CVD remain the leading cause of premature death and rising medical costs globally(Reference Moran, Forouzanfar and Roth1). In 2019, the number of prevalent cases of CVD was about 523 million and the number of CVD deaths was 18·6 million, showing a stable increasing trend compared with 1990(Reference Roth, Mensah and Johnson2). CVD involve disorders of the heart and blood vessels and include coronary artery diseases (CAD), stroke, heart failure, etc.(Reference Thomas, Diamond and Vieco3) CAD is the most common type of CVD(Reference Moran, Forouzanfar and Roth1,4) , and the main drivers of CAD include cardiometabolic, environmental, behavioural and social risk factors(Reference Roth, Mensah and Johnson2). There are some modifiable risk factors, such as high systolic blood pressure, high fasting plasma glucose, HDL-cholesterol and LDL-cholesterol, high BMI, impaired kidney function, ambient and household air pollution, tobacco, dietary risks, and low physical activity(Reference Roth, Mensah and Johnson2,Reference Steg and Ducrocq5) . It is estimated that more than 90 % of CAD could be prevented through early intervention(Reference McGill, McMahan and Gidding6) and then could reduce CAD mortality and burden(Reference Steg and Ducrocq5).
Methionine (Met) is a unique amino acid and also proteogenic amino acid necessary of the canonical twenty amino acids building proteins, which contains sulphur and can be transformed into other sulphur-containing molecules in vivo (Reference Brosnan and Brosnan7,Reference Stipanuk8) . Methionine is the initiating amino acid and plays a vital role as an initiator of protein synthesis in almost all prokaryotes and eukaryotes(Reference Parkhitko, Jouandin and Mohr9). In body, methionine can not only construct proteins but also have other special functions, such as modifying DNA, regulating methylation reaction and maintaining proper functioning of the cells(Reference Martinez, Li and Liu10,Reference Ravanel, Gakiere and Job11) . In addition, methionine metabolism is closely related to various metabolic pathways and plays an important role through its metabolism. It has been reported that dysregulation of methionine metabolism is related to a variety of diseases, such as obese(Reference Plaisance, Greenway and Boudreau12), cancer(Reference Cavuoto and Fenech13) and CVD(Reference Ostrakhovitch and Tabibzadeh14). The first step of methionine metabolism is the biosynthesis of S-adenosylmethionine (SAM) through methionine adenosyltransferase catalysing methionine and ATP. Then SAM donates its methyl group to substrates in the methylation process and generates S-adenosylhomocysteine (SAH) through methyltransferases(Reference Chiang, Gordon and Tal15). The conversion of SAH to homocysteine (Hcy) and adenosine is a reversible hydrolysis reaction, which is catalysed by S-denosylhomocysteine hydrolase (SAHH/AHCY). Hcy can be remethylated by the methionine synthase with 5-methyltetrahydrofolate as a methyl donor or betaine homocysteine methyltransferase with betaine as a methyl donor to form methionine(Reference Parkhitko, Jouandin and Mohr9). SAH and betaine are two important molecules in the methionine cycle. SAH has varying degrees of inhibition on different methyltransferases and is a known product inhibitor of SAM-dependent methylation reactions, so low levels of SAH are critical to maintaining normal methylation in cells. Betaine is distributed widely in many foods and sever as a methyl donor which may affect the re-methylation of methionine(Reference Craig16). The concentrations of betaine in wheat bran, wheat germ, spinach pretzels, shrimp and wheat bread are relatively high(Reference Zeisel, Mar and Howe17). Choline is the metabolic precursor of betaine, which can be easily obtained from beef liver, chicken liver, eggs, wheat germ, bacon, dried soyabeans and pork(Reference Zeisel, Mar and Howe17). Inadequate dietary intake of methyl donor food leads to hypomethylation of many metabolic pathways, leading to various diseases, such as diabetes, CVD and metabolic syndrome(Reference Ueland18). Previous studies have shown that dietary betaine intake may reverse alcoholic fatty liver(Reference Barak, Beckenhauer and Badakhsh19) and protect against CAD(Reference Mar and Zeisel20,Reference Vos21) . However, some literatures have indicated that dietary betaine metabolite trimethylamine N-oxide is related with the risk of CVD(Reference Tang, Wang and Levison22,Reference Senthong, Li and Hudec23) . The contradictory results of these studies raise the question of the role of dietary betaine in the risk of CVD. Epidemiological evidence for dietary betaine and mortality is limited, and there are few studies based on the Chinese population. In our study, we prospectively evaluated the relationship of dietary betaine intake with the risk of mortality in patients with CAD based on Chinese population.
Methods
Study population
The data for this study are from the Guangdong Coronary Artery Disease Cohort, which is a prospective observational cohort study investigating the influence of environmental, social, and genetic factors on the progression and prognosis of CAD. Participates were recruited between October 2008 and December 2011. Patients aged 40 to 85 years were enrolled from the cardiology departments of three major hospitals (General Hospital of Guangzhou Military Command of People’s Liberation Army, the First Affiliated Hospital and Second Affiliated Hospital of Sun Yat-sen University) in Guangzhou in South China (23° 16’ north latitude). This study was approved by the Ethical Committee of Sun Yat-sen University. Written informed consent was provided by all participants at the time of enrolment. All of the protocols adhered to institutional guidelines and to the Helsinki Declaration. The rationale and design of this cohort study, inclusion and exclusion criteria, methods, and definitions refer to the previous studies(Reference Xiao, Zhang and Wang24–Reference Yu, Xue and Wang28). For this analysis, additional exclusion criteria included participants taking supplements containing choline or betaine, and participants who were pregnant or breast-feeding at the time of enrolment. According to WHO 1999/2000 guidelines, a total of 1977 patients underwent coronary angiography were diagnosed with CAD. After excluding 389 participants with missing baseline data or lacking sufficient plasma samples and 296 patients with missing baseline FFQ data, 1292 patients were included for analysis in the present study (online Supplementary Fig. 1). Our cohort study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (S1 STROBE Checklist).
