INTRODUCTION
Acute diarrhoeal illness (ADI) is one of the most common childhood illnesses, with over 20 million cases per year in the USA [Reference Pont1]. While most of these are benign, self-limited infections, gastroenteritis in children results in 1·5 million healthcare visits and 200 000 hospitalizations annually [Reference Bhutta, Kliegman, Behrman, Jenson and Stanton2, Reference King3]. The recent introduction of a vaccine against rotavirus, one of the most common pathogens in paediatric gastroenteritis, is expected to reduce the incidence and burden of disease [Reference Tate4]. However, with many viruses and other pathogens implicated as causes of gastroenteritis, prevention strategies must also include efforts to limit exposure to infectious agents.
Most cases of gastroenteritis in children result from person-to-person transmission of the common pathogens. However, investigators have estimated that up to 12% of cases of gastroenteritis in the USA may be due to waterborne disease [Reference Messner5–Reference Reynolds, Mena and Gerba7]. Contamination of water sources may occur in a variety of ways: point sources of pollution; non-point sources such as storm water run-off or contamination of wells or distribution pipes in areas of old infrastructure; and sewage release into waterways in the form of combined or sanitary sewer overflows or sewage blending [Reference Reynolds, Mena and Gerba7–Reference Redman9]. Any of these factors can introduce human pathogens into ground- or surface-water sources, with potential health risks.
Our understanding of waterborne disease is hampered by several factors. First, water quality standards focus primarily on the measurement of indicator organisms, such as faecal coliform bacteria. These organisms are not themselves pathogenic, but indicate the presence of faecal contamination and therefore the likely presence of pathogens. Pathogenic organisms, specifically viruses, are not routinely monitored. Second, most of the research on waterborne illness has focused on disease outbreaks, such as the 1993 Cryptosporidium outbreak in Milwaukee, WI, which affected over 400 000 people [Reference MacKenzie10]. However, such events are likely to represent only a small minority of cases compared to endemic disease [Reference Yoder11]. The extent of and risk factors for endemic waterborne illness have been less well studied.
The aim of this study was to examine the association between water exposures and ADI in children under non-outbreak conditions in a major US metropolitan area. We hypothesized that there would be different risks depending on the child's water source, suggesting endemic waterborne disease. We further hypothesized that the use of bottled water or water filters would be associated with a lower risk of diarrhoeal illness, and that any such protective effect would vary depending on the primary water source.
METHODS
Design
This was a nested case-control study, in which cases and controls were selected from within a prospectively identified cohort. The study was reviewed and approved by the Children's Hospital of Wisconsin Human Research Review Board.
Setting and subjects
The study was conducted at an urban/suburban paediatric emergency department (ED) with an annual census of 60 000 visits. The ED serves a metropolitan area with a population of 1·5 million; about 25% of all ED visits in the metropolitan area for children aged <18 years are to this ED. The cohort consisted of all patients aged <18 years seen in the ED during the 24-month study period (1 January 2007 to 31 December 2008).
Eligible cases were children with ADI, defined as a change in stool consistency (loose or watery) and increased stool frequency, with a duration of ⩽7 days. Patients with vomiting in addition to diarrhoea were eligible, but those with vomiting alone were excluded. Eligible controls were those children seen in the ED for a non-gastrointestinal presenting complaint. Potential controls with diarrhoea in the 3 days prior to the ED visit were excluded, as were any children with a chronic gastrointestinal condition (e.g. inflammatory bowel disease, chronic constipation).
