Herbal cannabis contains over 400 compounds including over 60 cannabinoids, which are aryl-substituted meroterpenes unique to the plant genus Cannabis. The pharmacology of most of the cannabinoids is largely unknown but the most potent psychoactive agent, ▵9-tetrahydrocannabinol (▵9-THC, or THC), has been isolated, synthesised and much studied. Other plant cannabinoids include ▵8-THC, cannabinol and cannabidiol (Fig. 1, Table 1). These and other cannabinoids have additive, synergistic or antagonistic effects with THC and may modify its actions when herbal cannabis is smoked. Synthetic cannabinoids such as nabilone and others are also available for therapeutic and research purposes. Non-cannabinoid constituents of the plant are similar to those found in tobacco (with the exception of nicotine). Recent research on the pharmacology and effects of cannabis and cannabinoids is briefly reviewed here.
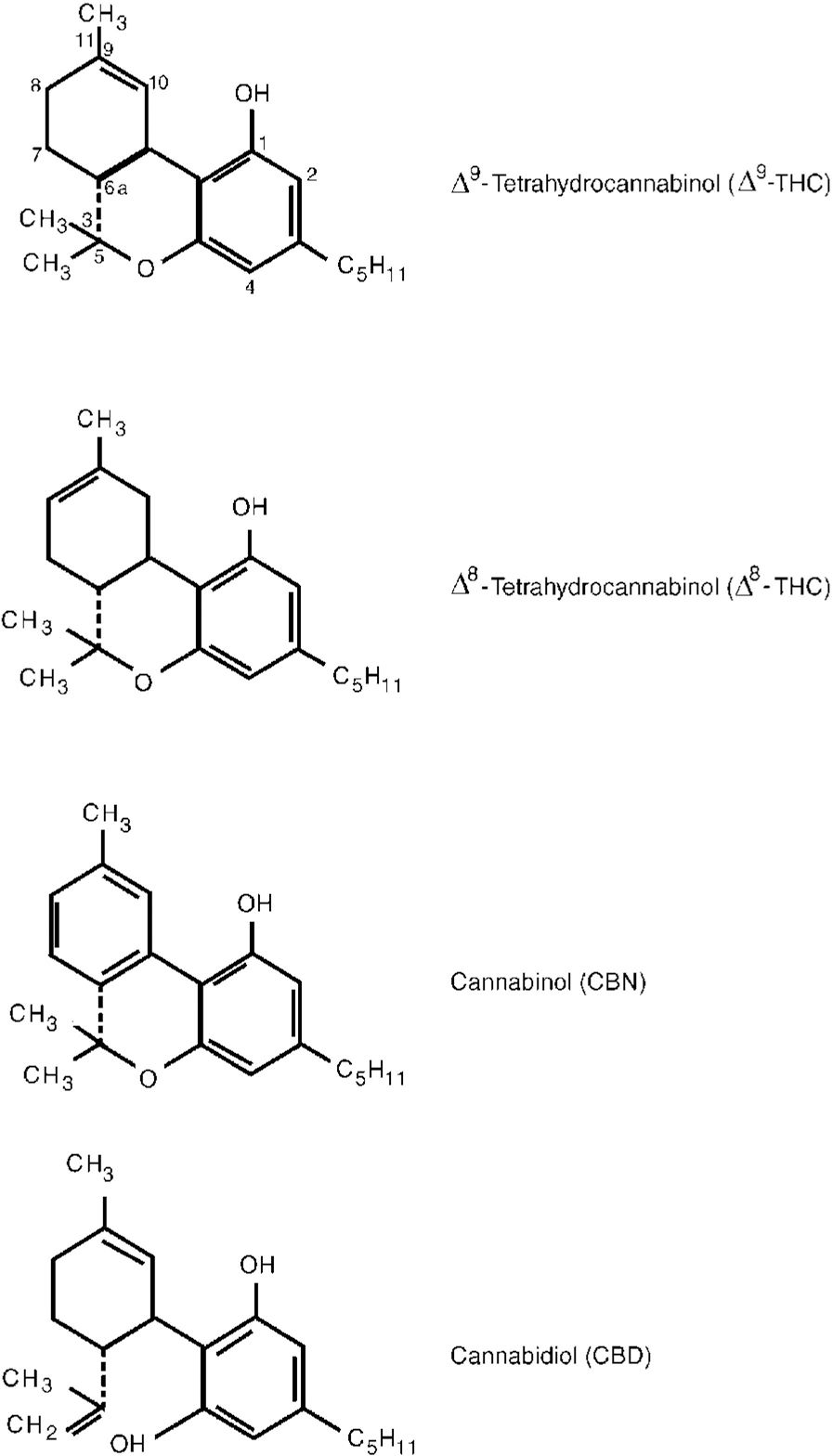
Fig. 1 Chemical structure of main cannabinoids in Cannabis sativa.