Analysis of coronary angiography
Coronary angiography was performed in all of the participants with the standard Judkins technique through the femoral artery or brachial artery and scored for luminal narrowing using a modified American Heart Association/American College of Cardiology classification of the coronaries(Reference Fihn, Gardin and Abrams29). The angiography was interpreted by at least two independent cardiologists who were blinded to the patient’s risk factors. CAD patients are defined in three levels, namely patients with CAD were defined in three levels, namely mild CAD (visible plaque resulting in > 20 % but < 50 % luminal narrowing stenosis), moderate (≥ 50–< 70 % stenosis) and obstructive CAD (≥ 50 % stenosis in the left main coronary artery, or ≥ 70 % in any other coronary artery, or both). Patients with obstructive CAD were further categorised by the number of diseased vessels, namely a single-, double- or triple-vessel distribution. Taken together, the Gensini score was used to comprehensively evaluate the extent of CAD in current study(Reference Sullivan, Marwick and Freedman30). The methods of coronary angiography analysis have been presented in our previous articles(Reference Liu, Liao and Dai31).
Dietary assessment
The dietary intake of all participants was collected using a paper-based semi-quantitative FFQ, which was used to assess the usual consumption of major nutrients and food groups(Reference Zhang and Ho32). The FFQ, which included eight food groups with a total of eighty-one food items, had been verified to have satisfactory reproducibility and reasonable validity in previous study(Reference Zhang and Ho32). The frequency and portion-size data of the participants were calculated using the China Food Composition Table 2004(Reference Yang, Wang and Pan33). The total amount of dietary betaine intake was assessed according to the US Department of Agriculture (USDA) database(Reference Rouge34) and the data of betaine in common foods(Reference Zeisel, Mar and Howe17). The residual method proposed by Willett and Stampfer was used to adjust the nutrient intake in the total energy intake(Reference Willett and Stampfer35).
Baseline measurements and biochemical analyses
General information about personal basic information, living habits, dietary, leisure-time physical activity, clinical data and measurement indexes, use of medications, and so on was collected by using standardised questionnaires during clinical face-to-face interviews. Menopause was defined as the absence of menstruation for ≥ 12 months, and women ≥ 55 years old without menopausal information were considered postmenopausal(Reference Kim, Chang and Ahn36). Medical records were reviewed by medical staff, and then the questionnaires were checked by a trained interviewer for missing data and completeness before the data were entered into a database. Anthropometric measurements methods, information presentation form and biochemical analyses have been described in our previous studies(Reference Liu, Liao and Dai31). The inter-assay and intra-assay CV of plasma lipid levels, creatinine, fasting blood glucose, plasma total cysteine (tCys) and total homocysteine (tHcy), and serum vitamin B12 were ≤ 8·6 %.
Plasma S-adenosylmethionine and S-adenosylhomocysteine measurements
Plasma SAM and SAH are more prone to degradation without any treatment. Therefore, the collected plasma samples should be immediately aliquoted, acidified(Reference Gellekink, van Oppenraaij-Emmerzaal and van Rooij37) and stored at −80°C until analysed. The treated samples can be stored at −80°C for at least 1 month. Based on HPLC-MS technology, plasma SAH and SAM were measured by stable-isotope dilution which allows sensitive and rapid measurement of the two molecular(Reference Gellekink, van Oppenraaij-Emmerzaal and van Rooij37).
Outcomes of follow-up
The main outcomes of this study were all-cause mortality and cardiovascular mortality. The final date of follow-up was 30 December 2019. Annual follow-up information was collected and confirmed during a median follow-up of 9·2 years (interquartile range: 8·5–10·2 years). Death certificates were coded by nosologists according to the International Classification of Diseases. Cardiovascular mortality was defined as death attributable to an ischemic cardiovascular cause (including fatal myocardial infarction, stroke and peripheral arterial disease) or sudden death due to an unknown but presumed cardiovascular cause in high-risk patient according to the International Classification of Diseases Tenth Revision codes I00-I99.
