Introduction
Worldwide, moth borers are among the most damaging pests of sugarcane (Saccharum spp. hybrids) with about 50 species, mostly belonging to the family Crambidae, reported damaging sugarcane stalks (Long and Hensley, Reference Long and Hensley1972). Eldana saccharina (Walker) (Lepidoptera: Pyralidae) and Diatraea saccharalis F. (Lepidoptera: Crambidae) are major pests in Africa and the American continent, respectively (Showler and Reagan, Reference Showler, Reagan, Gonclaves and Correia2012). Tunneling of stalks by larvae generally leads to deadheart formation and side shooting, disruption of nutrient flow, stalk breakage, loss of stalk weight, and introduction of pathogens, all of which contribute to reduced yield and juice quality (Long and Hensley, Reference Long and Hensley1972; Milligan et al., Reference Milligan, Balzarini and White2003; Reay-Jones et al., Reference Reay-Jones, Showler, Reagan, Legendre, Way and Moser2005; White et al., Reference White, Viator, Dufrene, Dalley, Richard and Tew2008; Showler and Reagan, Reference Showler and Reagan2017; Reagan and Mulcahy, Reference Reagan and Mulcahy2019). The genus Chilo spp. is distributed throughout Asia and Africa with Chilo sacchariphagus (Lepidoptera: Crambidae) being the major pest in China, India, Indonesia, Madagascar, Mauritius, Taiwan, Reunion and the Comoros, Borneo, Java, Bali, Sumatra, Celebes, Japan, Singapore, Sri Lanka, Malaysia, Thailand, and the Philippines (Sallam and Allsopp, Reference Sallam and Allsopp2003) with known yield losses of up to 40 t/ha (Goebel et al., Reference Goebel, Fernandez, Tibere and Alauzet1999).
In India, more than 14 species of moth borers are reported to attack sugarcane in both tropical and sub-tropical sugarcane-producing provinces (David and Nandagopal, Reference David, Nandagopal, David, Easwaramoorthy and Jayanthi1986). Among these, the internode borer Chilo sacchariphagus indicus (Kapur) (Lepidoptera: Crambidae), a subspecies of C. sacchariphagus (David, Reference David, David, Easwaramoorthy and Jayanthi1986; Sallam and Allsopp, Reference Sallam and Allsopp2003), is the most destructive borer pest in peninsular India (Srikanth and Kurup, Reference Srikanth and Kurup2011; Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). It generally attacks the crop from internode formation to harvest causing estimated yield losses of 10–35% (Yalawar et al., Reference Yalawar, Pradeep, Ajith Kumar, Hosamani and Rampure2010). Injury extending to three or more internodes or more than 10% length of cane can produce significant deterioration in juice quality (David, Reference David, David, Easwaramoorthy and Jayanthi1986). Destruction of apical meristem in young and weak shoots results in the production of deadhearts, axillary bud sprouting, and bunchy top formation (Figure 1) leading to greater crop losses (Mukunthan and Rakkiyappan, Reference Mukunthan and Rakkiyappan1989; Srikanth and Kurup, Reference Srikanth and Kurup2011). Insecticides are not the best option for the control of C. sacchariphagus indicus due to the concealed habit of larvae and unamenable crop canopy (Ananthanarayana and David, Reference Ananthanarayana, David, David, Easwaramoorthy and Jayanthi1986). Biological control and sex pheromone traps are known to minimize borer incidence levels but suffer from limitations (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). In this context, cultivar resistance, a major component of long-term integrated pest management strategy for sugarcane moths in many sugarcane growing regions of the world (Reay-Jones et al., Reference Reay-Jones, Showler, Reagan, Legendre, Way and Moser2005; Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018; Wilson et al., Reference Wilson, White, Richard and Johnson2021), can serve as a potential tool to reduce losses caused by borers.

Figure. 1. Chilo sacchariphagus indicus damage in sugarcane: (a) dead heart, (b) bore hole, (c) bunchy top, and (d) grown-up larva in leaf sheath.