Table 1 Some natural cannabinoids and their properties
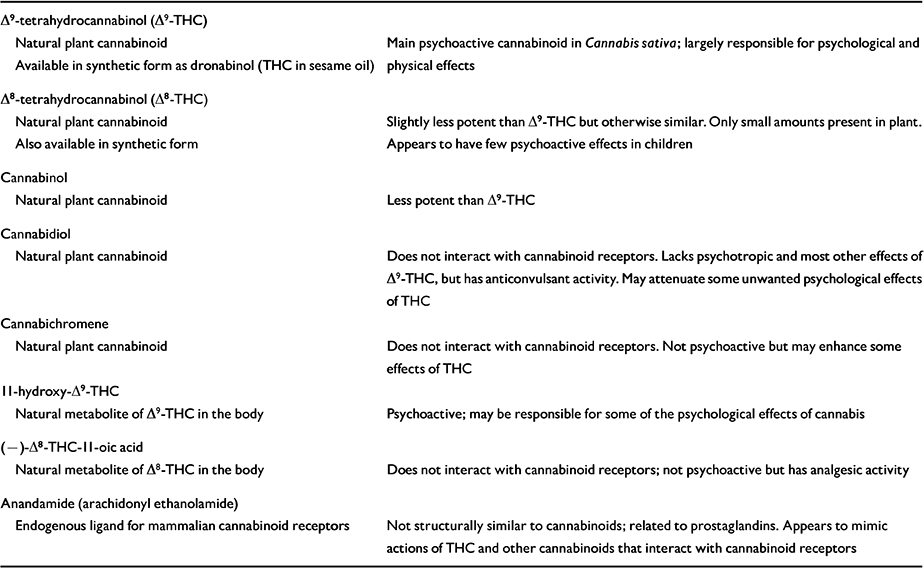
| Δ9-tetrahydrocannabinol (Δ9-THC) | |
| Natural plant cannabinoid | Main psychoactive cannabinoid in Cannabis sativa; largely responsible for psychological and physical effects |
| Available in synthetic form as dronabinol (THC in sesame oil) | |
| Δ8-tetrahydrocannabinol (Δ8-THC) | |
| Natural plant cannabinoid | Slightly less potent than Δ9-THC but otherwise similar. Only small amounts present in plant. |
| Also available in synthetic form | Appears to have few psychoactive effects in children |
| Cannabinol | |
| Natural plant cannabinoid | Less potent than Δ9-THC |
| Cannabidiol | |
| Natural plant cannabinoid | Does not interact with cannabinoid receptors. Lacks psychotropic and most other effects of Δ9-THC, but has anticonvulsant activity. May attenuate some unwanted psychological effects of THC |
| Cannabichromene | |
| Natural plant cannabinoid | Does not interact with cannabinoid receptors. Not psychoactive but may enhance some effects of THC |
| II-hydroxy-Δ9-THC | |
| Natural metabolite of Δ9-THC in the body | Psychoactive; may be responsible for some of the psychological effects of cannabis |
| (—)-Δ8-THC-II-oic acid | |
| Natural metabolite of Δ8-THC in the body | Does not interact with cannabinoid receptors; not psychoactive but has analgesic activity |
| Anandamide (arachidonyl ethanolamide) | |
| Endogenous ligand for mammalian cannabinoid receptors | Not structurally similar to cannabinoids; related to prostaglandins. Appears to mimic actions of THC and other cannabinoids that interact with cannabinoid receptors |
SOURCES OF CANNABINOIDS
Cannabinoids are present in the stalks, leaves, flowers and seeds of the plant, and also in the resin secreted by the female plant. The THC content varies tremendously between different sources and preparations of cannabis (Table 2). Over the past 20 years, sophisticated cultivation (such as hydroponic farming) and plant-breeding techniques have greatly increased the potency of cannabis products. In the ‘flower power’ days of the 1960s and 1970s an average reefer contained about 10 mg of THC. Now a joint made out of skunkweed, netherweed and other potent subspecies of Cannabis sativa may contain around 150 mg of THC, or 300 mg if laced with hashish oil. Thus, the modern cannabis smoker may be exposed to doses of THC many times greater than his or her counterpart in the 1960s and 1970s (Reference Mendelson and MeltzerMendelson, 1987; Reference Gold and MillerGold, 1991; Reference Schwartz, Nahas and LatourSchwartz, 1991; World Health Organization, 1997; Reference SolowijSolowij, 1998). This fact is important since the effects of THC are dose-related and most of the research on cannabis was carried out in the 1970s using doses of 5-25 mg THC (World Health Organization, 1997). Gold (Reference Gold and Miller1991, p. 356) remarks: “ This single fact has made obsolete much of what we once knew about the risks and consequences of marijuana use”.