Statistical analyses
Participants were categorised according to the tertiles of dietary betaine intake. Continuous variables for baseline characteristics are presented as means ± sd or median with interquartile range, and categorical variables of baseline characteristics are presented as counts and percentages (%). Differences between groups were compared by using one-way ANOVA and the χ2 test. The relationships between dietary betaine intake and plasma concentrations of methionine metabolites, including SAH, SAM, SAM/SAH, tHcy, tCys, folate and vitamin B12, are based on partial correlational analysis. The cumulative event plot for all-cause mortality and cardiovascular mortality according to the tertiles of dietary betaine intake was estimated by using the Kaplan–Meier method, and P-values were compared by using the log-rank test. Betaine was not only obtained from diet but also from the oxidation of dietary choline in vivo (Reference Hamlin, Pauly and Melnyk38). Therefore, as dietary choline might affect the plasma level or dietary intake of betaine, models would be adjusted for dietary choline intake. The hazard ratio (HR) and 95 % CI of the outcomes of all-cause mortality and cardiovascular mortality according to increasing dietary betaine intake were calculated with Cox proportional hazards models adjusted for traditional cardiovascular risk factors (model 1) and traditional cardiovascular risk factors plus metabolites related to methionine cycle (model 2). Model 1 was adjusted for age, sex, BMI, smoking status, alcohol drinker, hypertension, diabetes mellitus, physical activity, family history of CAD, systolic blood pressure, Gensini score, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, use or non-use of statins, aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, dietary energy intake, dietary protein intake and dietary choline intake. Model 2 was adjusted for variables in model 1 plus SAH, SAM, tHcy, tCys, folate, and vitamin B12. The restricted cubic splines were used to estimate non-linear dose–response relationship between dietary betaine intake and HR for the outcomes. Additionally, we performed a further analysis on subgroups stratified by baseline covariates including age, sex and other traditional CVD risk factors with risk of all-cause mortality and cardiovascular mortality. Using the group categorised according to the tertiles of dietary betaine intake, we calculated crude and three adjusted HR with 95 % CI.
All statistical analyses were performed with SPSS 25.0 (IBM SPSS Inc.), and the restricted cubic splines were plotted by R language (version R-4.0.3). A two-sided P-value < 0·05 was considered statistically significant.
Results
Baseline characteristics
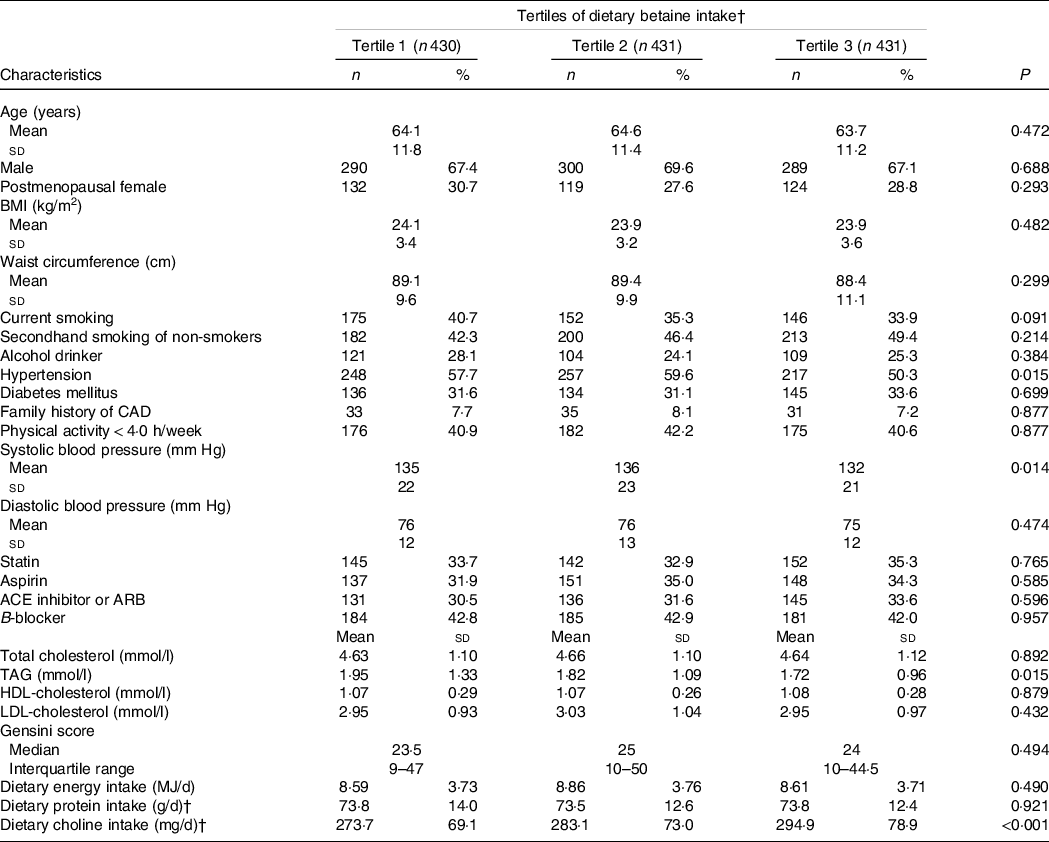
After excluding the patients without enough plasma samples or with missing data, a total of 1292 participants were included in this analysis. At the baseline, the average age was 64·1 ± 11·5 years, and 68 % of the participants were male. The cut-off points of tertiles of dietary betaine intake were 212 and 257 mg/d. Table 1 presents the baseline characteristics of all the 1292 patients according to the tertiles of dietary betaine intake. Among tertiles, there were no significant difference in sex, age and BMI. Participants in the first and second tertiles of dietary betaine intake were more likely to be higher prevalence of hypertension and systolic blood pressure than those in the third tertile (P < 0·05). According to tertiles of dietary betaine intake, participants with a higher dietary betaine intake had lower level of TAG. Dietary choline intake was positively correlated with the dietary betaine intake.
Table 1. Baseline characteristics of CAD patients by tertiles of dietary betaine intake*
CAD, coronary artery disease; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
* Values expressed as mean ± sd values, median (interquartile range) values and percentage (%) unless otherwise stated. Significance tests for comparisons by tertiles of dietary betaine intake based on ANOVA for continuous variables and Pearson’s χ 2 test for categorical variables. The cut-off points of tertiles of dietary betaine intake were 212 and 257 mg/d.
