In the diets of developed nations, one-quarter of total daily energies is provided by fatty acids that contain one or more double bonds in the molecules. These double bonds are typically positioned in the 3, 6, 7 or 9 carbon atoms from the terminal methyl group. Their geometry is usually cis, i.e. the two hydrogen atoms on the carbons adjacent to the double bond are located in the same side of the carbon chain, resulting in a bent shape and a liquid state at room temperature. However, some fatty acids have one or more double bonds in the trans configuration, i.e. the hydrogen atoms on the carbons adjacent to the double bonds are in opposite sides, resulting in a straight configuration and a solid state at room temperature. Trans-fatty acids are abundant in dairy fat and ruminant meats, but the main source is partially hydrogenated vegetable or fish oils. Production of partially hydrogenated fats to form margarines and shortenings began early in the twentieth century and increased steadily until about the 1960s. The processed vegetable fats displaced animal fats in the diets of most people in industrialised countries. The initial motivation was lower cost, but health benefits were also purported. The average per person consumption of trans-fatty acids from partially hydrogenated oils has remained at about 2 % of energies since the 1960s, because of the increased use of these fats in commercially baked products and fast foods. Since 1990, there has been increasing public-health concern about epidemiological studies, showing that trans-fatty acids increase the risk of CHD such as acute myocardial infarction, cardiovascular risk factors and sudden cardiac death(Reference Steinhert, Rickert and Winkler1–Reference Merchant, Kelemen and de Koning3).
Neutrophils are the first cells that migrate to tissues in response to invading micro-organisms. The antimicrobial function of these phagocytes depends on the release of lytic enzymes stored in cytoplasmatic granules and on the production of reactive oxygen species (ROS). In phagocytes, superoxide is mainly generated by the reaction of oxygen and NADPH through the NADPH oxidase complex(Reference Babior4–Reference Hatanaka, Levada-Pires and Pithon-Curi6). This enzyme system comprises of cytosolic components, p47phox and p67phox, a low molecular weight G-protein, Rac2 and a membrane-associated cytochrome b558. In resting neutrophils, the subunits of the oxidase complex are distributed between cytosol (granules) and the membranes. When the phagocytes are activated, cytosolic components become heavily phosphorylated and migrate to the membrane, where they bind to cytochrome b558 to assemble the active oxidase. Superoxide anion and hydrogen peroxide (H2O2) generated by the NADPH oxidase complex give rise to strong cytolytic agents, such as hypochlorous acid and hydroxyl radical. Neutrophil activation involves a variety of plasma membrane receptors that interact with several compounds including bacterial products, components of the complement and soluble compounds such as cytokines released by other cells. The cascade that leads to ROS production by neutrophils involves G-protein and via phospholipase C the release of inositol trisphosphate and diacylglycerol. Diacylglycerol activates protein kinase C (PKC) that catalyses the phosphorylation of p47phox. NADPH oxidase can also be activated via PI3K. There is evidence that PI3K can regulate the assembly and activity of the neutrophil NADPH oxidase complex in vitro, acting to recruit the enzyme components to phagosome(Reference Marshall, Booth and Stambolic7).
Fatty acids have been shown to regulate immune and inflammatory responses(Reference Yaqoob and Calder8–Reference Gorjão, Cury-Boaventura and de Lima10). In fact, fatty acids have been employed for the treatment of various oxidative stresses involving diseases, such as CHD and rheumatoid arthritis(Reference Wanten and Calder11, Reference Calder12). A recent study from our laboratory has shown that the C18 fatty acids (OA, linoleic and γ-linolenic acids) stimulate ROS production, through NADPH oxidase activation, by human and rat neutrophils(Reference Hatanaka, Levada-Pires and Pithon-Curi6). Evidence has been accumulated that OA and linoleic acid either in vivo and ex vivo induce leucocyte death(Reference Cury-Boaventura, Kanunfre and Gorjão13, Reference Cury-Boaventura, Gorjão and de Lima14), and this effect may occur through oxidative stress(Reference Pompéia, Cury-Boaventura and Curi15–Reference Patel, Ghanim and Ravishankar17). Therefore, these fatty acids are able to modulate neutrophil function(Reference Pereira, Hatanaka and Martins18, Reference Kang, Lee and Jeung19) in addition to their well-known effects on macrophage function, lymphocyte proliferation, cytokine production and modification of natural killer cell activity(Reference Otton, Graziola and Souza20–Reference Waitzberg, Lotierzo and Logullo23).