Table 2 Preparations of cannabis (USA and UK)
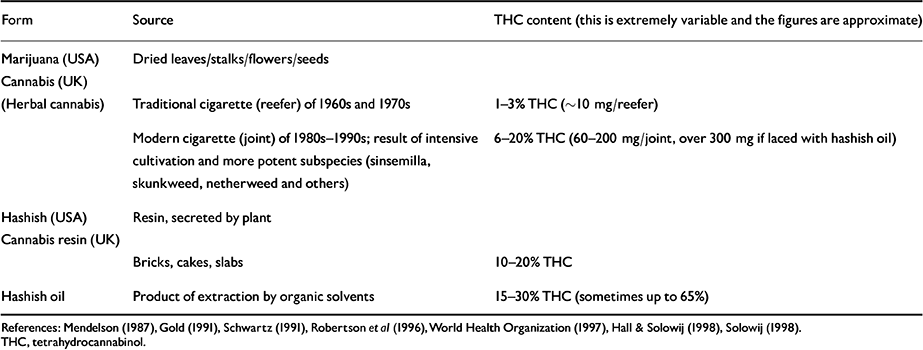
| Form | Source | THC content (this is extremely variable and the figures are approximate) |
|---|---|---|
| Marijuana (USA) | Dried leaves/stalks/flowers/seeds | |
| Cannabis (UK) | ||
| (Herbal cannabis) | Traditional cigarette (reefer) of 1960s and 1970s | 1-3% THC (∼10 mg/reefer) |
| Modern cigarette (joint) of 1980s-1990s; result of intensive cultivation and more potent subspecies (sinsemilla, skunkweed, netherweed and others) | 6-20% THC (60-200 mg/joint, over 300 mg if laced with hashish oil) | |
| Hashish (USA) | Resin, secreted by plant | |
| Cannabis resin (UK) | ||
| Bricks, cakes, slabs | 10-20% THC | |
| Hashish oil | Product of extraction by organic solvents | 15-30% THC (sometimes up to 65%) |
In the UK at present, many recreational users grow their own supplies of high-potency cannabis (exact details of how to grow it can be obtained on the internet). Another main source is imports from Holland (also high-potency) and home growers can obtain seeds in Amsterdam at £10-£50 for 10 seeds, depending on potency. Cannabis can be smoked as joints, from pipes, or from ‘buckets’, by inhaling from a mass of plant or resin ignited in a sawn-off plastic bottle. It can also be eaten, baked into cookies or cakes or occasionally drunk as an extract. It is unsuitable for intravenous use as it is relatively water insoluble, although it has been dissolved in alcohol and delivered as a fast-flowing saline infusion for research purposes.
PREVALENCE OF CANNABIS USE
The prevalence of cannabis use has increased markedly over the past decade in young people in the UK, although patterns of consumption vary between different social groups. A survey of 3075 university students from 10 UK universities (Reference Webb, Ashton and KellyWebb et al, 1996) found that about 60% had some experience with cannabis; nearly 25% had tried it more than once or twice and 20% of students reported regular use (weekly or more frequently). Experience with cannabis had usually started at school, and other surveys have shown that 30-40% of 15- to 16-year-olds have tried it (Reference Miller and PlantMiller & Plant, 1996).
Among 785 second-year medical students from seven UK medical schools surveyed in 1996, 46% reported cannabis use and 10% were taking it at least once a week (Reference Webb, Ashton and KellyWebb et al, 1998). A survey of 90 house officers found that nearly 30% reported current cannabis use and 11% used it weekly or monthly (Reference Birch, Ashton and KamaliBirch et al, 1998).
