INTRODUCTION
At the Oxford Radiocarbon Accelerator Unit (ORAU), the vast majority of radiocarbon (14C) measurements on archaeological bones are performed on bulk protein extracted from the mineral matrix of the sample. This bulk protein, known as “collagen” (following De Niro and Weiner Reference DeNiro and Weiner1988), is purified following chemical pretreatment protocols specific to the condition of the sample (see Brock et al. Reference Brock, Higham, Ditchfield and Ramsey2010). The purpose of these protocols is to remove as much carbon contamination as possible, which can be derived directly from the burial environment, from handling of the specimen during excavation, from laboratory activities and from museum conservation efforts. The removal of contamination is essential if accurate dates are to be obtained—even trace amounts of modern carbon contamination can have severe effects on the accuracy of dates obtained for samples nearing or beyond the limit of the method. For example, just 1% modern carbon contamination in collagen extracted from an infinitely aged bone would cause the measured age to be no greater than 38,000 years (Bowman Reference Bowman1990).
However, routine pretreatment protocols are not always sufficient to entirely remove exogenous carbon. Although ultrafiltration has been shown to significantly reduce the amount of contamination in many cases (Higham et al. Reference Higham, Jacobi and Ramsey2006; Higham Reference Higham2011), it cannot remove contaminants that exceed 30 kD (the molecular weight cut off for the Vivaspin 15™ 30 kD MWCO Sartorius ultrafilters in routine use by the ORAU) or those that are chemically cross-linked to collagen. Compound specific 14C analysis (CSRA) has therefore emerged as an alternative pretreatment method that involves isolating and dating a single amino acid present in bone collagen, thereby providing a highly pure source of autochthonous carbon for dating. Hydroxyproline is the targeted amino acid since it constitutes around 12% of the total amino acids in mammalian collagen (Eastoe Reference Eastoe1955) and is almost unique in such high abundances elsewhere in nature (Marom et al. Reference Marom, McCullagh, Higham, Sinitsyn and Hedges2012).
At the ORAU, hydroxyproline is isolated from hydrolyzed bone collagen using an optimized high performance liquid chromatography (HPLC) protocol described in full by Deviese et al. (Reference Deviese, Comeskey, McCullagh, Bronk Ramsey and Higham2018). This protocol is the culmination of more than a decade of work focused on improving the quality of the chromatographic separation and the efficiency of the process (Tripp et al. Reference Tripp, McCullagh and Hedges2006; McCullagh et al. Reference McCullagh, Marom and Hedges2010; Marom et al. Reference Marom, McCullagh, Higham, Sinitsyn and Hedges2012; Nalawade-Chavan et al. Reference Nalawade-Chavan, McCullagh, Hedges, Bonsall, Boroneanţ, Ramsey and Higham2013; Devièse et al. Reference Deviese, Comeskey, McCullagh, Bronk Ramsey and Higham2018). Much of this work has involved the testing of new columns, which can often be problematic due to the stringent requirements for hydroxyproline dating. The column needs to be able to separate amino acids; many of which exhibit similar properties and must be able to do so with the use of a water-only mobile phase, so as to avoid introducing carbon contamination from other solvents. The column must also be able to handle large injection volumes (between 10–50 mg of hydrolyzed collagen) in order for a sufficient amount of hydroxyproline to be isolated and collected for subsequent 14C measurements. The speed at which hydroxyproline elutes must also be taken into account—a column with a particularly long elution time will slow down throughput and hinder progress both in research and commercial settings.
Early studies describe the use of a two-stage separation HPLC procedure in order to isolate hydroxyproline (Tripp et al. Reference Tripp, McCullagh and Hedges2006). This method involves the initial use of aqueous reverse-phase (RP) chromatography in order to isolate and collect the polar amino acids, for subsequent reinjection onto the same column using 0.5 mM pentadecafluorooctanoic acid (PDFOA) as the mobile phase. During this stage, hydroxyproline is isolated and collected through an ion-pairing (IP) mechanism. The use of carbon-containing PDFOA as the mobile phase, however, is not ideal due to the difficulty in removing it from the desired amino acid fractions, and the potential for extraneous carbon to be introduced. An alternative procedure was outlined by McCullagh et al. (Reference McCullagh, Juchelka and Hedges2006), whereby a mixed-mode column combining RP interactions with ion-exchange interactions can be used to isolate hydroxyproline in one stage instead of two using only Milli-Q water as the mobile phase. The chosen column is the Primesep A (SIELC technologies, Wheeling, USA), which has the advantage of facilitating the baseline separation of amino acids without the use of carbon-containing mobile phase solvents. The Primesep A column has been used in the ORAU hydroxyproline dating protocol since then.