Screening of sugarcane cultivars against D. saccharalis in Louisiana, USA (Bessin et al., Reference Bessin, Reagan and Martin1990; Posey et al., Reference Posey, White, Reay-Jones, Gravois, Salassi, Leonard and Reagan2006; Reagan et al., Reference Reagan, Way, Beuzelin and Akbar2008), and Brazil (Tomaz et al., Reference Tomaz, Coutinho, Soares, Peternelli, Pereira and Barbosa2018), Eoreuma loftini (Dyar) (Lepidoptera: Crambidae) in Texas (Reay-Jones et al., Reference Reay-Jones, Way, Tamou, Legendre and Reagan2003; Wilson et al., Reference Wilson, Van Weelden, Beuzelin, Reagan, Way, White, Wilson and Showler2015; Salgado et al., Reference Salgado, Wilson, Richard, Penn and Way2022a), E. saccharina in South Africa (Keeping, Reference Keeping2006), and other moth borers in Papua New Guinea (Korowi and Samson, Reference Korowi and Samson2013) have demonstrated significant differences in the resistance of cultivars to various borers. Commercial sugarcane cultivars display a range of resistance with susceptible ones often suffering 5–10 fold greater levels of injury than resistant cultivars (Salgado et al., Reference Salgado, Wilson, Villegas, Richard and Penn2022b). The Research Center of ICAR-Sugarcane Breeding Institute (ICAR-SBIRC) located at Kannur, Kerala State, India, currently maintains the world’s largest collection of sugarcane germplasm. This vast germplasm of diverse genetic makeup includes assemblages of Indian hybrids, foreign hybrids, Saccharum spp., and allied genera, constituting a crop island (Mahesh et al., Reference Mahesh, Srikanth, Salin, Singaravelu, Chandran and Mahendran2019). In earlier work, attempts were made to identify sources of resistance among limited accessions of five Saccharum spp., namely S. barberi, S. robustum, S. sinense, S. spontaneum, and S. officinarum available at the Research Center, against C. sacchariphagus indicus under field conditions (Jayanthi, Reference Jayanthi1988; Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). However, resistance among hybrids to this borer has not been examined seriously hitherto except for the screening of a few commercial hybrids (Jhansi and Rao, Reference Jhansi and Rao1996; Rajendran et al., Reference Rajendran, Gopalan and Hanifa1996; Albert et al., Reference Albert, Thirumalai, Krishnamurthi, Ramya, Lourdusamy and Gopinathan2007) and prerelease clones (Jayakumar, Reference Jayakumar2018; Pasupathy et al., Reference Pasupathy, Shanmuganathan, Ravichandran and Babu2021). Hence, the present study was undertaken to investigate the resistance of a large collection of Indian hybrids to C. sacchariphagus indicus in a series of field screening trials followed by the assessment of selected resistant hybrids in pot culture. Besides, oviposition preference, relative survival and comparative biology of the borer were examined in the laboratory to determine the underlying resistance mechanisms and enable identification of genetic stock for introgression of borer resistance in sugarcane breeding programs.
Materials and methods
Field screening of Indian hybrids
Study site and field layout
A series of four field experiments was conducted to screen Indian hybrids against C. sacchariphagus indicus under natural pest pressure at ICAR-SBIRC, Kannur, Kerala State, India, and research farm of M/s Rajshree Sugars and Chemicals Ltd (RSCL), Mundiyampakkam, Villupuram district, Tamil Nadu State, India, from 2013 to 2016 (Supplementary Material Table S1). The hybrids were screened adopting a process of elimination of those found susceptible in any one replication in successive years of screening (Mukunthan, Reference Mukunthan, Sreenivasan, Amalraj and William Jebadas2001). In the first year trial (2013), hybrids showing above 30% incidence level were excluded whereas in the subsequent trials (2014–2016), hybrids showing more than 15% incidence were eliminated as a more rigorous method of screening.