These users are fairly moderate compared with some others. Some users report daily cannabis use, smoking up to 15 or more joints daily. Many of these are unemployed youths who smoke to obtain a high level of intoxication and may be exposed to several hundreds of milligrams of THC daily. Other groups with a high prevalence of cannabis use are alcohol and polydrug misusers and psychiatric patients.
PHARMACOKINETICS OF CANNABINOIDS
The pharmacokinetics of cannabinoids are reviewed by Agurell et al (Reference Agurell, Halldin and Lindgren1986) and Maykut (Reference Maykut1985) and others. About 50% of the THC in a joint of herbal cannabis is inhaled in the mainstream smoke; nearly all of this is absorbed through the lungs, rapidly enters the bloodstream and reaches the brain within minutes. Effects are perceptible within seconds and fully apparent in a few minutes. Bioavailability after oral ingestion is much less; blood concentrations reached are 25-30% of those obtained by smoking the same dose, partly because of first-pass metabolism in the liver. The onset of effect is delayed (0.5-2 hours) but the duration is prolonged because of continued slow absorption from the gut.
Once absorbed, THC and other cannabinoids are rapidly distributed to all other tissues at rates dependent on the blood flow (Fig. 2). Because they are extremely lipid soluble, cannabinoids accumulate in fatty tissues, reaching peak concentrations in 4-5 days. They are then slowly released back into other body compartments, including the brain. Because of the sequestration in fat, the tissue elimination half-life of THC is about 7 days, and complete elimination of a single dose may take up to 30 days (Reference MaykutMaykut, 1985). Clearly, with repeated dosage, high levels of cannabinoids can accumulate in the body and continue to reach the brain. Within the brain, THC and other cannabinoids are differentially distributed. High concentrations are reached in neocortical, limbic, sensory and motor areas.
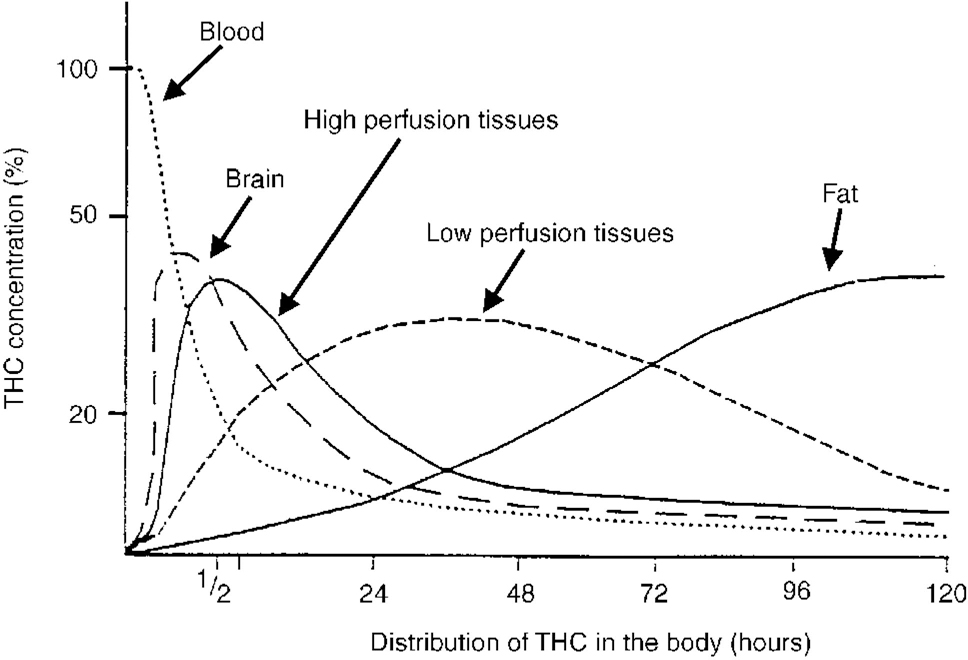
Fig. 2 Distribution of THC in the body. The distribution of THC after a single administration in plasma and body tissues. Note the ‘biphasic’ disappearance in plasma. The rapid phase (in minutes) indicates a rapid uptake of the drug by fat-containing tissues. The slow phase (in days) shows the release of THC by these tissues (Reference Nahas and RichterNahas, 1975). THC, tetrahydrocannabinol.
