level of 8·9 to 12·2 mg/d(Reference He, Li and Liu13). Our findings imply that obesity status must be considered when determining the association between dietary Zn intake and incident T2DM. It was found in the present research that dietary Zn intake with a median intake of 12·0 mg/d decreased the risk of T2DM among subjects with normal weight, but this amount of dietary Zn was not sufficient to reduce the risk of T2DM in overweight and obese subjects; however, this issue was not supported by other studies(Reference Drake, Hindy and Ericson23,Reference Joo, Kim and Lee24) . Additionally, Zn deficiency is a common problem in obese subjects(Reference Esposito, Pontillo and Di Palo50), and if it is not treated, it might result in obesity-related disorders, such as insulin resistance(Reference Kaidar-Person, Person and Szomstein49). However, the higher dose of Zn supplementation – 30 mg/d – improved cardiometabolic risk factors like insulin resistance and glycaemic indices in this population(Reference Khorsandi, Nikpayam and Yousefi51,Reference Marreiro, Geloneze and Tambascia52) . Therefore, Zn supplements seem to be necessary for the prevention of insulin resistance and T2DM only in this population; however, more research is required to confirm the findings.
In the current study, no significant association was found between dietary Mg and the risk of T2DM. In contrast to our findings, evidence from a systematic review and meta-analysis suggested that dietary Mg might protect against T2DM. However, in later studies, heterogeneity was substantially high(Reference Zhao, Deng and Li14,Reference Schulze, Schulz and Heidemann15,Reference Xu, Chen and Zhai53) . The reason for this contradiction may be that in our study, the median intake of Mg in the first tertile of our subjects was 306 mg/d, which is near the recommended dietary allowance. Therefore, an intake of 500 mg/d in the third tertile compared with 300 mg in the first tertile did not provide a greater benefit in terms of the prevention of T2DM, consistent with the data reported in the previous study(Reference Ma, Lawson and Liese54). However, only in studies where Mg consumption is often lower than the recommended dietary allowance(Reference Gröber, Schmidt and Kisters55), dietary Mg was associated with a lower incidence of T2DM(Reference van Dam, Hu and Rosenberg56). Furthermore, it is yet unknown how the presence or absence of obesity, as a recognised risk factor for T2DM, impacts this association. In a meta-analysis, the beneficial effects of Mg on the risk of T2DM were shown to be more evident among individuals with a BMI ≥ 25 kg/m2; however, the interaction test was not significant(Reference Dong, Xun and He21). BMI status was not a potential modifier for the association between Mg intake and T2DM in another meta-analysis either(Reference Zhao, Zeng and Zhao25). In the present study, in line with the findings of Women’s Health Study, BMI modified this association(Reference Song, Manson and Buring22); however, in a large cohort of American women, the inverse association was more pronounced among subjects with BMI ≥ 25 kg/m2, whereas in the present study, this relationship was seen in individuals with normal weight. We previously reported that the Tehranian overweight and obese population had a significant odds ratio of 1·7 and 4·0 for incident T2DM(Reference Harati, Hadaegh and Saadat57), and the issue might lead to a neutral effect of dietary antioxidant consumption among these individuals.
The main strengths of the present study included the population-based prospective design, gathering dietary data using the validated FFQ by well-trained nutritionists, the use of a cumulative approach rather than a single measurement(Reference Hu, Stampfer and Rimm30), the adjustment of micronutrients for total energy intake using residual model(Reference Hu, Stampfer and Rimm30) and diagnosis of T2DM based on FPG, 2-h-PCPG and the use of blood glucose-lowering medication rather than self-reported data.
In interpreting the results, several limitations should be acknowledged. Observational studies cannot prove a causal association and residual confounding remains a concern, even after wide-range adjustments for potential confounders(Reference Willett and Leibel58). The amount of dietary antioxidants, even with the valid and reliable FFQ, was underestimated(Reference Zhang, Iso and Ohira59), likely weakening the association between dietary intake and chronic diseases(Reference Bingham, Luben and Welch60,Reference Mayer-Davis, Costacou and King61) . Although using a cumulative average diet, which best represents a long-term diet, minimised measurement errors(Reference Hu, Stampfer and Rimm30). Additionally, the usage of multivitamin/mineral supplements was not taken into account when estimating antioxidant intake from foods, culminating in biased results of the observed association. Another limitation is the short follow-up period; hence, studies with long-term follow-up periods are required. Additionally, we lacked any information on how cooking techniques could alter food’s antioxidant concentration. Last but not least, since this study was conducted in Tehran’s metropolitan area, its findings might not be generalisable to the rural population.
Conclusion
In conclusion, the results of this population-based prospective analysis suggest that dietary antioxidant intakes, when accompanied by healthy weight, may bring benefits to the prevention of T2DM. The public health message for the prevention of T2DM should emphasise the importance of maintaining a healthy weight along with consumption of an antioxidant-rich diet. However, more investigation is required to determine how BMI status affects the association between dietary antioxidants and T2DM in different population groups.
Acknowledgement
We express our appreciation to the participants of this study for their collaboration.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
S. H-N. and F. H. conceptualised and designed the study. S. H-N. and M. G. analysed and interpreted the data; S. H-N., M. G., P. H., F. H., P. M. and F. A. drafted the initial manuscript; F. H. supervised the project and all authors have read and approved the final version of the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523002854