In the first field trial (2013) at ICAR-SBIRC, 535 hybrids (Table S2) were planted in 2 m rows each with a row-to-row spacing of 90 cm. In the subsequent trials (2014–2016) at M/s Rajshree Sugars and Chemicals Ltd., an area endemic for C. sacchariphagus indicus (Puthira Prathap et al., Reference Puthira Prathap, Karpagam, Bhaskaran and Nair2014), hybrids found resistant in the previous trial were carried forward and screened against the borer in replicated randomized block design. In the 2014 trial, 187 test hybrids, including the two susceptible hybrids, namely Co 86032 (Nalawade et al., Reference Nalawade, Indi, Bhilare, Thorvae and Raskar2022) and Co 1060 (Table S1), were planted in four blocks serving as replications, each containing two rows of all entries randomized within the block. In the 2015 trial, 50 hybrids and the two susceptible checks 86032 and Co 1060 were planted in five blocks with two rows for each entry. In the 2016 trial, four hybrids and the two susceptible checks Co 86032 and Co 1060 were planted in six blocks with six rows for each plot. All test entries were planted in 6 m rows with 1.8 m spacing between rows and 1.2 m alley between plots. In all the trials, planting was done in January following recommended agronomical practices (Sundara, Reference Sundara1998) but without pesticide application.
Damage assessment
The attack of C. sacchariphagus indicus begins at fifth month age of the crop and extends almost until harvest. Neonate larvae, after feeding on the inside of leaf sheath tissue for 7–8 days, typically bore into the top internodes egesting out wet frass from the bore holes which indicates fresh attack; the presence of large bore holes, often blackened by the growth of saprophytes, in the lower internodes (Figure 1) characterizes old attack (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). At harvest, millable canes in all rows of each replication were counted, cut at the ground level, examined for the presence of injury symptoms, and the number of canes with bore holes was recorded. In the injured canes, total number of internodes and number of internodes with bore holes were recorded and the following three injury parameters were computed.
While percent incidence indicates between-plant dispersal of the borer, percent intensity signifies within-plant spread and repeated attack by successive generations of the borer. Infestation index accommodates variation in both types of attack and may serve as a composite indicator, and in many cases, the index reflects percent incidence (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018).
Screening under artificial infestation
Internode borer culture
Chilo sacchariphagus indicus required for artificial infestation studies was obtained from a laboratory culture maintained at the Section of Entomology, ICAR-Sugarcane Breeding Institute, Coimbatore, Tamil Nadu, India. The culture originated from borer larvae collected in sugarcane fields which were reared on shoot bits in the laboratory until pupation. After determining the sex of pupae (Duttamajumder, Reference Duttamajumder2020), equal numbers of male and female pupae (10 : 10) were placed in oviposition cages (45 cm L x 45 cm W x 45 cm ht) provisioned with 20 cm leaf bits of the susceptible Co 86032 as substrate for oviposition. Egg masses laid on leaves were surface sterilized with 1% formaldehyde at black head stage and emerging neonate larvae were reared in artificial diet in 10 cm ht x 2.5 cm dia. glass tubes at 25 ˚C and 65% RH (Mehta and David, Reference Mehta and David1978; Anonymous, 2018).
Artificial inoculation
After eliminating a large number of susceptible entries under natural conditions of infestation in the 4-year field-screening process, four apparently resistant hybrids, namely Co 293, Co 389, Co 62019, and Co 62213, together with the two susceptible hybrids Co 86032 and Co 1060, were subjected to artificial infestation in pot culture under greenhouse conditions. Single-budded sugarcane setts from 7-month-old crop of the six hybrids were planted in 45 cm diameter pots filled with a 1:1:1 mixture of sand, silt, and cattle farmyard manure (0.5% N - 0.2% P2O5 - 0.5% K2O). Each hybrid was planted in six pots serving as six replications, and a uniform plant stand of six canes was maintained in each pot.
When plants attained 6 months age, neonate larvae of C. sacchariphagus indicus obtained from laboratory culture were released on the inner side of leaf sheath of +4 or +5 leaf (Clements and Ghotb, Reference Clements and Ghotb1969) at 10 per plant using a fine camel hair brush. A month after inoculation, the entries were evaluated based on the appearance of bore holes or deadhearts.
Categorization of hybrids
Percent incidence, percent intensity and infestation index were considered to assess borer damage as described above. However, in view of its predominance in the computation of infestation index, only percent incidence was used as a rapid and low sampling-cost parameter to group hybrids into three categories of resistance (Table S3) (Mukunthan, Reference Mukunthan, Sreenivasan, Amalraj and William Jebadas2001; Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018) under both natural and controlled conditions.