In 2020, after the purchase of two new Primesep A columns, consistently high backgrounds were observed during routine testing. Hydroxyproline isolated from background Alaskan permafrost bison bone collagen (P18802) returned consistently young dates of around 30–35 ka BP. Communication with SIELC revealed that the manufacturing method (and some reagents) for the Primesep A stationary phase ligands had recently been changed in order to streamline the synthetic process. While testing performed by SIELC demonstrated that their new method did not affect the selectivity or the retention characteristics of the Primesep A column, the high backgrounds recently observed at the ORAU suggest that the new ligands may be more susceptible to column bleed.
Since the older Primesep A columns are no longer available and the newer version is prone to unacceptable levels of carbon-containing column bleed, we are left with two options. The first is to attempt removal of the column bleed from the hydroxyproline fractions after the HPLC separation procedure, as has been demonstrated by Ishikawa et al. (Reference Ishikawa, Itahashi, Blattmann, Takano, Ogawa, Yamane, Yokoyama, Nagata, Yoneda, Haghipour and Eglinton2018). The second option is to identify a suitable replacement column that permits the efficient separation of key amino acids under aqueous conditions, and is not as susceptible to column bleed as the new Primesep A.
Here, we report background and blank data obtained during the testing of the “New” Primesep A columns versus the “Old” pre-manufacturing change Primesep A columns to demonstrate the unsuitability of the former following the existing Deviese et al. (Reference Deviese, Comeskey, McCullagh, Bronk Ramsey and Higham2018) hydroxyproline dating protocol. We also present preliminary background and blank data for a potential alternative mixed-mode column, the Newcrom AH (SIELC Technologies, Wheeling, IL, USA).
MATERIALS AND METHODS
Permafrost bison bones from Alaska (P18801 and P18802) with an age of between 60–80 ka BP (beyond the limit of the 14C dating method; ca. 50 ka BP) and a known-age pig bone (P46648.9) from the Mary Rose shipwreck (sank 1545 AD) were used to monitor the amount of carbon contamination introduced by column bleed.
The most recent ORAU hydroxyproline dating protocol is described in full by Deviese et al. (Reference Deviese, Comeskey, McCullagh, Bronk Ramsey and Higham2018) but is summarized here. The initial collagen extraction procedure follows a modified Longin (Reference Longin1971) method outlined in full by Brock et al. (Reference Brock, Higham, Ditchfield and Ramsey2010), whereby bone samples of approximately 500 mg was initially surface cleaned and crushed, and then demineralized in 0.5M HCl over the course of two days. The samples were then subjected to a 0.1M NaOH wash in order to remove humic acids, and finally rinsed in 0.5M HCl to remove any dissolved CO2 and base-insoluble contaminants. Once gelatinized, the samples were filtered through Ezee™ filters and freeze-dried. Between 40–50 mg of freeze-dried collagen from each sample was then hydrolyzed in 6M HCl (1 mL per 10 mg) at 110ºC for 24 hr, and subsequently evaporated to dryness. Samples were then reconstituted in 700 μl of 0.2M NaOH in order to regulate the pH to 3 prior to injection and filtered into a 2 mL vial using a Millex® syringe-driven filter unit (0.22 μm membrane). 600 μL of ultrapure water was subsequently flushed through the same filter unit and into the vial in order to maximize recovery. Prior to injection, the pH of each sample is checked.