The afore-mentioned information led us to investigate the effect of C18 trans-MUFA (VA and EA) on rat neutrophil function. The results were compared with those of C18 OA (cis-monounsaturated) and SA (saturated). The following neutrophil functions were studied: phagocytic capacity; production of ROS; candidacidal activity. The involvement of NADPH oxidase, PKC, phosphatidylinositol-3 kinase (PI3K), mitogen-activated protein kinase and NADPH oxidase H+ channel in the effects of the fatty acids was also examined.
Materials and methods
Chemicals
Fatty acids were purchased from MP Biochemicals LLC, Irvine, CA; wortmannin, 3-[1-[3-(dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (GF109203X), diphenyleneiodonium (DPI), rotenone, nitroblue tetrazolium (NBT), zymosan (ZY) A (Saccharomyces cerevisiae), oyster glycogen type II, cytochrome c and sodium nitrite were obtained from Sigma-Aldrich Corporation, St Louis, MO, USA; Roswell Park Memorial Institute (RPMI)-1640 medium and fetal calf serum were from Invitrogen, Carlsbad, CA, USA.
Animals
Male Wistar rats weighing 180–200 g were used throughout the study. These animals were obtained from the Institute of Biomedical Sciences, University of São Paulo. The procedure used in the present study was approved by the Ethical Committee of the Institute of Biomedical Sciences, University of São Paulo.
Peritoneal cell preparation
Male Wistar rats weighing 180–200 g were killed by decapitation without anaesthesia. Neutrophils were obtained by intraperitoneal lavage with 40 ml sterile PBS, 4 h after the intraperitoneal injection of 20 ml sterile oyster glycogen solution (1 %) in PBS. The cells were centrifuged (850 g for 10 min) three times in PBS. The number of viable neutrophils, over 95 % of the total cells harvested from intraperitoneal cavity, was counted in a Neubauer chamber by optical microscopy, using a 1 % Trypan blue solution in saline(Reference Pithon-Curi, Melo and Palanch24–Reference Alba-Loureiro, Hirabara and Mendonça26).
Culture of neutrophils
The cells (5·0 × 106 cells/ml) were seeded in RPMI-1640 medium supplemented with 10 % (v/v) fetal calf serum, 2 mm-glutamine and 20 μg penicillin–streptomycin per ml. The medium was supplemented with OA, EA, VA and SA dissolved in ethanol (0·5 %) at final concentrations of 10–150 μm, as indicated in the Results section. This range of fatty acid concentration was chosen so as to highlight the differences in the effects of C18 fatty acid on neutrophil function. The fatty acids were fully soluble in the concentrations and conditions used.
Preparation of zymosan (Saccharomyces cerevisiae) for the measurement of phagocytosis
Thirty-five milligrams of ZY in 100 ml PBS were boiled for 30 min and washed twice with PBS before use. For opsonisation, 0·5 ml ZY particles (14 mg/ml PBS) were mixed with 0·5 ml rat serum and incubated for 30 min at 37°C. The opsonised ZY particles were then washed and resuspended in PBS at a concentration of 1 mg/ml.
Phagocytosis assay
Neutrophils, which were previously cultured with fatty acids, were incubated (2 × 106 cells per flask) in 1 ml PBS with 2 % (w/v) defatted bovine serum albumin, in the presence of glucose (5 mm), containing opsonised ZY at 37°C for 30 min. The percentage of phagocytosis was determined by counting the cells that had phagocytosed three or more particles of ZY in a counting chamber. We used a procedure similar to that used in our previous studies(Reference Pithon-Curi, Melo and Palanch24, Reference Sampaio, Sousa-e-Silva and Borelli27).
Neutrophil candidacidal activity assay
The candidacidal activity was assessed by the method described by Lehrer & Cline(Reference Lehrer and Cline28). Neutrophils (1 × 106 cells per flask) were incubated in 1 ml RPMI-1640 medium with 10 % (v/v) fetal calf serum, containing 75 μm OA, EA, VA or SA in the presence of Candida albicans (1:1 proportion) for 60 min at 37°C. A control sample was treated with 0·5 % ethanol instead of the fatty acids. After incubation, 100 μl Triton X-100 (5·5 %) were added followed by the addition of 1·9 ml methylene blue solution (0·01 %). At this concentration, Triton causes immediate lysis of neutrophils without causing damage to the Candida cells, whose viability was assessed by methylene blue exclusion in a Neubauer chamber. The candidacidal activity was expressed as a percentage of stained yeast cells in relation to total. Similar procedure was used in our previous study(Reference Sampaio, Sousa-e-Silva and Borelli27).