† Nutrient intake was adjusted for energy intake using the residual method.
Association of dietary betaine intake with plasma concentrations of methionine metabolites and vitamins
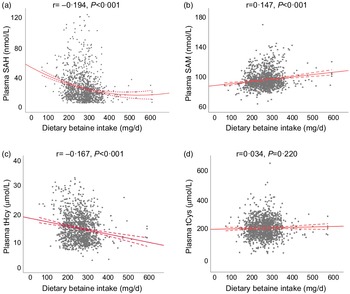
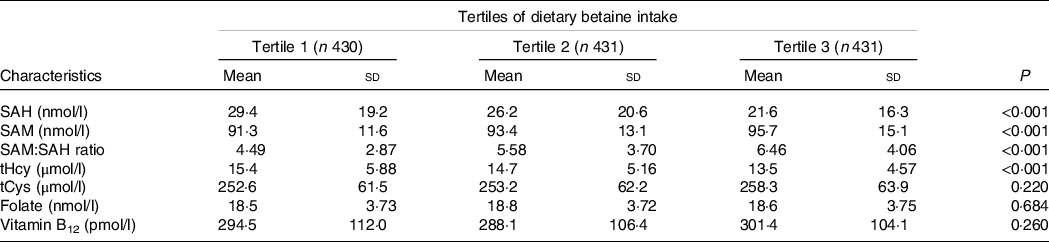
Table 2 showed the relationship of dietary betaine intake with plasma concentrations of methionine metabolites and vitamins according to tertiles of dietary betaine intake. For the methionine metabolites in plasma, participants in higher tertiles of dietary betaine intake had a significantly lower level of SAH and tHcy, but higher level of SAM and higher ratio of SAM/SAH. Plasma tCys concentration did not differ significantly among the three tertiles of dietary betaine intake, nor did the plasma vitamins concentrations (folate and vitamin B12). And results of the partial correlation analysis of the associations between dietary betaine intake and plasma concentrations of methionine metabolites and vitamins were showed in Fig. 1 and Supplementary Table 1, and dietary betaine intake was inversely associated with the level of plasma SAH (r = –0·194, P < 0·001, Fig. 1(a)) and tHcy (r = –0·167, P < 0·001, Fig. 1(c)), but positively associated with the level of plasma SAM (r = 0·147, P < 0·001, Fig. 1(b)) and SAM:SAH ratio (r = 0·240, P < 0·001), which was the same as in univariate analysis. There was no significant correlation between dietary betaine intake and plasma tCys, folate and vitamin B12 in both univariate analysis and multivariate analysis.
Table 2. Plasma concentrations of methionine metabolites and vitamins in patients with CAD by tertiles of dietary betaine intake*
SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; tHcy, total homocysteine; tCys, total cysteine.
* Adjusted for age, sex, BMI, smoking status, alcohol drinker, hypertension, diabetes mellitus, physical activity, family history of coronary artery disease, systolic blood pressure, Gensini score, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, use or non-use of statins, aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, dietary energy intake, dietary protein intake, and dietary choline intake.
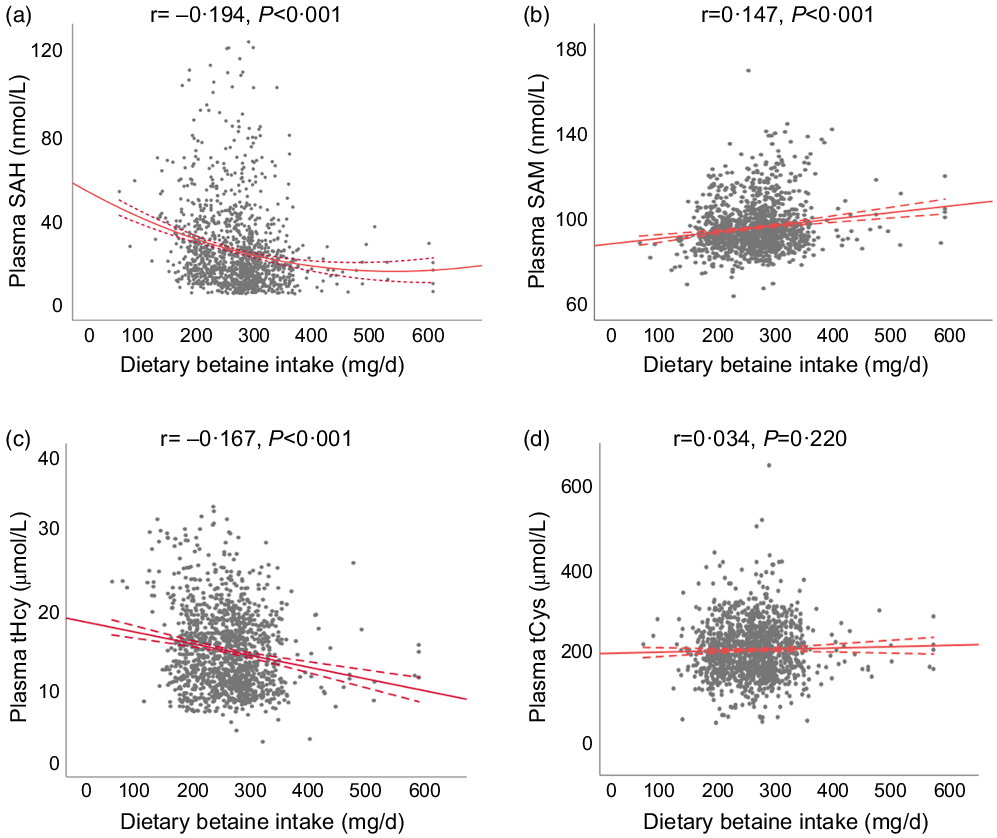
Fig. 1. The relationships between dietary betaine intake and plasma concentrations of methionine metabolites (a) SAH, (b) SAM, (c) tHcy and (d) tCys in patients with coronary artery disease. SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; tHcy, total homocysteine; tCys, total cysteine.