Subject enrolment
Trained clinical research assistants were available in the ED for 16 h each day (08:00 to 24:00 hours) Monday–Friday, and 12 h per day (12:00 to 24:00 hours) on weekends. Eligible cases seen when the research assistant was on duty were approached for enrolment into the study and informed consent was obtained. Upon enrolment of a case, new ED patients were screened for eligible controls, matched on age in five age groups (<1 year, 12–23 months, 24 months-4 years, 5–10 years, 11–17 years), and date of visit (controls were seen no more than 48 h after the case). Then the next eligible control patient was approached for enrolment and consent obtained; screening for controls continued until a control subject was successfully enrolled. One control was generally enrolled for each case; however, because we were separately studying associations between diarrhoeal illness and combined sewer overflows, additional controls (up to four per case) were enrolled in the week after a sewer overflow (three such events occurred during the study period). In addition, if a case had to be excluded after enrolment, that case's control would be assigned to another case meeting the matching criteria. In a few instances, if an eligible control could not be found within the specified 48-h window, two cases were assigned to the same control. Informed consent was obtained from the parent or legal guardian of all subjects, and assent was obtained from subjects aged ⩾8 years.
Because research assistants were not available at all times, we attempted to ascertain completeness of enrolment of potentially eligible subjects. We estimated the number of eligible subjects during the study period by two methods. First, we queried the ED tracking system for subjects with a chief complaint of diarrhoea. Because the system only allows entry of one complaint, and some children who might meet eligibility criteria could have had a different primary complaint (e.g. fever), we considered this to be a lower estimate of eligible subjects, yielding a maximum estimated capture rate. We also identified all children with a discharge diagnosis of gastroenteritis based on any of the following International Classification of Diseases, 9th revision (ICD-9) codes being recorded as the ED discharge diagnosis: specified gastrointestinal infections (ICD-9 codes 001–009·9), or unspecified gastroenteritis (558·9). A sample of these charts was reviewed to determine whether they would have met inclusion criteria. Since some children with these diagnoses might have met exclusion criteria that were not recorded in the chart (e.g. prolonged duration of illness), we considered this to be an upper estimate of eligible subjects, from which we calculated a minimum estimated capture rate.
Data collection
One parent or caregiver of each subject was asked to complete a previously validated water-use survey [Reference Gorelick, Wagner and McLellan12]. The survey included demographic information, and questions about exposure to water for drinking (15 questions), hygiene (four questions) and recreation (five questions). Drinking-water questions included the child's usual use of bottled and tap water (including use of water to mix formula for infants), and use of filters. Detailed data on the type of bottled water (e.g. bottled individual serving vs. large jugs) was not collected. Water source was determined based on the zip code of the child's primary residence: these zip codes were categorized as surface water or ground water. Surface water in our region is provided by municipal water utilities obtaining their water from Lake Michigan, using either chlorination or ozonation for disinfection. Ground water could come from either municipal or private wells. Because water source was determined by zip code and not by specific questions to families, we were unable to specify whether a given subject's source was a private or municipal well, although most homes in our hospital's service area are served by municipal wells that all utilize chlorination for disinfection. There were also questions about other putative risk factors for ADI (presence of ill contacts at home with diarrhoea, attendance at day care). Family income was estimated based on median income for zip code of residence, using US Census Bureau data. Surveys were self-administered, but research assistants were available to provide assistance upon request. A Spanish-language version of the survey was also available, along with a certified interpreter, for Spanish-speaking families with limited English proficiency. Completed surveys were returned at the end of the ED visit; no follow-up after the visit was attempted.
Data analysis
Standard summary statistics were generated. Continuous variables were compared using ANOVA, and proportions were compared using χ2. To account for matching in the design, conditional logistic regression was performed, with case status as the outcome variable. The main effects of interest were household water source [surface water vs. ground (well) water], primary drinking-water type (tap vs. bottled), and use of water filters. Because effect modification was assumed based on clinical grounds (i.e. the effect of one variable would depend on the level of the others), we included interaction terms for these main effects a priori. Potential confounders were included in the model as covariates. We did not perform stepwise analysis. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated. For the three main effects with interactions, stratum-specific odds ratios were calculated and level of significance was set at α<0·05. Analyses were performed using Stata version 8.0 (Stata Corporation, USA).