Measurement of hydrogen peroxide production
The measurement of H2O2 production was performed in the cells that were previously incubated with 100 μm fatty acids. H2O2 production was measured by using the method of phenol red as described by Pick & Mizel(Reference Pick and Mizel29). This is based on the horseradish peroxidase-dependent conversion of phenol red by H2O2 into a coloured compound. Briefly, the cells were incubated in the presence of phenol red and horseradish peroxidase, under an atmosphere of 5 % CO2–95 % air at 37°C during 1 h. After this period, the reaction was stopped by adding 10 μl NaOH solution (1 m) and the amount of H2O2 formed was measured at 620 nm (Spectramax plus; Molecular Devices, Sunnyvale, CA, USA). The production of H2O2 was always determined against standard curves prepared for each experiment.
Nitroblue tetrazolium reduction
Superoxide production was estimated by reduction of NBT, a yellow water-soluble powder that becomes blue and insoluble upon reduction(Reference Madhavi, Das and Prabha30, Reference Schrenzel, Serrander and Banf31). Cells (4 × 106 cells/ml) were incubated for 1 h at 37°C in 0·1 % NBT solution in phosphate-buffered saline with glucose solution: 0·13 mm-NaCl, 2·7 mm-KCl, 1·0 mm-MgCl2, 5 mm-glucose and 10 mm-NaH2PO4/Na2HPO4, pH 7·4. The reaction was stopped by placing the samples on ice. After cell centrifugation, reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication (one pulse of 5 s). Cell debris was pelleted and absorbance of the supernatant was determined at 560 nm in a microtitre plate reader (Spectramax plus; Molecular Devices). Freshly obtained neutrophils were incubated for the determination of NBT reduction in the presence of phorbol myristic acetate (PMA; 162 nm) and GF109203X (5 μm). A similar procedure was used in our previous study(Reference Pompéia, Cury-Boaventura and Curi15).
The main advantage of this method is the possibility to determine both intra- and extracellular superoxide production. Reducing agents such as NO− can also reduce NBT and could jeopardise the measurement of O2− ∙ production(Reference Vasquez-Vivar, Hogg and Martasek32). In a control experiment, the cells were pretreated with N-nitro-l-Arg, an inhibitor of NO synthase. The addition of N-nitro-l-Arg to the incubation medium did not affect the results of NBT reduction (data not shown).
Protein determination
Protein content of cell preparations was measured by the method of Bradford(Reference Bradford33), using bovine serum albumin as a standard.
Statistical analysis
All the experiments were performed in triplicate and repeated at least three times. The results are presented as means and standard deviations. Statistical significance of the differences between groups was assessed by the two-tailed, unpaired Student's t test or ANOVA as appropriate. Significance was considered for P < 0·05.
Results
Phagocytosis capacity
Neutrophils treated with 100 μm EA and VA had their phagocytic capacity enhanced by 51 %, whereas OA caused an increase of 61 % (Table 1). The addition of SA to the incubation medium did not cause any effect.
Table 1 Effect of the fatty acids on neutrophil phagocytic capacity†
(Mean values and standard deviations of nine determinations)
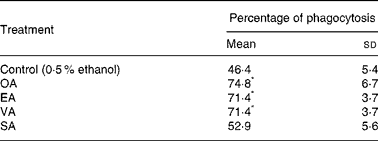
OA, oleic acid; EA, elaidic acid; VA, vacceric acid; SA, stearic acid; RPMI, Roswell Park Memorial Institute; PBS, phosphate-buffered saline.
* Mean value was significantly different from that of the control (p < 0·05).
† Neutrophils (5 × 106 cells/ml) were incubated for 3 h at 37°C in RPMI-1640 culture medium in the presence of the fatty acids at 100 μm or 0·5 % ethanol. After this period, the cells were pelleted and washed twice with PBS. The cells (2 × 106 cells/ml) were then incubated for 40 min in PBS containing 5 mm-glucose, 2 % albumin and zymosan particles.
Candidacidal activity
Trans-MUFA increased the killing of opsonised C. albicans by neutrophils by 30–40 %, after 60 min incubation, when compared with control cells treated with 0·5 % ethanol (Table 2). Stearic acid also did not cause a significant effect.