Association of dietary betaine intake with risk of mortality
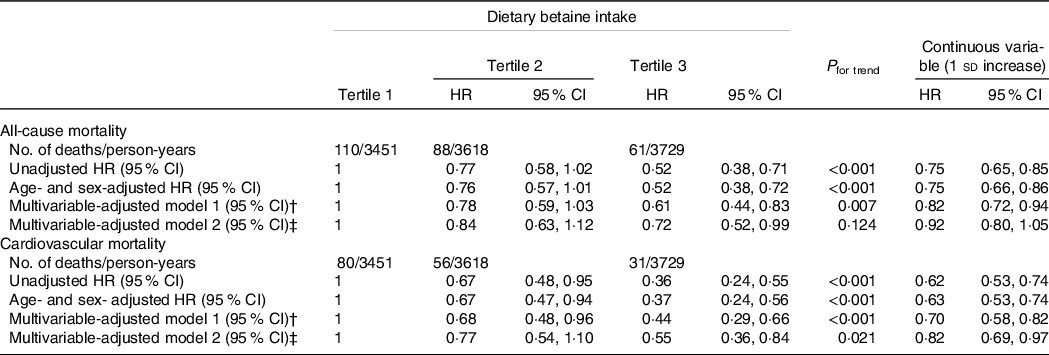
During a median follow-up of 9·2 years (interquartile range: 8·5–10·2 years), there were 259 deaths (20·0 %) recorded in 1292 participants, of which 167 (12·9 %) died of CVD. Kaplan–Meier analysis showed that dietary betaine intake as tertiles was significantly inversely correlated with all-cause mortality risk (log-rank, P < 0·001, Fig. 2(a)) and cardiovascular mortality risk (log-rank, P < 0·001, Fig. 2(b)). The unadjusted HR for each 1 sd increase in daily betaine intake were 0·75 (95 % CI 0·65, 0·85; P < 0·001) for all-cause mortality and 0·62 (95 % CI 0·53, 0·74; P < 0·001) for cardiovascular morality, respectively (Table 3). Compared with patients in the lowest tertile, patients in the highest tertile of dietary betaine intake had a lower risk of all-cause death (adjusted HR, 0·52; 95 % CI 0·38, 0·72; P < 0·001) and cardiovascular death (adjusted HR, 0·37; 95 % CI 0·24, 0·56; P < 0·001) in age- and sex-adjusted analysis (Table 3). Assessment of continuous values of daily betaine intake showed that 1 sd increase in the daily betaine intake was associated with a 18 % lower risk of all-cause mortality (adjusted HR, 0·82; 95 % CI 0·72, 0·94; P = 0·007) and a 30 % smaller risk of cardiovascular death (adjusted HR, 0·70; 95 % CI 0·58, 0·82; P < 0·001) in the multivariable-adjusted model 1 analysis (Table 3). In the multivariable-adjusted model 2, HR across tertiles of dietary betaine intake were 1·00, 0·84 and 0·72 for all-cause mortality (P for trend = 0·124), and 1·00, 0·77 and 0·55 for cardiovascular mortality (P for trend = 0·021) (Table 3). The restricted cubic splines further showed a dose–response association between dietary betaine intake and all-cause or cardiovascular mortality of multivariable-adjusted model 1 and multivariable-adjusted model 2 analysis, although the inverse association trends were not significant (P > 0·05) (Fig. 3). We further analysed the relationship between daily betaine intake and all-cause and cardiovascular mortality based on stratified baseline characteristics including age, sex, BMI, smoking status, alcohol drinking, hypertension and diabetes mellitus. However, among the above parameters, there was no significant interaction between daily betaine intake and all-cause and cardiovascular mortality (online Supplementary Table 2).