Hydroxyproline was isolated using a Varian Prostar HPLC system (Varian Analytical Instruments, Walnut Creek, CA, USA) equipped with twin Prostar 210 Solvent Delivery Modules, a Prostar 320 UV/VIS detector set at 205 nm, a Prostar 510 column oven set at 30ºC and a Prostar 701 X/Y fraction collector. The existing protocol uses a preparative scale mixed-mode Primesep A column (22 × 250 mm, particle size 5 μm; manufactured by SIELC Technologies, Wheeling, IL, USA and distributed by Hichrom, Theale, UK). The Primesep A stationary phase contains reverse-phase C18 alkyl chains with embedded acidic ion-pairing groups, which interact with the base moieties in the amino acids. A Primesep A guard column (22 × 50 mm, particle size 5 μm, SIELC Technologies, Wheeling, IL, USA) is also installed upstream. However, a made-to-order, preparative-scale Newcrom AH column (22 × 250 mm, particle size 5 μm; SIELC Technologies, Wheeling, IL, USA) is also tested here. The Newcrom AH is a mixed-mode column with embedded ion-exchange groups positioned at the terminal end of the reverse-phase alkyl chain, unlike the Primesep A in which these groups are positioned more closely to the surface of the silica gel. The hydrolyzed collagen was injected into an 18 mL min–1 flow of 100% Milli-Q water as solvent A. Using the Primesep A under these conditions, hydroxyproline elutes between approximately 15–17 min and was collected. The fractions were subsequently evaporated to dryness. The mobile phase was then switched to 100% 0.3 M H3PO4 (solvent B) for the remainder of the program (275 min) in order to flush the column of remaining amino acids. After evaporation of the solvent, the hydroxyproline was combusted following the ORAU graphite production protocols described in Brock et al. (Reference Brock, Higham, Ditchfield and Ramsey2010). A C:N value between 4.9 and 5.0 is considered indicative of purified hydroxyproline. Samples were then dated using the ORAU MICADAS 200 kV AMS system (Ionplus AG) and determinations are corrected for combustion and graphitization following Wood et al. (Reference Wood, Ramsey and Higham2010). This method was used for both the Primesep A and the Newcrom AH column.
Before use, new columns were initially flushed with 100% Milli-Q water for 24 hr to equilibrate them. In order to characterize and quantify the amount of bleed from the columns, 1 mL dilute HCl blanks (pH3) were injected following the same HPLC conditions described above. pH3 blanks were chosen over Milli-Q blanks in order to better replicate the bleed produced by the acidic conditions present during routine injections of hydrolyzed collagen. Fractions were collected based on the time usually taken for hydroxyproline to elute and were evaporated to dryness. Once dried, the blanks were reconstituted in 25 uL of Milli-Q water and transferred into a tin capsule (8 mm × 5 mm, OEA Laboratories Limited, Cornwall, UK) containing approximately 12 mg of diatomaceous silica (Chromosorb WAW 60/80 mesh; Supelco, PA, USA) for analysis via EA-IRMS.
RESULTS AND DISCUSSION
Pretreatment backgrounds, determined as hydroxyproline isolated from the P18802 bison collagen using the Old and New Primesep A columns and the Newcrom AH column, are presented in Table 1.
Table 1 AMS 14C determinations obtained using the Primesep A and Newcrom AH columns.
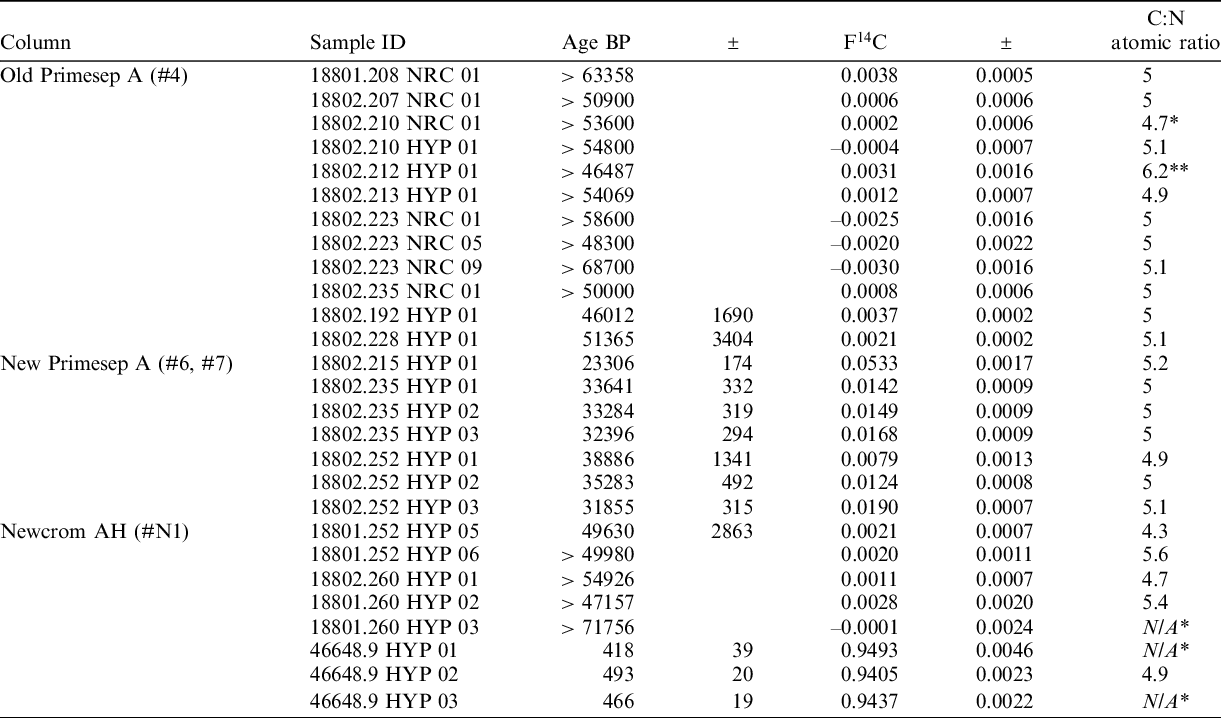
*Data unavailable due to an error during combustion. Results are listed in chronological order (by injection date). *Low C:N likely due to low target size in relation to alanine standards. **High C:N due to IRMS error.