Table 2 Effect of the fatty acids on neutrophil candidacidal activity†
(Mean values and standard deviations of nine determinations)
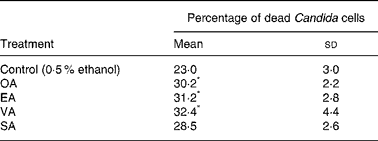
OA, oleic acid; EA, elaidic acid; VA, vacceric acid; SA, stearic acid; RPMI, Roswell Park Memorial Institute.
* Mean value was significantly different from that of the control (p < 0·05).
† Neutrophils (1 × 106 cells/ml) were incubated for 3 h at 37°C in 1 ml RPMI-1640 culture medium in the presence of the fatty acids at 100 μm or 0·5 % ethanol (control) and Candida albicans at 1:1 proportion. After this period, 100 μl Triton-X-100 (5·5 %) and 1·9 ml methylene blue (0·01 %) were added to the incubation medium to evaluate the proportion of dead Candida cells.
Hydrogen peroxide production
MUFA increased H2O2 production by PMA-stimulated neutrophils but had no effect on unstimulated cells (Fig. 1). Stearic acid did not increase H2O2 production when compared with the control group.
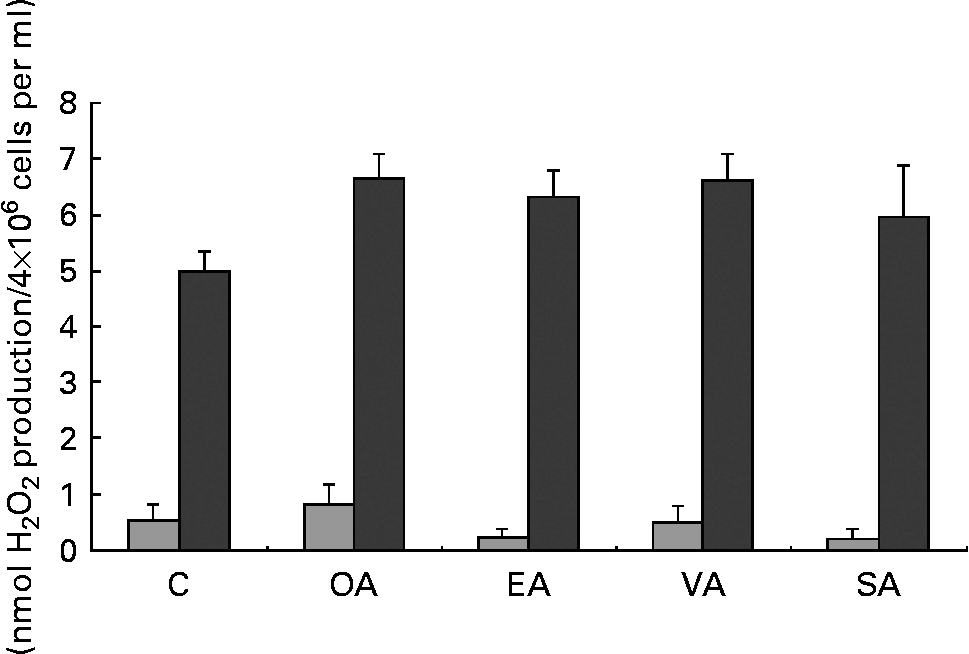
Fig. 1 Production of hydrogen peroxide (H2O2) by neutrophils treated with OA, EA, VA or SA. Cells (4 × 106 cells/ml) were incubated for 3 h in Roswell Park Memorial Institute (RPMI)-1640 culture medium with 10 % fetal calf serum and 100 μm fatty acids. Control cells were incubated with 0·5 % ethanol. The bars represent the results of H2O2 production obtained under basal (![]() ) and phorbol myristic acetate-stimulated (■) conditions. The values are presented as means and standard deviations of nine determinations (n 9). *Mean value was significantly different from that of the control (p < 0·05). C, control; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid.
) and phorbol myristic acetate-stimulated (■) conditions. The values are presented as means and standard deviations of nine determinations (n 9). *Mean value was significantly different from that of the control (p < 0·05). C, control; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid.