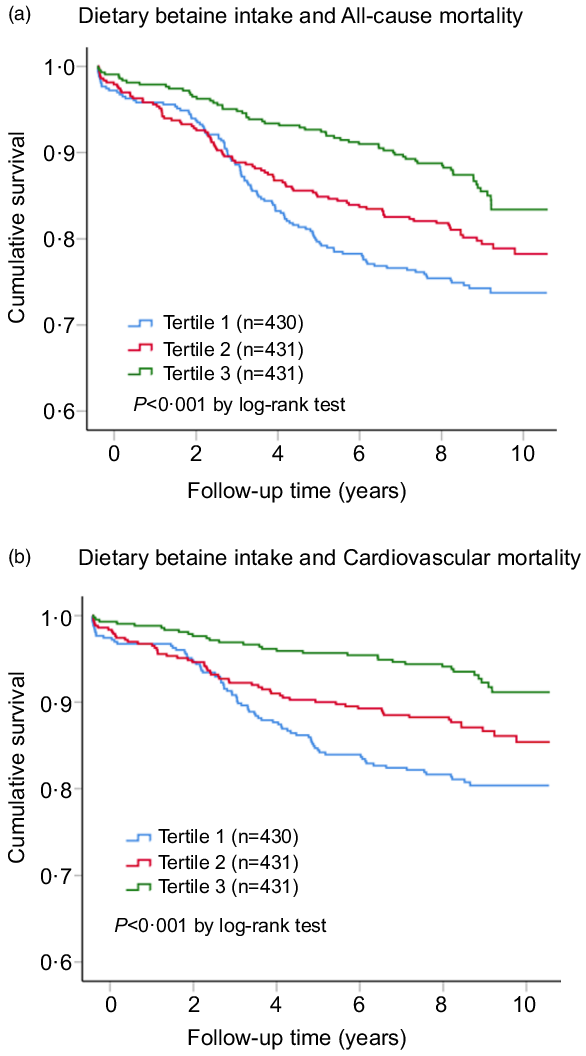
Fig. 2. Kaplan–Meier plots for all-cause mortality (a) and cardiovascular mortality (b) according to tertiles of dietary betaine intake among patients with coronary artery disease. The cut-off points of tertiles of dietary betaine intake were 212 and 257 mg/d. P-values were compared by using the log-rank test.
Table 3. HR for all-cause and cardiovascular mortality according to dietary betaine intake in patients with CAD*
CAD, coronary artery disease; HR, hazard ratio.
* HR and 95 % CI were estimated by Cox proportional hazards regression models.
† Adjusted for age, sex, BMI, smoking status, alcohol drinker, hypertension, diabetes mellitus, physical activity, family history of coronary artery disease, systolic blood pressure, Gensini score, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, use or nonuse of statins, aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, dietary energy intake, dietary protein intake, and dietary choline intake.
‡ Adjusted for variables in model 1 plus S-adenosylhomocysteine, S-adenosylmethionine, total cysteine; total homocysteine, folate and vitamin B12.
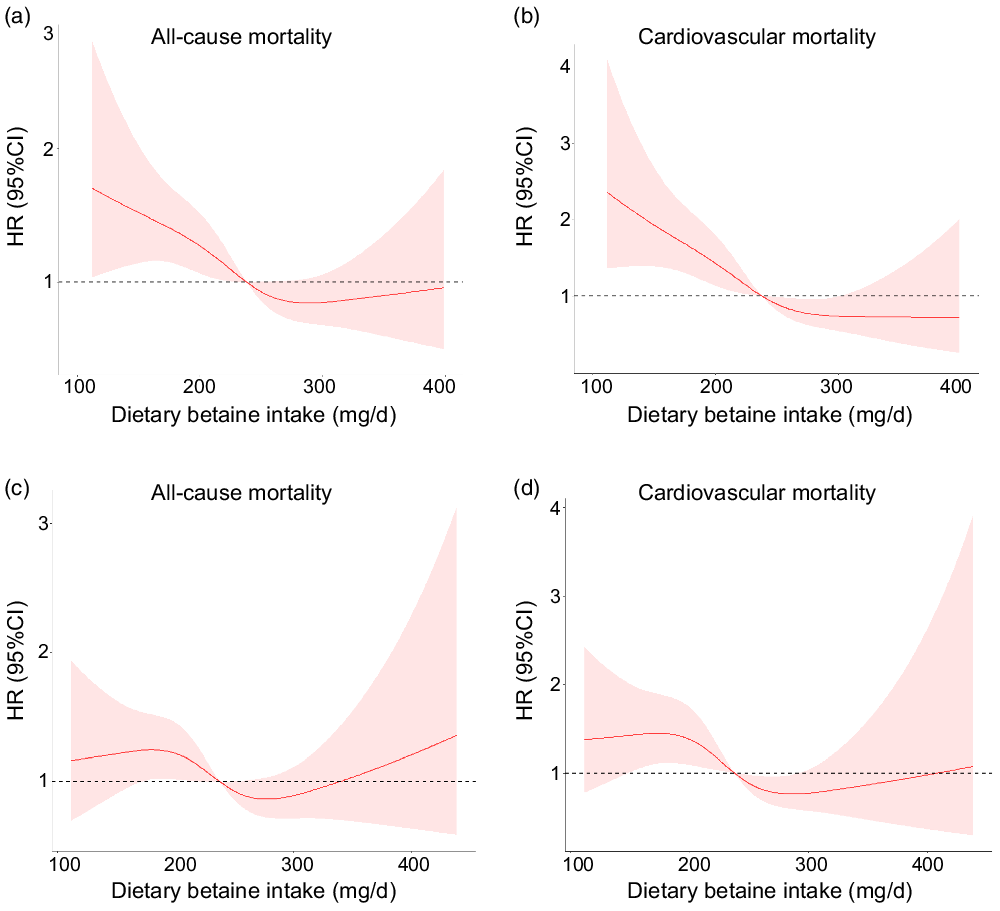
Fig. 3. Multivariable-adjusted spline functions for model 1 show the relationship between dietary betaine intake and all-cause mortality (a), cardiovascular mortality (b), multivariable-adjusted spline functions for model 2 show the relationship between dietary betaine intake and all-cause mortality (c), and cardiovascular mortality (d). HR and 95 % CI were estimated by Cox proportional hazards regression models (n 1292). Model 1 was adjusted for age, sex, BMI, smoking status, alcohol drinker, hypertension, diabetes mellitus, physical activity, family history of coronary artery disease, systolic blood pressure, Gensini score, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, use or non-use of statins, aspirin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, dietary energy intake, dietary protein intake, and dietary choline intake. Model 2 was adjusted for variables in model 1 plus S-adenosylhomocysteine, S-adenosylmethionine, total cysteine, total homocysteine, folate and vitamin B12.
