An international team of geologists led by Monika Kusiak of the Polish Academy of Sciences, has discovered that metallic lead is distributed inhomogeneously in the form of nanoclusters inside zircon crystals. This has significant implications for zircon geochronology, as described in the April 21 issue of Proceedings of the National Academy of Sciences (DOI: 10.1073/pnas.1415264112; p. 4958).
Geochronology is the scientific study of the age of minerals and rocks. Zircon or zirconium silicate, a commonly used geochronometer, is a tetragonal nesosilicate that plays a prominent role in this field, mainly because its unit cell can accommodate uranium and thorium atoms that leave behind stable lead isotopes upon radioactive decay. The large lead ion is not accommodated into the zircon structure, which means that all of the lead found in zircon must be the product of radioactive decay alone. The ratio of the lead-to-uranium content of a zircon should be a measure of its age of formation and hence the age of the rock in which it was found. Due to the long half-lives of 238U and 235U—4.47 and 0.704 billion years, respectively—the range of this method is quite extensive.
Complicating this simple picture are other properties of zircon that make the material equally attractive and difficult for geologists. Zircons are chemically and mechanically inert, often surviving the melting of rocks that contain them and recrystallizing in the new rock that forms around the zircon inclusions. Different parts of the same rock can contain zircon grains of different ages. However, high temperature increases lead mobility in these crystals, possibly allowing lead to leach out and affect the age determined using the U-Pb isotope method.
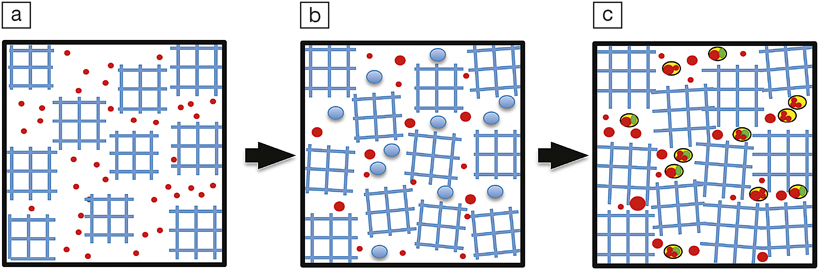
The formation of lead nanospheres in zircon: (a) Metamict zircon with inclusions of incompatible elements like Pb and Si; (b) inclusions grow in size due to Ostwald ripening; and (c) Pb precipitates out of melt inclusions forming nanospheres. Reprinted with permission from Proc. Natl. Acad. Sci. U.S.A. 112 (16) (2015), DOI: 10.1073/pnas.1415264112; p. 4958. © 2015 National Academy of Sciences.
The alpha emissions from centuries of decay processes can also severely disrupt the crystal structure, creating small regions of disorder where lead can move even more freely. Above a certain rate of alpha decay, termed the first percolation point, a number of these amorphous islands form and they start connecting, effectively inverting the picture into that of small island crystals embedded in an amorphous matrix.
Such a zircon is considered metamict—that is, it falls between a crystal and a glass. It was thought that when such a crystal undergoes thermal annealing (e.g., if the rock melted), zircons could recrystallize and eliminate all of the lead in them. This effectively creates a new zircon crystal—with the clock reset—that has no lead in it. However, when the researchers analyzed a zircon specimen from the Napier complex of the Enderby Land of Antarctica, they discovered numerous lead spheres of 5–30-nm diameter. This affects the fidelity of radiometric dating, which is conducted over small regions by a micrometer-sized ion probe. When cast over a nanocluster, the probe measured very high lead content, but over a crystalline region it measured a lower lead content. The measurement will be incorrect regarding the age in both cases. In a prior study, the same researchers found that this difference can be as large as 500 million years.
Kusiak and colleagues used high-resolution transmission electron microscopy to show that these lead clusters are cubic crystals of metallic lead. Pure lead is extremely rare in nature. This conclusion was based on the study of 31 different zircon samples that were all in different stages of percolation. Based on these observations the researchers propose that these nanoclusters are formed from inclusions of new mineral phases from elements like lead and silicon that broke apart from the zircon crystal.
“The presence of nanospheres of lead indicates the separation of a metallic liquid phase from a silicate before the crystallization of native lead. Once phase separation occurred, the Pb droplets embedded in silica melt inclusion grew in size and decreased in number through the process of Ostwald ripening thus minimizing surface energy,” the researchers state in their article.
“This work has alarmed all in situ geochronologists to be careful of nanoscale heterogeneity,” Keewook Yi of the Korea Basic Science Institute said in a communication to MRS Bulletin.
John Valley of the University of Wisconsin, who has done much work on zircon heterogeneity, said, “It has long been recognized that radiogenic lead can be concentrated in clusters within zircon as a result of radiation damage. This paper shows for the first time that under extreme conditions, lead can be concentrated as nano-inclusions of lead metal and that such metamict zircons are not reliable for geochronology.”