Determination of nitroblue tetrazolium reduction
Neutrophils exposed to 100 μm MUFA for 1 h showed a 3-fold increase in NBT reduction when compared with the control. Stearic acid also promoted an increase in NBT reduction but at much less extend by 60 % (Fig. 2). To better characterise the effect of the fatty acids on superoxide production, dose–response curves and kinetics of NBT reduction were performed (Figs. 3 and 4, respectively). The concentrations used were 10, 20, 50, 100 and 150 μm and the incubation periods were 15, 30, 45 and 60 min. As shown by the dose–response curve, the effect of the fatty acids on NBT reduction was as follows: OA>EA = VA>SA (Fig. 4). The effect of the fatty acids was observed already after 15 min incubation (about 70 % increase), and it did not markedly change afterwards (Fig. 5).

Fig. 2 Nitroblue tetrazolium (NBT) reduction by neutrophils incubated in the presence of OA, EA, VA or SA. Neutrophils (4 × 106 cells/ml) were incubated for 1 h at 37°C in phosphate-buffered saline with glucose solution containing 0·1 % NBT in the presence of 100 μm fatty acids under constant stirring. The reaction was stopped by placing the samples in ice. After centrifugation, reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication (one pulse of 5 s). Cell debris was pelleted and absorbance of the supernatant was determined at 560 nm. The values are expressed as means and standard deviations of nine determinations (n 9). * Mean value was significantly different from that of the control (p < 0·05). C, control; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid.

Fig. 3 Dose–response curve of nitroblue tetrazolium (NBT) reduction in neutrophils incubated in the presence of oleic (–♦–), elaidic (–□–), vaccenic (–▲–) or stearic (– × –) acid. Neutrophils (4 × 106 cells/ml) were treated with different concentrations (10, 20, 50, 100 and 150 μm) of fatty acids in phosphate-buffered saline with glucose solution containing 0·1 % NBT for 1 h at 37°C under constant stirring. After centrifugation, reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. Cell debris was pelleted and absorbance of the supernatant was determined at 560 nm. The results are expressed as percentage of increase when compared with the control group (100 %) from two experiments.

Fig. 4 Kinetics of nitroblue tetrazolium (NBT) reduction induced by (a) oleic, –■–; ethanol, –⋄–, (b) elaidic, –♦–; ethanol, –⋄–, (c) vaccenic, –♦–; ethanol, –⋄– and (d) stearic, –▲–; ethanol, –⋄– acids in incubated neutrophils. Neutrophils (4 × 106 cells/ml) were incubated in the presence of the fatty acids (75 μm) in phosphate-buffered saline with glucose solution containing 0·1 % NBT for 15, 30, 45 and 60 min at 37°C under constant stirring. After centrifugation, reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. Cell debris was pelleted and absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9).

Fig. 5 Effect of diphenyleneiodonium (DPI), an NADPH oxidase inhibitor, on nitroblue tetrazolium (NBT) reduction in neutrophils treated with fatty acids, phorbol myristic acetate (PMA) or zymosan (ZY). Cells (4 × 106 cells/ml) were pre-incubated in phosphate-buffered saline with glucose (PBSG) solution with 0·5 % ethanol (control;![]() ) or DPI chloride (20 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, 0·5 % ethanol, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were then incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid; PMA, phorbol myristic acetate; ZY, zymosan. *P < 0·05 due to the effect of DPI.
) or DPI chloride (20 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, 0·5 % ethanol, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were then incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid; PMA, phorbol myristic acetate; ZY, zymosan. *P < 0·05 due to the effect of DPI.
Mechanisms by which oleic, elaidic and vaccenic acids enhance superoxide anion production
In order to investigate whether superoxide production induced by the fatty acids involves the activation of this system, an NADPH oxidase inhibitor, DPI, was used(Reference O'Donnell, Tew and England34). The cells were also treated with two compounds that activate NADPH oxidase by different mechanisms, PMA and ZY. PMA activates PKC, which phosphorylates p47phox, a cytosolic component of NADPH oxidase, initiating the activation of the NADPH oxidase complex(Reference El Benna, Han and Park35). ZY, in turn, interacts with a membrane receptor and leads to NADPH oxidase activation.
The decrease in NBT reduction observed in the cells treated with PMA and ZY stimulation was much greater than that induced by the treatment with MUFA. DPI caused a 70 % reduction in superoxide production induced by the fatty acids (Fig. 6). This effect of DPI was still more pronounced in the presence of PMA and ZY (Fig. 6).