Cannabinoids are metabolised in the liver. A major metabolite is 11-hydroxy-THC which is possibly more potent than THC itself and may be responsible for some of the effects of cannabis. More than 20 other metabolites are known, some of which are psychoactive and all of which have long half-lives of several days. The metabolites are partly excreted in the urine (25%) but mainly into the gut (65%) from which they are reabsorbed, further prolonging their actions. Because of the pharmacokinetic characteristics of cannabinoids — both the sequestration in fat and the presence of active metabolites — there is a very poor relationship between plasma or urine concentrations and degree of cannabinoid-induced intoxication.
PHARMACODYNAMICS OF CANNABINOIDS
Cannabinoids exert their effect by interaction with specific endogenous cannabinoid receptors, discovered by Devane et al (Reference Devane, Dysarz and Johnson1988). Neuronal cannabinoid receptors are termed CB1 receptors and have been found in rat, guinea pig, dog, monkey, pig and human brains and peripheral nerves. A second cannabinoid receptor, the CB2 receptor, was identified by Munro et al (Reference Munro, Thomas and Abu-Shaar1993) in macrophages in the spleen and is also present in other immune cells. The distribution of CB1 receptors is very similar to that of injected THC and includes cerebral cortex, limbic areas (including hippocampus and amygdala), basal ganglia, cerebellum, thalamus and brainstem (Reference Herkenham and PertweeHerkenham, 1995).
The discovery of cannabinoid receptors naturally stimulated a search for an endogenous ligand with which the receptors naturally interact. Such a substance was isolated from the pig brain by Devane et al (Reference Devane, Hanus and Breuer1992). It was found to be chemically different from plant cannabinoids: it is a derivative of the fatty acid arachidonic acid (arachidonyl ethanolamide) related to the prostaglandins (Fig. 3). This endogenous substance was named anandamide after the Sanskrit word for bliss, ananda. It has a high affinity for CB1 receptors and has most of the actions of THC. Thus, the story of opium, opioid receptors and endogenous opioids is now repeated with cannabis, cannabinoid receptors and anandamides.
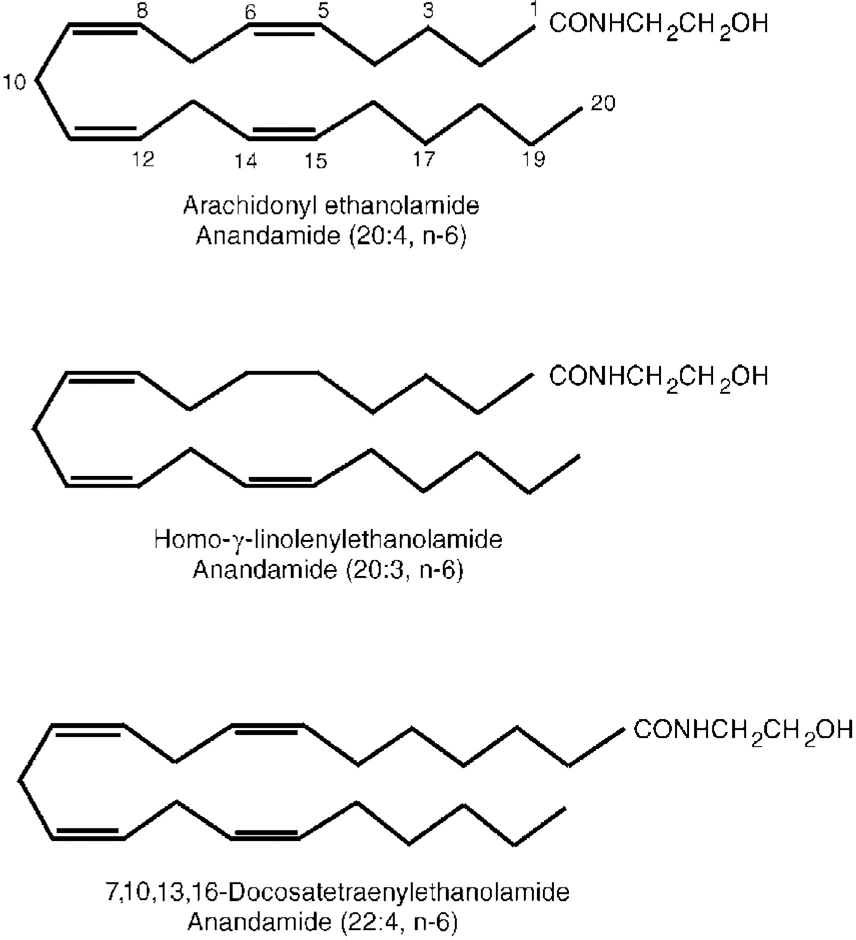
Fig. 3 Chemical structure of anandamides.