Relative resistant ratio
A modified relative resistance ratio (RRR) was computed for four test hybrids and two susceptible hybrids following Wilson et al., (Reference Wilson, Van Weelden, Beuzelin, Reagan, Way, White, Wilson and Showler2015). In the field trial and the test conducted under controlled conditions, hybrids within each replication were ranked for percent of incidence and percent of intensity and RRR was computed as
where:
n : number of hybrids evaluated
RR(Inc.): relative resistance ranking based on percent of incidence (the lowest percent has an
RR(Inc.)=1 and the highest has an RR(Inc.)= n)
RR(Int.): relative resistance ranking based on percent of intensity (the lowest percent has an
RR(Int.)=1 and the highest has an RR(Int.)= n)
Oviposition choice tests
Oviposition preference of C. sacchariphagus indicus was examined with Co 293, the hybrid found to be resistant in the field and moderately resistant under controlled conditions, and the susceptible hybrids Co 1060 and Co 86032. The hybrids were maintained in micro-plots following normal agronomic practices (Sundara, Reference Sundara1998) and +4 or +5 leaves (Clements and Ghotb, Reference Clements and Ghotb1969) excised from 5-month-old plants were used in oviposition choice tests. The tests were conducted in plastic containers (20 cm ht x 18 cm dia.) with wire mesh lid serving as oviposition cages. Since the borer was found to prefer middle part of +4 or +5 leaves for oviposition in the wild (Anonymous, 2018), five 17 cm long leaf bits cut from the center of excised leaves of each hybrid were placed vertically by inserting them in moist sand placed in a small plastic cup. The three cups containing leaf bits of each hybrid were placed equidistant from one another in the oviposition cage and freshly eclosed (<24 h) adults (1 male: 1 female) of C. sacchariphagus indicus selected from the laboratory culture were released in each cage. The test was replicated 22 times with oviposition cages maintained under ambient laboratory conditions (25 ˚C and 65% RH). Leaf bits were replaced with fresh ones every 24 h after examining for oviposition. The number of leaf bits oviposited upon, and the number of egg masses and eggs on each leaf bit of the three hybrids were recorded every day until the death of female in about 7 d. From the cumulative data compiled over the observation period for the three hybrids, percent of leaf bits oviposited, and percent of egg masses and egg numbers deposited were calculated for the 22 replications. Besides, egg number in individual egg masses laid in all three hybrids was also assessed.
Oviposition preference index (OPI)
An oviposition preference index (OPI) on per leaf bit basis, per cm2 area of leaf bit, and per gram weight of leaf bit for egg mass number and egg number was derived using the equation of Reay-Jones et al., (Reference Reay-Jones, Wilson, Showler, Reagan and Way2007). For each hybrid, area of leaf bit was calculated as the product of length (17 cm) and broadest width measured from four samples and mean area computed; mean weight of leaf bit was computed from seven samples. OPI was computed as
where
α ij = estimated preference shown for the ith hybrid for the jth dataset
n ij= mean number of egg masses or eggs laid per unit area or unit weight of leaf bit on the ith treatment and jth dataset
max n j = mean maximum number of egg masses or eggs laid per unit area or unit weight of leaf bit across treatments for the jth dataset
Larval development and survival
After screening under artificial inoculation in pot culture, the resistant Co 293 and the susceptible Co 1060 and Co 86032 hybrids were assessed for borer development and survival in the laboratory under ambient conditions (25 ˚C, 65% RH and 12L:12D). Neonate larvae obtained from stock culture were placed in rearing boxes (8.5 cm ht x 6.0 cm dia.) lined with moist filter paper at the bottom. Five larvae were released per box and provisioned with three 5.0 cm long tender shoot bits obtained from 5-month old plants of the three hybrids maintained in a micro-plot. Fifteen boxes were maintained for each hybrid as replicates in a completely randomized design. Shoot bits were changed and larval mortality per box was recorded on alternate days. The experiment was continued until death of larvae or pupation (≈ 6 weeks), and cumulative weekly mortality rates were computed.
Comparative biology on resistant and susceptible hybrids
Life cycle in shoots
Life cycle of the borer was examined in shoots of the field-resistant (Co 293) and field-susceptible hybrids (Co1060 and Co 86032). Five neonate larvae obtained from stock culture were released in each of 10 rearing boxes and reared until pupation as described above. Freshly formed pupae were sexed, weighed, and kept individually in plastic boxes lined with moist filter paper at the bottom for adult emergence. Moths were maintained on 50% honey solution dispensed on cotton wad until death. Total larval duration, pupal period, pupal weight, sex ratio, and adult longevity were recorded for larvae that completed development which were used as replications (n=17-22) for analysis. Emerging males and females were held in pairs in oviposition cages until female death and fecundity was recorded as described under oviposition choice tests above.