Fig. 6 Effect of 3-[1-[3-(dimethylamino)propyl]-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (GF109203X), a protein kinase C inhibitor, on nitroblue tetrazolium (NBT) reduction induced by the fatty acids, phorbol myristic acetate (PMA) and zymosan (ZY). Neutrophils (4 × 106 cells/ml) were incubated in phosphate-buffered saline with glucose (PBSG) solution containing 0·5 % DMSO (control;![]() ) or GF109203X (10 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, 0·5 % DMSO, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were then incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of GF109203X. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid; PMA, phorbol myristic acetate; ZY, zymosan.
) or GF109203X (10 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, 0·5 % DMSO, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were then incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of GF109203X. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; SA, stearic acid; PMA, phorbol myristic acetate; ZY, zymosan.
Neutrophils were also treated with GF109203X, a PKC inhibitor(Reference Toullec, Pianetti and Coste36). PMA-induced NBT reduction was completely abolished by GF109203X, whereas the effect of ZY was only partially reversed (Fig. 7). However, GF109203X did not affect the NBT reduction induced by the fatty acids.
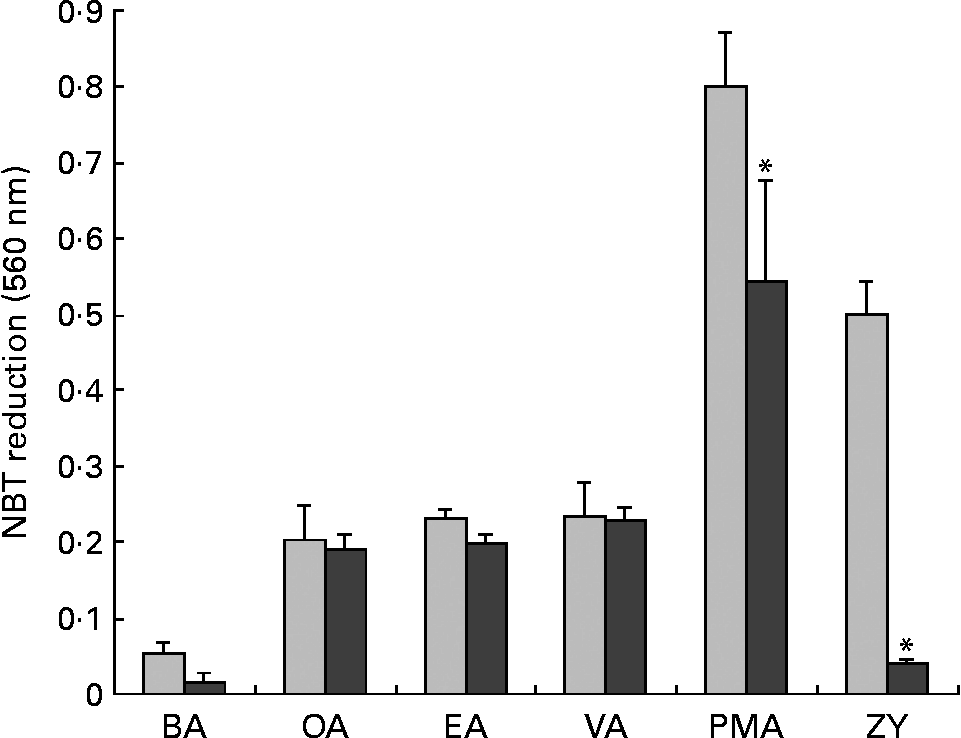
Fig. 7 Effect of wortmannin, a phosphatidylinositol-3 kinase inhibitor, on nitroblue tetrazolium (NBT) reduction induced by fatty acids, phorbol myristic acetate (PMA) or zymosan (ZY). Neutrophils (4 × 106 cells/ml) were incubated in phosphate-buffered saline with glucose (PBSG) solution in the absence (PBS;![]() ) or presence of wortmannin (200 nm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, fatty acids (150 μm), PMA (40 nM) or ZY (100 particles per cell) was added and the cells were again incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of wortmannin. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; PMA, phorbol myristic acetate; ZY, zymosan.
) or presence of wortmannin (200 nm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, fatty acids (150 μm), PMA (40 nM) or ZY (100 particles per cell) was added and the cells were again incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of wortmannin. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid; PMA, phorbol myristic acetate; ZY, zymosan.