RESULTS
During the study period, a total of 1446 cases were enrolled initially. One hundred sixty-eight were excluded because of prolonged duration of illness, and 69 were excluded because a matched control could not be found, leaving 1209 cases and 1263 controls.
During the 2-year period, there were 1596 visits with a chief complaint of diarrhoea, yielding an upper estimate of the capture rate of 91%. There were 5257 visits with a discharge diagnosis of gastroenteritis. Of a sample of 30 of these, 18 (60%) met criteria for eligibility based on the review of the chart. We thus estimated a maximum of 3154 eligible patients, with a minimum estimated capture rate of 46%.
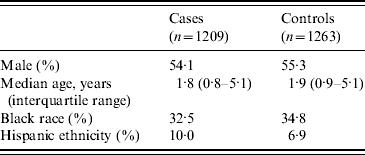
Demographic characteristics of enrolled subjects are shown in Table 1. Although the age range was 0–17·9 years, most subjects were in the younger age groups. For cases, the median duration of symptoms prior to the ED visit was 3 days. The expected seasonal peak during January–April (51% of cases) was observed.
Table 1. Characteristics of study subjects
Complete data were available for all covariates for 90·4% of subjects. The rate of missing data was highest for type of drinking water used (5·7%). Rates of missing data for all variables were similar for cases and controls.
Nearly 80% of subjects lived in zip codes served by a municipal water utility that obtained its water from a surface-water source; 20·5% lived in homes served by ground water from wells. Twenty-seven subjects lived in zip codes served by a mixture of surface and ground water, and since their water source could therefore not be determined, they were excluded from the analysis.
The type of drinking water used by subjects is shown in Table 2. For analysis, we combined the categories of ‘only bottled water’ or ‘mostly bottled water’ into a single category of ‘primarily bottled water’, while the remainder (except those with missing data) were combined and considered as ‘primarily tap water’. We included those reported as drinking no water in the tap-water group, since exposures to tap water would be expected to occur from use of water in cooking, brushing teeth, etc. Race and ethnicity were tied to bottled-water use, with those of black race, Hispanic ethnicity, and other races all having higher (and similar) rates of reported bottled-water use compared to non-Hispanic whites. We therefore included race/ethnicity as a potential confounder in the regression model, using an indicator variable for ‘non-Hispanic white’ vs. ‘all other race/ethnicity’. Use of some type of filter was reported by 17·7% of subjects.
Table 2. Drinking-water use by subjects

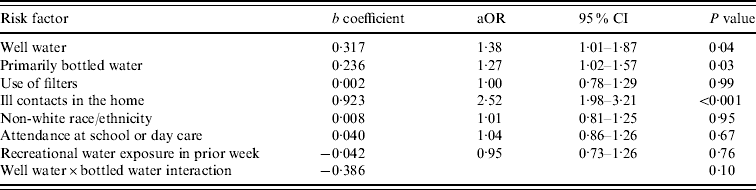
The results of the conditional logistic regression model are shown in Table 3. Although we had a priori reasons for considering interactions between water source and both water type and use of filters, the interaction term between water source and filter use was not statistically significant (P=0·55), we retained the model that included only the interaction between water source and water type. The results for all covariates were essentially unchanged. When adjusted for confounders, use of well water was associated with increased odds of ADI, as was use of bottled water. However, the effect of bottled water differed depending on the household source of drinking water. Of children living in homes served by surface water, the use of primarily bottled water was associated with an increased odds of illness compared to use of primarily tap water (aOR 1·27, 95% CI 1·03–1·58). In contrast, for those whose household is served by well water, bottled water was not significantly associated with diarrhoeal illness (aOR 1·17, 95% CI 0·82–1·68). Use of filters was not associated with a decreased odds of illness.
Table 3. Adjusted odds ratios from conditional logistic regression
aOR, Adjusted odds ratio; CI, confidence interval.