Two similar endogenous fatty acids have since been isolated (Fig. 3) and it now appears that there may be a whole system of multiple cannabinoid receptors and anandamide-related substances. Their physiological function has yet to be elucidated (see Reference Pertwee and PertweePertwee, 1995, for a review). It appears that both anandamides and their receptors reside within neuronal lipid membranes and act as neuromodulators through intracellular G-proteins controlling cyclic adenosine monophosphate formation and Ca2+ and K+ ion transport. In this role the system may have important interactions with other neurotransmitters, including γ -aminobutyric acid, opioid systems and monoamines. In particular, THC has been shown to increase the release of dopamine from the nucleus accumbens and prefrontal cortex (Reference Tanda, Pontieri and Di ChiaraTanda et al, 1997). This effect, which is common to many drugs of misuse (including heroin, cocaine, amphetamine and nicotine), may be the basis of its reinforcing properties and its recreational use. It is reversed by naloxone, suggesting an opioid link.
ACTIONS OF CANNABIS IN HUMANS
Cannabis affects almost every body system. It combines many of the properties of alcohol, tranquillisers, opiates and hallucinogens; it is anxiolytic, sedative, analgesic, psychedelic; it stimulates appetite and has many systemic effects. In addition, its acute toxicity is extremely low: no deaths directly due to acute cannabis use have ever been reported. Only a selection of cannabis effects are described in this review; other actions are reviewed by Paton & Pertwee (Reference Paton, Pertwee and Mechoulam1973), Pertwee (Reference Pertwee and Pertwee1995), Adams & Martin (Reference Adams and Martin1996) and many others.
Psychological effects
Effect on mood
The main feature of the recreational use of cannabis is that it produces a euphoriant effect or ‘high’. The high can be induced with doses of THC as low as 2.5 mg in a herbal cigarette and includes a feeling of intoxication, with decreased anxiety, alertness, depression and tension and increased sociability (if taken in friendly surroundings). The high comes on within minutes of smoking and then reaches a plateau lasting 2 hours or more, depending on dose. It is not surprising that the overwhelming reason for taking cannabis given by recreational users is simply ‘pleasure’ (Webb et al, Reference Webb, Ashton and Kelly1996, Reference Webb, Ashton and Kelly1998). However, cannabis can also produce dysphoric reactions, including severe anxiety and panic, paranoia and psychosis. These reactions are dose-related and more common in naïve users, anxious subjects and psychologically vulnerable individuals. (Psychiatric reactions including aggravation or precipitation of schizophrenia are described by Reference JohnsJohns, 2001, this issue).
Effects on perception
Accompanying the high, and often contributing to it, cannabis produces perceptual changes. Colours may seem brighter, music more vivid, emotions more poignant and meaningful. Spatial perception is distorted and time perception is impaired so that perceived time goes faster than clock time. Hallucinations may occur with high doses.
Effects on cognition and psychomotor performance
Not surprisingly, cannabis impairs cognitive and psychomotor performance. The effects are similar to those of alcohol and benzodiazepines and include slowing of reaction time, motor incoordination, specific defects in short-term memory, difficulty in concentration and particular impairment in complex tasks which require divided attention. The effects are dose-related but can be demonstrated after relatively small doses (5-10 mg THC in a joint), even in experienced cannabis users, and have been shown in many studies across a wide range of neurocognitive and psychomotor tests. These effects are additive with those of other central nervous system depressants.