In order to investigate whether MUFA induce NBT reduction through PI3K activation, neutrophils were pre-incubated with wortmannin, an inhibitor of PI3K(Reference Ellson, Davidson and Anderson37). The NBT reduction induced by PMA was significantly reduced and that by ZY was fully abolished. However, fatty acid-induced NBT reduction was not affected by the treatment with wortmannin (Fig. 7).
The administration of Zn2+, an H+ channel inhibitor(Reference Henderson38, Reference Levy, Lowenthal and Dana39), caused a 60 % decrease in the fatty acid- and ZY-induced NBT reduction, whereas the decrease in the reduction induced by PMA was only 30 % (Fig. 8).

Fig. 8 Effect of zinc, an NADPH oxidase H+ channel inhibitor, on nitroblue tetrazolium (NBT) reduction induced by fatty acids, phorbol myristic acetate (PMA) or zymosan (ZY)-stimulated neutrophils. Neutrophils (4 × 106 cells/ml) were incubated in phosphate-buffered saline with glucose (PBSG) solution in the absence (phosphate-buffered saline; ![]() ) or in the presence of (zinc chloride 100 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were again incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of Zn2+. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid.
) or in the presence of (zinc chloride 100 μm; ■) for 30 min at 37°C. One volume of PBSG solution containing 0·1 % NBT, fatty acids (150 μm), PMA (40 nm) or ZY (100 particles per cell) was added and the cells were again incubated at 37°C for 1 h. After this period, the cells were pelleted and reduced NBT was solubilised in 100 μl acetic acid solution (50 %) upon sonication. After centrifugation, absorbance of the supernatant was determined at 560 nm. The results are expressed as means and standard deviations of nine determinations (n 9). *P < 0·05 due to the effect of Zn2+. BA, basal; OA, oleic acid; EA, elaidic acid; VA, vaccenic acid.
The priming role of the MUFA on the PMA and ZY responses needs to be better discussed with respect to previous reports on this aspect.
Discussion
The effect of C18 cis- and trans-monounsaturated and SFA on neutrophil function was investigated. Phagocytic capacity of neutrophils was increased by MUFA, and this effect did not depend on the double-bond space configuration cis or trans. The position of the double bond in the aliphatic chain did not have any influence either. In fact, neutrophil phagocytosis capacity was not different between EA (18 : 1-9t) and VA (18 : 1-11t).
Neutrophils treated with cis- and trans-MUFA for 3 h showed a significant increase of H2O2 production. Thus, the increase in phagocytic capacity induced by MUFA was also followed by an increment of H2O2 production. Again, SA did not cause important change in H2O2 production. So far, the presence of double bonds in the C18 fatty acid regardless of the cis or trans configuration seems to play an important role in neutrophil activation.
Treatment with cis- and trans-MUFA also enhanced candidacidal activity. This result indicates that the increase in phagocytic capacity and ROS production effectively contributes to micro-organism destruction. Other fatty acids have also been shown to control neutrophil cytolytic activity. Neutrophils treated with long-chain PUFA, in particular arachidonic, eicosapentaenoic and DHA, have their antiparasitic activity against Plasmodium falciparum enhanced(Reference Kumaratilake, Ferrante and Robinson40). On the other hand, bacterial killing activity of neutrophils is significantly reduced by the treatment with medium-chain triacylglycerol emulsion(Reference Waitzberg, Lotierzo and Logullo23, Reference Bellinati-Pires, Waitzberg and Salgado41, Reference Wanten, Curfs and Meis42).
Neutrophils pre-exposed to the MUFA showed an increased response to PMA (Fig. 1), suggesting that these fatty acids prime the cells. Similar findings have been previously reported for PUFA(Reference Ferrante, Carman and Nandoskar43, Reference Huang, Hii and Rathjen44). This priming effect may be associated with the increased neutrophil phagocytosis capacity and microbial killing activity induced by MUFA treatment.
Hydrogen peroxide is formed upon dismutation of superoxide anion (O2− ∙). The exposure of neutrophils to MUFA caused a marked increase of NBT reduction. This difference can be explained by the fact that the basal values of O2− ∙ production were about four times greater in pre-incubated neutrophils when compared with freshly obtained ones. Therefore, cells pre-incubated with MUFA show increased capacity to produce O2− ∙. Oleic acid had a slightly more pronounced effect than trans-fatty acids on NBT reduction. Previous study has shown that OA stimulates neutrophil adherence and degranulation even at lower concentrations(Reference Bates, Ferrante and Smithers45).