The mean background obtained using the Old Primesep A column (#4) from January 2019 up until the purchase and installation of the New Primesep A in November 2020 was F14C = 0.000633 ± 0.002309 (n=12). This suggests that little to no modern carbon contamination was introduced during the HPLC procedure, or alternatively, that any carbon contamination introduced to the hydroxyproline fractions was of background age. Marom et al. (Reference Marom, McCullagh, Higham, Sinitsyn and Hedges2012) reported that the procedural blank for the Primesep A column prior to the manufacturing change was 3.3 ± 1.4 µg, suggesting that column bleed contributed very little carbon contamination to the hydroxyproline fraction during the isolation process.
The mean background obtained using the two New Primesep A columns (#6 and #7) (purchased after the change in manufacturing method) was F14C = 0.019760 ± 0.015172 (n=7). The measured 14C ages range from 23,306 BP to 38,886 BP, with a mean of 32,664 ± 4747 BP. This suggests that younger carbon is being introduced to the hydroxyproline fractions at some point during the HPLC process. Based on the average procedural blank, the amount of carbon contamination per HYP fraction collected is 7.3 ± 1.4 µg; more than double the amount calculated for the Old Primesep A column (#4)—though it should be noted that these blanks may not necessarily be accurate reflections of the amount of bleed produced during a sample injection due to variations in pH. Nevertheless, based on the F14C values and given that the ligand manufacturing method has changed to involve the use of new reagents, it seems likely that while the chromatography has not been affected, the susceptibility of the column to shed C18 alkyl chains from the stationary phase has increased, therefore introducing carbon contamination of a younger age to hydroxyproline fraction.
The mean background obtained using the Newcrom AH column (#N1) is F14C = 0.001564 ± 0.000785 (n=5); an order of magnitude lower than the New Primesep A columns (#6 and #7). All but one of these backgrounds gave an infinite age, with the single finite age equivalent to 49630 ± 2863 BP. This is a significant improvement on the backgrounds obtained for the New Primesep A columns #6 and #7 and suggests that there is minimal bleed of modern carbon from the stationary phase. However, the mean obtained for the known-age Mary Rose samples dated using the Newcrom AH column (#N1) (n=3) is F14C = 0.944483 ± 0.004426, equivalent to a mean age of 459 ± 38 BP. This may suggest that a small amount of dead carbon is being added to the HYP fraction, resulting in an older age than expected for the Mary Rose standards—likely as a result of ligand bleed. The average procedural blank for the Newcrom AH column suggests 4.8 ± 2.5 µg of carbon is introduced during the collection of each HYP fraction—again, though, it should be noted that these blanks may not be an accurate representation of the amount of bleed produced during a real sample injection. The data for the Newcrom AH are preliminary, but the results suggest that this column could be suitable alternative to the Primesep A. It should be noted that the CN ratios reported for the Newcrom AH in Table 1 are varied at this time due to the process of refining the chromatographic separation of aspartic acid and hydroxyproline.