Driving and piloting skills
These effects combine to affect skills related to driving a vehicle or flying an aeroplane. Numerous studies have shown that cannabis impairs road-driving performance and have linked cannabis use with increased incidence of road traffic accidents. In the UK, USA, Australia, New Zealand and many European countries, cannabis is the most common drug, apart from alcohol, to be detected in drivers involved in fatal accidents or stopped for impaired driving. A large proportion of such drivers have not taken alcohol or have concentrations below the legal limit. For example, in two studies from the UK Department of Transport (Reference Everest, Tunbridge and WiddopEverest et al, 1989; Department of Environment, Transport and the Regions, 1998), no alcohol was detected post-mortem in 70% and 80%, respectively, in road traffic accident fatalities testing positive for cannabis. In Australia (Road Safety Committee, 1995) only half of surviving drivers of vehicle collisions involving death or life-threatening injuries who tested positive for cannabis had also taken alcohol. In Norway, 56% of a sample of drug-impaired drivers negative for alcohol gave positive blood samples for THC (Reference Gjerde and KinnGjerde & Kinn, 1991). From the USA, McBay (Reference McBay1986) had earlier found that 75% of a sample of drivers with cannabinoids in their blood were also intoxicated with alcohol. The World Health Organization (1997, p. 15) concluded:
“There is sufficient consistency and coherence from experimental studies and studies of cannabinoid levels among accident victims…to conclude that there is an increased risk of motor vehicle accidents among persons who drive when intoxicated with cannabis…. The risk is magnified when cannabis is combined with intoxicating doses of alcohol”.
Piloting an aeroplane is an even more complex task than driving a car and cannabis has been shown in several investigations seriously to impair aircraft piloting skills. The results of one placebo-controlled study are shown in Fig. 4 (Reference Leirer, Yesavage and MorrowLeirer et al, 1991). The subjects were nine licensed pilots, highly trained in a flight simulator task, who were current cannabis users. They received a cannabis cigarette containing 20 mg THC (a moderate dose by present-day standards). This dose caused a significant decrement in performance compared with placebo and the impairment lasted over 24 hours after this single dose. Furthermore, most of the pilots were unaware that their performance was still impaired at 24 hours. Several pilots reported that they had actually flown while high on cannabis, and the authors noted that in at least one aeroplane crash the pilot was known to have taken cannabis some hours before flying and to have made a similar landing misjudgement (poor alignment on the runway) as was noted in experimental studies.
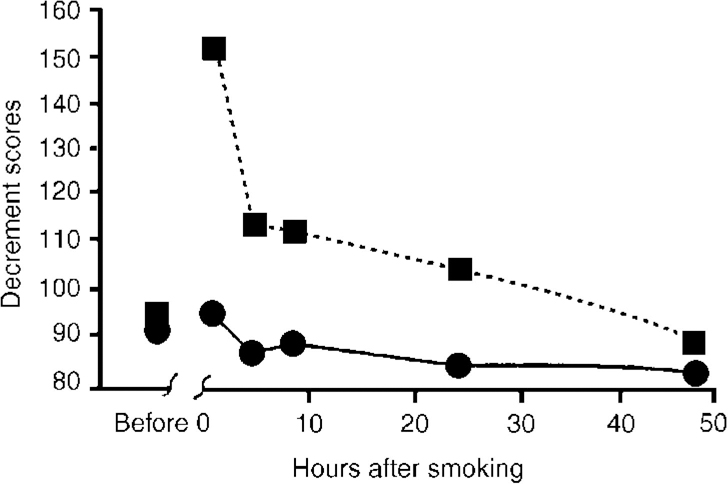
Fig. 4 Effect of smoking a cannabis cigarette containing 20 mg tetrahydrocannabinol (THC) on pilot performance in a flight simulator landing task (Reference Leirer, Yesavage and MorrowLeirer et al, 1991). - - ▪ - -, 20 mg THC; —[UNK]—, placebo.
There is evidence that similar longlasting impairments apply to motor cyclists, train drivers, signal operators, air traffic controllers and operators of heavy machinery. However, a problem is that because of the very slow elimination of cannabinoids, there is no accurate way of relating blood, urine, saliva or sweat concentrations to the degree of intoxication of the driver or pilot at the time of an accident, no way of telling exactly when the last dose was taken and no proof that cannabis was actually the cause of an accident.
Long-term effects of chronic use
There is considerable evidence, reviewed by Hall et al (Reference Hall, Solowij and Lemon1994), that performance in heavy, chronic cannabis users remains impaired even when they are not actually intoxicated. These impairments, especially of attention, memory and ability to process complex information, can last for many weeks, months or even years after cessation of cannabis use (Reference SolowijSolowij, 1998). Whether or not there is permanent cognitive impairment in heavy long-term users is not clear.