Badway et al. (Reference Badway, Curnute and Robinson46) reported that cis-unsaturated fatty acids stimulate the release of O2− ∙ from neutrophils. This effect was not observed for saturated SA. Other studies(Reference Poulos, Robinson and Ferrante47–Reference Robinson, Hll and Ferrante49) showed that the intensity of the response increases with the unsaturation degree of the fatty acids. The present observations and those from others support the proposition that superoxide production induced by C18 fatty acids in neutrophils increased with the presence of unsaturation in the molecule, but was not influenced by the position of the double bond in the carbon chain. By contrast, however, at lower concentrations (from 2·5 to 10 μm), cis- and trans-MUFA had opposite effects on neutrophil function(Reference Steinbeck, Robinson and Karnovsky50). The differences in the rate of incorporation of the fatty acids into the plasma membrane under these latter conditions may explain these observations.
Diphenyleneiodonium, an NADPH oxidase inhibitor, lowered NBT reduction induced by MUFA. The same effect of DPI was observed in the cells treated with PMA and ZY, confirming that the source of superoxide was NADPH oxidase. Although DPI can inhibit several other flavoproteins, such as those of the mitochondrial electron transport chain, a specific inhibitor of this superoxide-producing site (rotenone) was ineffective to abolish the increase in NBT reduction induced by the fatty acids (data not shown). These results suggest that cis- and trans-MUFA stimulate ROS production through the activation of NADPH oxidase in neutrophils.
Nitroblue tetrazolium reduction induced by PMA was strongly inhibited also by the GF109203X treatment. This drug also inhibited NBT reduction in cells stimulated with ZY. However, NBT reduction induced by OA, EA and VA was not altered by GF109203X. These observations support the proposition that activation of NADPH oxidase by MUFA did not involve PKC as one could expect(Reference Bosca, Diaz-Guerra and Mojena51, Reference Padma and Das52). Trans- and cis-MUFA also did not activate respiratory burst in neutrophils through the activation of PI3K. Wortmannin treatment completely inhibited NBT reduction induced by ZY. However, the drug did not affect NBT reduction in cells treated with OA, EA and VA. These findings indicate that PI3K is not the mechanism involved in the activation of neutrophil NADPH oxidase induced by trans- and cis-MUFA. This contrasts with the requirement for PKC(Reference Hii, Huang and Bilney53) and PI3K(Reference Hii, Moghadammi and Dunbar54) with PUFA such as arachidonic acid.
The possibility that MUFA may lead to opening of NADPH oxidase H+ channel in neutrophils was investigated. Single-electron transfer from internal NADPH to external oxygen is an electrogenic process and the efflux of H+ through the H+ channel is necessary for charge compensation(Reference Henderson38). The channel is voltage gated, i.e. the depolarisation is required to initiate H+ efflux. However, the H+ channel may be gated by ΔpH or by a combination of both membrane potential and ΔpH, i.e. the proton motive force. Henderson & Chappell(Reference Henderson and Chappell55) reported that arachidonate activates the H+ channel. The addition of Zn2+, an H+ channel inhibitor, caused a 60 % decrease in superoxide production stimulated by the fatty acids. These findings indicate that fatty acids may activate NADPH oxidase via the H+ channel. It remains to be investigated whether MUFA activate NADPH oxidase directly or by releasing arachidonic acid via phospholipase A2 stimulation.
The results presented herein indicate that OA, EA and VA stimulate neutrophil phagocytosis, candidacidal activity and production of ROS. The effect of the fatty acids on ROS production occurred by the activation of NADPH oxidase through the H+ channel opening. There was no marked difference in the effects of cis- and trans-MUFA, but SA had no significant effect. These findings support the proposition that undesirable effects of trans-MUFA, such as induction of insulin resistance(Reference Simopoulos56), may be associated with their pro-inflammatory actions.
Acknowledgements
The authors declare no conflict of interest. R. P. developed the experimental work of his PhD thesis and R. C. was his supervisor. The present research has been supported by FAPESP, CNPq, CAPES and UNICSUL University. The authors are grateful to Drs L. R. Lopes, S. C. Sampaio and C. M. Peres for their valuable contribution during the development of the experiments and to the technical assistance of J. R. Mendonça and G. de Souza. The authors are indebted to E. P. Portioli Silva for revision and final preparation of the manuscript.