As shown in Table 3, of the other factors studied, only presence of ill contacts at home with diarrhoea was independently associated with increased odds of illness. Attendance at school or day care was not associated with diarrhoeal illness. When the analysis was restricted to infants aged <3 years, the association with day care remained non-significant (aOR 1·13, 95% CI 0·89–1·44), and the adjusted odds ratio for ill contacts was unchanged. We considered the possibility that the association between bottled water and illness could be due to cross-contamination by ill contacts in the home. We therefore performed an analysis stratified by the presence of ill contacts; there was no change in the results.
We did not ask specifically about breastfeeding. It is possible that some infants with no reported exposure to water may have been exclusively breastfed and therefore genuinely not have any water exposure. There were 60 infants aged <6 months who were reported to have no water exposure. We repeated our analysis with these infants excluded, and the point estimates for the adjusted odds ratios and tests of significance remained unchanged.
We were concerned about possible confounding by socioeconomic status (SES), specifically, that lower SES might account for higher disease occurrence in certain zip codes. However, the median income in zip codes served by surface water was 53% lower than in the ground-water zip codes ($36 990 and $56 753, respectively).
DISCUSSION
While water in the USA is generally regarded as very safe, concerns about waterborne illness remain [Reference Reynolds, Mena and Gerba7, Reference MacKenzie10, 13]. In this study, we observed significantly elevated odds of ED visits for ADI in children with household exposure to ground water compared to surface water, suggesting that waterborne transmission plays a role in causing disease in this population living in a major US metropolitan area served by municipal treated-water sources. While the risk of illness is increased in those using ground water, we cannot exclude the possibility of waterborne illness in surface-water users. During the study period, there were no reports of waterborne disease outbreaks in Wisconsin. Our results, therefore, suggest that waterborne transmission is endemic, occurring under baseline conditions.
It is important to note that municipal water sources in our region are fully compliant with Environmental Protection Agency and state regulations; no violations of drinking-water standards were reported by the Milwaukee Water Works, the primary supplier of surface water to the population served by our ED, or by any of the other water utilities in the five counties of southeastern Wisconsin that constitute our primary service area, during the study period. However, drinking water is not routinely monitored for the presence of enteric viruses, which are the cause of most cases of ADI, especially in children. Moreover, our results are consistent with estimates of endemic waterborne illness from community and household intervention studies that have demonstrated decreased risk of gastroenteritis with increases in water treatment, even when water meets relevant drinking-water quality standards [Reference Colford6, Reference Calderon and Craun14]. In the intervention studies published to date, the water source has been surface water. Data on endemic risk from ground-water sources has come from observational studies such as the current study. The existing literature showing increased illness risk due to well water has focused almost exclusively on specific pathogens, primarily protozoa (such as Giardia and Cryptosporidium) and bacteria (e.g. Campylobacter, Legionella) [Reference Craun and Calderon15]. Such infections account for a relatively small proportion of ADI in children. One previous study in Norway, by Kuusi et al., examined gastroenteritis of all causes [Reference Kuusi16]. They found that in children aged <15 years, drinking water from a private well was associated with increased risk of acute gastrointestinal illness (relative risk 3·1, 95% CI 1·4–7·1).
Ground-water sources, especially deep wells, have historically been believed to be relatively free of pathogens due to natural filtration, making an association of ground water with increased illness risk a surprising finding [Reference Reynolds, Mena and Gerba7]. However, our results, are also consistent with microbiological surveillance data that have found substantial proportions (8–42%) of wells tested to have detectable human enteric viruses including hepatitis A, norovirus, enteroviruses, and rotavirus [Reference Reynolds, Mena and Gerba7, Reference Borchardt, Haas and Hunt17, Reference Abbaszagedan, LeChevallier and Gerba18].