Tolerance, dependence, withdrawal effects
Tolerance has been shown to develop to many effects of cannabis including the high and many systemic effects, and a cannabis withdrawal syndrome has been clearly demonstrated in controlled studies in both animals and man (Reference Jones, Fehr and KalantJones, 1983; Reference Kouri, Harrison and PopeKouri et al, 1999). The withdrawal syndrome has similarities to alcohol, opiate and benzodiazepine withdrawal states and includes restlessness, insomnia, anxiety, increased aggression, anorexia, muscle tremor and autonomic effects. A daily oral dose of 180 mg of THC (one or two modern, good-quality joints) for 11-21 days is sufficient to produce a well-defined withdrawal syndrome (Reference Jones, Fehr and KalantJones, 1983). The development of tolerance leads some cannabis users to escalate dosage, and the presence of withdrawal syndrome encourages continued drug use. Thus, chronic cannabis use can lead to drug dependence, and reports from the USA, UK and New Zealand (Reference Roffman and BarnhartRoffman & Barnhart, 1987; Reference Stephens, Roffman and SimpsonStephens et al, 1993) indicate that many cannabis users are now seeking treatment for cannabis dependence.
Systemic effects
Cardiovascular effects
Cannabinoids produce a dose-related tachycardia which may reach rates of up to 160 beats/minute or more, although tolerance develops with chronic use. There is also a widespread vasodilation and reddening of the conjunctivae, a characteristic sign of cannabis use (Reference Paton, Pertwee and MechoulamPaton & Pertwee, 1973). Postural hypotension and fainting may occur. These and other cardiovascular effects may carry a risk for individuals with preexisting cardiac disease, and several cases of acute and sometimes fatal cardiac incidents have been reported in young cannabis smokers.
Effects on the respiratory system
The smoke from herbal cannabis preparations contains all the same constituents (apart from nicotine) as tobacco smoke, including carbon monoxide, bronchial irritants, tumour initiators (mutagens), tumour promoters and carcinogens (British Medical Association, 1997). The tar from a cannabis cigarette contains higher concentrations of benzanthracenes and benzpyrenes, both of which are carcinogens, than tobacco smoke. It has been estimated that smoking a cannabis cigarette results in approximately a five-fold greater increase in carboxyhaemoglobin concentration, a three-fold greater amount of tar inhaled and retention in the respiratory tract of one-third more tar than smoking a tobacco cigarette (Reference Wu, Scott and BurnettWu et al, 1988; Reference Benson and BentleyBenson & Bentley, 1995). This is mainly due to the way a cannabis joint is smoked, with deep and prolonged inhalation and no filter. In addition, cannabis has a higher combustion temperature than tobacco.
Chronic cannabis smoking is associated with bronchitis and emphysema. It has been calculated that smoking 3-4 cannabis cigarettes a day is associated with the same evidence of acute and chronic bronchitis and the same degree of damage to the bronchial mucosa as 20 or more tobacco cigarettes a day (Reference Benson and BentleyBenson & Bentley, 1995). Prospective studies of the long-term effects on the lungs of chronic cannabis smoking are lacking, but some authors suggest that chronic airways disease and bronchogenic carcinoma may be as great a risk as with tobacco smoking. In addition, there appears to be an increased incidence of rare forms of oropharyngeal cancer in young people who smoke cannabis chronically.
Effects on other systems
Cannabis also has immunosuppressant and endocrine effects although the clinical significance of these is still not clear. Chronic cannabis use appears to carry reproductive risks, both to the mother during pregnancy and childbirth and to the foetus and neonate, although these areas need further study. The full extent of long-term health risks of chronic cannabis use (if today's young smokers continue the habit) may require a latent period of 10-20 years to be revealed.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Cannabis use is associated with increased risk of road, rail and air traffic accidents.
-
▪ Chronic cannabis use can result in tolerance, dependence, withdrawal effects and possibly long-term cognitive impairment.
-
▪ Long-term cannabis use carries respiratory, cardiovascular and other health risks.
LIMITATIONS
-
▪ There is no clear relationship between cannabinoid concentrations in body fluids and degree of psychomotor impairment, making traffic-control policies difficult.
-
▪ Long-term prospective controlled studies are needed to quantify the health risks of chronic cannabis use.
-
▪ Further research is needed on the effects of individual cannabinoids and their interactions with tetrahydrocannabinol.









eLetters
No eLetters have been published for this article.