Discussion
In our prospective cohort study, we explored the relationship between dietary betaine intake and the risks of all-cause mortality and cardiovascular morality in patients with CAD. Daily betaine intake was negatively correlated with the risks of all-cause and cardiovascular morality, but the association between dietary betaine intake and risk of all-cause mortality was not statistically significant after further adjusting for plasma methionine metabolites and vitamins.
Baseline characteristics analysis showed that patients with higher daily betaine intake were more likely to be lower prevalence of hypertension and systolic blood pressure and showed lower level of TAG but had a higher level of dietary choline intake. High blood pressure caused blood vessels to narrow, and then blood flowed to the heart may slow down or even stop, making it difficult for the heart to pump blood. Hypertension was the risk factor for premature CVD(39,Reference Kjeldsen40) , and almost half of cardiovascular events was related to hypertension globally(Reference Lawes, Vander Hoorn and Rodgers41). Animal experiments showed that betaine could improve hypertension by inhibiting inflammatory response(Reference Yang, Zhou and Zhang42). In previous cohort study, researcher found that betaine possibly contributed to blood pressure regulation in female patients(Reference Wang, Zhao and Liu43). SBP were associated with the CVD mortality risks, and hypertension and higher systolic blood pressure could significantly increase the CVD mortality risk(Reference Wu, Hu and Chou44). In Chinese adults, blood pressure was independent association with the risk of CVD and systolic blood pressure was predictor of CVD risk(Reference Gu, Kelly and Wu45). Most of the plasma TAG were packed in lipoprotein particles (chylomicrons). Elevated TAG-rich remnant lipoproteins promoted responsible for atherosclerosis formation(Reference Stalenhoef and de Graaf46). Elevated plasma TAG levels were strongly associated with smaller size of LDL-particle, which had a powerful atherogenic effect(Reference Ye, Kong and Zafar47). TAG could stimulate atherosclerosis by producing pro-inflammatory cytokines and impairment of fibrinolysis(Reference Kim, Ahn and Kang48). Population-based prospective studies showed that increased plasma TAG level was associated with an increase incident CVD(Reference Austin, Hokanson and Edwards49,Reference Miller, Stone and Ballantyne50) and risk of all-cause mortality(Reference Liu, Zeng and Liu51). In general, the risk of CVD was associated with the prevalence of hypertension and systolic blood pressure and higher level of TAG, which could be improved by increased betaine intake, thereby reducing the risk and mortality of CVD.
For the methionine metabolites in plasma, dietary betaine intake was positively correlated with the levels of plasma SAM and SAM:SAH ratio and negatively associated with the concentrations of plasma SAH and tHcy. Betaine is distributed widely in many foods and severed as a methyl donor, which may affect the re-methylation of methionine and in turn affect the level of metabolites in the methionine cycle. Betaine could methylate Hcy and decrease the level of Hcy released by the liver(Reference Barak, Beckenhauer and Mailliard52). In our cohort study, we also found that the increase of betaine intake was inversely correlated with the level of tHcy. Hcy had adverse effects on vascular endothelial cells and smooth muscle cells and further lead to inflammatory reaction and promoted the formation of atherosclerosis(Reference Refsum, Ueland and Nygård53). The increase of daily betaine intake can increase the concentration of SAM in plasma. Other studies had shown that betaine treatment could increase the level of plasma SAM(Reference Bostrom, Sweta and James54), and there was a strong positive correlation between plasma betaine and SAM(Reference Imbard, Smulders and Barto55). As a methylation donor, SAM could regulate the methylation reaction of the body. Administration of exogenous SAM to mice could change the inflammatory process, reduce oxidative stress(Reference Lim, Moon and Shin56) and prevent endothelial dysfunction(Reference Kim, Hong and Kim57), which were all related to the occurrence of CVD. The results of our cohort study showed that the level of plasma SAM was negatively correlated with the risk of death(Reference Liu, Liao and Dai31). In this study, we found that higher intake of betaine was correlated with a decrease in plasma SAH levels, which was consistent with other reported studies(Reference Barak, Beckenhauer and Mailliard52). On the one hand, SAH could disrupt the DNA methylation(Reference Mirbahai, Southam and Sommer58) by inhibiting the expression of DNA methyltransferase (DNMT1) and then promoted oxidative stress and induced vascular endothelial dysfunction via activating the expression of p66shc gene(Reference Xiao, Xia and Cheng59). The activation of oxidative stress could further induce the proliferation and migration of vascular smooth muscle cells to promote atherogenesis(Reference Luo, Xiao and Song60). Moreover, SAH induced macrophage apoptosis and accelerated the formation of atherosclerosis by modulating histone methylation(Reference Xiao, Huang and Zhang61). Previous studies had shown that elevated plasma SAH concentrations were associated with an increased risk of CVD and atherosclerosis(Reference Xiao, Zhang and Wang24). Betaine supplementation were effective in increasing the ratio of SAM:SAH in hepatocytes(Reference Kharbanda, Rogers and Mailliard62). The decrease in the intracellular SAM:SAH ratio inhibited the activity of SAM-dependent methyltransferase(Reference Barak, Beckenhauer and Mailliard52) and affected the normal methylation reaction, which played an important role in the regulation of atherosclerosis(Reference Turunen, Aavik and Yla-Herttuala63). On the whole, the increase of tHcy and SAH and the decrease of SAM and SAM:SAH ratio were related to the risk of CVD, and betaine intake might affect the change of their levels and reduced the risk of CVD.