In order to further investigate the cause of the high backgrounds obtained for the New Primesep A columns, bulk samples of the mixed-mode packing used in the Old Primesep A, New Primesep A and the Newcrom AH columns were dated following the ORAU combustion and graphitization protocol outlined in Brock et al. (Reference Brock, Higham, Ditchfield and Ramsey2010). Samples of each were provided by SIELC Technologies (Wheeling, USA). 14C measurements and elemental data obtained for these ligands are provided in Table 2. The elemental data for the Old and New Primesep A columns suggests that the reagents used to manufacture the latter contain a higher proportion of carbon (5.3% more), and the difference of 4‰ in δ13C of both ligand samples implies that the source of this carbon is also different—consistent with personal communication with SIELC. The similarity of the F14C values of the Old and New Primesep A ligands (0.6090 and 0.6658 respectively) is therefore particularly interesting, as one might expect that different carbon sources would result in a greater difference in fraction modern values. Conversely, the F14C suggests that the high backgrounds associated with the New Primesep A columns (columns #6 and #7) are more likely to be a result of increased susceptibility to bleed, rather than equal degree of bleed with an increased contribution from new, modern reagents. On the other hand, elemental data for the Newcrom AH ligands reflect a similar carbon content to the Old Primesep A. The δ13C value of the Newcrom AH ligands may suggest a greater carbon contribution from petroleum sources (Yeh and Epstein Reference Yeh and Epstein1981), which is consistent with the lower F14C content compared to both the Old and New Primesep A columns. This may also explain the slightly older than expected ages for the Mary Rose standards.
Table 2 AMS 14C determinations obtained for column ligand samples. These samples were provided by SIELC and do not correspond to the columns listed in Table 1.
Newcrom AH Chromatography
Based on blank measurements and 14C measurements obtained for known age standards, the Newcrom AH column is less susceptible to modern carbon bleed and is therefore promising as a replacement for the Primesep A. As such, further method development is ongoing in order to optimize the isolation of hydroxyproline in hydrolyzed bone collagen.
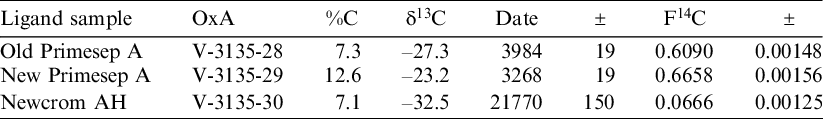
Under the conditions outlined by Deviese et al. and with a collagen injection of between 45–50 mg, aspartic acid and hydroxyproline co-elute at around 7 min. Attempts were not made to improve separation by changing the composition of the mobile phase, as this would further complicate the process of purifying the hydroxyproline fractions after elution. Separation can instead be achieved by reducing the mass of hydrolyzed collagen to between 10 and 15 mg (see Figure 1), which generally yields between 200–500 µg of carbon. Work is ongoing with the aim of improving the separation of aspartic acid (ASP) and hydroxyproline.

Figure 1 Example of a chromatogram obtained using the Newcrom AH column and a 15 mg hydrolyzed collagen injection. The program parameters are outlined in the Methods section.
CONCLUSIONS
The Primesep A column (SIELC Technologies, Wheeling, IL, USA) has been central to the ORAU hydroxyproline dating protocol since 2010 (Brock et al. Reference Brock, Higham, Ditchfield and Ramsey2010). This mixed-mode, preparative scale column permits the isolation of hydroxyproline and a number of other key amino acids under fully aqueous conditions, and until recently, excellent backgrounds could be achieved. Following a change in ligand manufacturing method by SIELC, increased column bleed has rendered the column unusable for material close to the limit of the method without post-separation purification steps.
An alternative preparative mixed-mode column, the Newcrom AH, is introduced as a potential alternative to the Primesep A. Preliminary results suggest that while the Newcrom AH demonstrates excellent backgrounds and may be more suitable for the dating of samples close to the limit of the method than the new Primesep A, some minimal bleed of old carbon may occur and may render the column unsuitable for younger material. This bleed is likely due to the use of 14C-dead reagents used in the ligand manufacturing process. Optimization of the method in order to improve amino acid separation is ongoing, but basic isolation of hydroxyproline is possible under fully aqueous conditions.
This report outlines the great need for clarity and open communication with column manufacturers. Protocols that rely on commercially made columns are at the mercy of manufacturers, as products may be discontinued or changed to an extent that renders them unusable by the community. In the future, further development of post-separation purification steps and the in-house manufacturing of columns by the 14C community may provide a more long-term solution for this.
ACKNOWLEDGMENTS
The authors would like to thank the whole ORAU team for their ongoing support. Particular thanks go to Thomas Blattmann, Thibaut Devièse, Tom Higham, and Lorena Becerra Valdivia for helpful discussion. We would also like to thank SIELC for their communication and for providing the ligand samples.
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.