Contamination of drinking water may also occur within the distribution system, due to intrusion of pathogen-containing water from the soil into the water pipes through leakage points, especially during transient low or negative pressure events that commonly occur [Reference LeChevalllier19, Reference Tinker20]. One possible explanation for our findings is that the differences in illness occurrence between people from different zip codes is due not to contamination of the respective water sources, but rather due to difference in the integrity of the water distribution infrastructure. However, in our region, the areas served by surface-water sources tend to be older than those utilizing well water; greater infrastructure problems would be expected in those older areas, which would produce a risk pattern contrary to what we found.
Bottled-water use was associated with increased odds of illness in children whose source of household water is surface water, a result we had not anticipated. Bottled water is regulated by the Food and Drug Administration, not the Environmental Protection Agency, and quality and monitoring standards differ. Thus, one potential explanation is relatively greater contamination of bottled water compared to municipal tap water from Lake Michigan. A study in Cleveland found substantially higher average bacterial counts in bottled water compared to the local tap water (also from a Great Lakes surface source) [Reference Lalumandier and Ayers21]; another study from Europe identified norovirus by polymerase chain reaction (PCR) in 33% of mineral water samples tested [Reference Beuret22]. Contamination may also occur after the product is opened by the consumer. This may be especially true for bottled water that is obtained from large jugs either delivered to the home, or from which smaller containers are refilled at the store. We do not have information available on the specific types of bottled water used by our study subjects, and therefore cannot differentiate risks; however, preliminary data from another study in the same ED show that 83% of bottled-water users reported using individual single-serving bottles. An alternative explanation is suggested by Frost et al. who identified lower levels of antibodies to Cryptosporidium in people drinking primarily bottled water, and those using municipal well water [Reference Frost23]. In a subsequent study, antibody levels were inversely correlated with risk of acute gastrointestinal illness. They concluded that repeated exposure to low levels of antigen (i.e. non-viable cysts) in surface-water users conveys protective immunity [Reference Frost24]. Whether a similar protective immunity to other pathogens, specifically viruses, could explain the differential risks based on water source and type in our study remains speculative.
The use of filters was not associated with a decreased risk of illness, even among the consumers of higher-risk well water. Since many point-of-use filtration devices are not designed to remove microbial pathogens [Reference Reynolds, Mena and Gerba7], this result is perhaps not surprising.
Our study is limited by the fact that we examined only ADI resulting in an ED visit, with no data on clinic visits or illness that did not receive medical attention. While other studies have also used ED visits as a marker of community disease occurrence [Reference Tinker20, Reference Schwartz, Levin and Hodge25], our results could reflect simply an association between water use and more severe illness. Conversely, by focusing only on children seeking ED care, we may have underestimated the association between water use and disease incidence. During our study period, only 9% of children with gastroenteritis were admitted to the hospital, suggesting that the cases are not very skewed towards higher levels of severity.
Another limitation is the reliance on self-reported data for water use. The use of a validated questionnaire was designed to reduce any resulting information bias. Most importantly, we have no data on pathogens in either the patients or water. Such data would be helpful in establishing whether the observed association is truly causal. We were unable to distinguish between municipal and private wells; the association between water use and illness may differ based on the type of well. However, we do know that most homes in our region with well water are served by municipal wells that utilize chlorination for disinfection. Since it is generally believed that private wells would pose a greater risk [13], our results may underestimate the risk from such sources. In a case-control study, residual confounding by unmeasured covariates can lead to biased estimates of association. In addition, while matching on age and season minimizes confounding due to these factors, it also makes it impossible to examine any association between these factors and disease risk. Finally, this study was performed in a single geographic area, and the results may not be generalizable to other regions with different water systems and hydrology.
CONCLUSION
We conclude that well-water use and bottled-water use are associated with increased odds of ADI in children in a major US metropolitan area served by municipal treated-water systems. While these results suggest endemic waterborne transmission of gastroenteritis in this setting, further work is needed to confirm these findings and explore the causes.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Children's Research Institute.
DECLARATION OF INTEREST
None.