Our results indicated that daily betaine intake was inversely correlated with and the risk of all-cause and cardiovascular death. After adjustment for cardiovascular risk factors and other potential confounders, the association between dietary betaine intake and risk of all-cause and cardiovascular death was still statistically significant. Higher dietary betaine intake was associated with a decreased risk all-cause and cardiovascular death after fully adjustment for cardiovascular risk factors, other potential confounders and plasma methionine metabolites and vitamins. However, the trend for all-cause mortality was not statistically significant (P = 0·124) after adjustment for plasma methionine metabolites and vitamins. This may be due to that dietary betaine participates in the metabolism of methionine and affects the level of intermediate metabolites, such as SAH, SAM, and tHcy. These metabolites had an impact on health and death risk, after adjusting for these factors, weakening the predictability of betaine. However, our results were not completely consistent with previous studies. Betaine supplementation of mouse during the lactation could increase the content of betaine in breast milk, which could improve glucose homoeostasis and led to reduce lower adiposity of the mouse offspring, while betaine supplementation could increase the Akkermansia abundance in the gut, so as to improve the long-term metabolic health of human offspring(Reference Ribo, Sanchez-Infantes and Martinez-Guino64). In a cohort study of Japanese population, betaine intake was negatively associated with the risk of death from coronary heart disease mortality but not inversely correlated with the risk of mortality from stroke in men, but dietary betaine intake was not associated with the risk of mortality from stroke and coronary heart disease in women(Reference Nagata, Wada and Tamura65). There was a negative relationship between higher betaine consumption and all-cause and breast cancer mortality in an observational population-based Long Island Breast Cancer study(Reference Xu, Gammon and Zeisel66). In a 10·6-year follow-up study with 2606 adults, the researchers found that the increase of dietary betaine has no relationship with the incidence of CVD(Reference Golzarand, Mirmiran and Azizi67). But some studies showed that increased intake of food containing betaine could increase the plasma trimethylamine N-oxide levels and then increase the risk of CVD mortality(Reference Yang, Li and Yang68). This inconsistency may be due to different populations, different health status, follow-up time, and so on. The subjects in our cohort study were patients diagnosed with CAD, which was different with previous studies. Therefore, dietary betaine intake had certain prerequisites for predicting the risk of cardiovascular mortality and the risk of all-cause mortality.
Limitations
Some limitations of this study must also be acknowledged. First, we use self-reported FFQ to record dietary intake of foods which may lead to bias in their estimates of usual food consumption. Hence, the residual confounding factor that over or under report of dietary intake might affect the evaluation of dietary betaine intake was not completely excluded. However, over or under report of dietary intake was associated with age, sex and BMI(Reference Niswah, Rah and Roshita69). In this study, the distribution of age, sex and BMI were not significantly different between the tertiles of dietary betaine intake. So, this confounding factor might be partially balanced among the tertiles of dietary betaine intake. Second, we just collect the dietary intake of betaine at baseline, and it may be changed over the follow-up. Third, other residual confounding cannot completely be ruled out even after carefully adjusting for possible confounders in our studies. Fourth, although we did not measure plasma choline and betaine levels, previous researches have shown that serum betaine level was positively associated with dietary betaine intake(Reference Hamlin, Pauly and Melnyk38,Reference Machek, Zawieja and Heileson70) , and intake of foods with high choline content could double plasma choline levels(Reference Hamlin, Pauly and Melnyk38,Reference Zeisel, Growdon and Wurtman71) . Therefore, it can be assumed that dietary intakes of betaine and choline may approximately reflect the serum status of betaine and choline in individuals. Fifth, subjects in the studies are all patients with CAD in China, and the results are not applicable to other ethnicities. And last, the observational nature of our study makes it impossible to estimate the causality between dietary betaine intake and mortality.
Conclusions
Daily betaine intake was inversely correlated with the risk of all-cause and cardiovascular death. After adjustment for cardiovascular risk factors and other potential confounders, the association between dietary betaine intake and risk of all-cause and cardiovascular death was still statistically significant. Higher dietary betaine intake was associated with a decreased risk of cardiovascular death after fully adjustment for cardiovascular risk factors, other potential confounders and plasma methionine metabolites and vitamins. However, the trend for all-cause mortality was not statistically significant after adjusting for plasma methionine metabolites and vitamins.
Acknowledgements
The authors are particularly grateful to all patients and volunteers for participating in the present study and for their kind assistance in collecting the data and samples.
This work was supported by National Natural Science Foundation of China (82073530, 81730090, 81402672), Guangdong Basic and Applied Basic Research Foundation (No.2022B1515020108), Guangdong Provincial Medical Research Fund (A2019017), and Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006).
The authors’ responsibilities were as follows: Y. X. and Y. T. designed the research; Y. X., S. L., D. W. and B. L. collected the data; Y. X., S. L., K. L., X. D. and J. W. provided essential reagents and materials; Y. X., T. H., L. C. and J. W. analysed the data; YX and Y. T. wrote the manuscript; and all authors read and approved the final manuscript.
None of the authors had a conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522002975