Relative suitability ratio
A relative suitability ratio (RSR) was developed on the lines of RRR (Wilson et al., Reference Wilson, Van Weelden, Beuzelin, Reagan, Way, White, Wilson and Showler2015) using larval and pupal durations obtained from replicated laboratory rearing on shoots of the three hybrids (n=17). Since developmental durations are generally prolonged on unsuitable hosts, longest developmental period was assigned the lowest rank and shortest developmental period was given the highest rank; in case of equal developmental periods, mean ranking was assigned. Consequently, lower RSR indicates unsuitability of the host and vice versa.
where:
n: number of hybrids evaluated
RS(Larva): relative suitability ranking based on larval period (the highest duration has an
RS(Larva) = 1 and the lowest has an RS(Larva) = n)
RS(Pupa): relative suitability ranking based on pupal period (the highest period has an
RS(Pupa) = 1 and the lowest has an RS(Pupa) = n)
Morphological characters vs. borer incidence
To establish the relationship between cane morphological characters and borer injury parameters, selected quantitative and qualitative morphological traits were recorded at 9 months age in healthy canes of hybrids maintained at ICAR-SBIRC, Kannur. Leaf characters were measured on third leaf from visible dewlap from each of three randomly selected plants and the means calculated. Leaf length (cm) was measured from ligule to leaf tip, and leaf width (cm) was measured in the middle part of leaf. Cane parameters were measured on three randomly selected canes to compute means. While cane length (cm) was measured from ground level to the top-breaking point, cane thickness (cm) was calculated as the mean of three measurements recorded in top, middle, and bottom parts of the cane using a digital vernier caliper. Internode hardness was measured in top, middle and bottom portions of the cane with a ‘rind hardness tester’ developed at ICAR-Sugarcane Breeding Institute, Coimbatore, India (Babu et al., Reference Babu, Koodalingam, Natarajan, Shanthi and Govindaraj2009; Govindaraj et al., Reference Govindaraj, Parthiban, Hari, Naik, Kotwaliwale, Viswanathan, Bhaskaran, Hemaprabha, Ramasubramanian and Nair2014); fiber content was estimated gravimetrically. Yield traits, namely single cane weight (kg), brix and sucrose percentage, were recorded at cane maturity, i.e., 12 months age. Brix was recorded in the middle portion of three independent canes using a hand refractometer (ATAGO, Japan).
Besides, qualitative plant characters such as rind color (14 classes), pith (three classes), sheath-clasp (four classes), and leaf hairiness (two classes) were recorded at 9 months age as per the descriptor classes and descriptor states adopted for sugarcane germplasm (Artschwager and Brandes, Reference Artschwager and Brandes1958).
Estimation of phenolics
Sample collection and preparation
Phenolics in shoots of the three hybrids Co 293, Co 1060, and Co 86032 were estimated (Tonnesen and Karlsen, Reference Tonnesen and Karlsen1983) at 6 months age. Top shoots were collected from three uninfested canes for each hybrid maintained in the field and processed immediately. Shoots were chopped finely, macerated in liquid nitrogen, extracted with methanol, and filtered through a filter paper (47 mm, Whatmann, UK) to remove residues and pigments. The extract was stored at -20°C until further analysis in HPLC.
HPLC analysis
Shoot phenols were estimated in Shimadzu LC-8A HPLC system with C18 column (Phenomenex Gemini 250 x 4.6 mm; 5 μm particle size) and photodiode array detector. Methanol extracts were filtered through 0.2 μ membrane, and 25 μl of sample was injected using Rheodyne injector. A gradient mixture of methanol and 0.1% phosphoric acid (in water) as the mobile phase at a flow rate of 1 ml/min was maintained; the detection was performed at 285 nm. The ratio of methanol : 0.1% phosphoric acid in water was increased from 0 : 100 to 5 : 95 in the first 5 min, 10 : 90 in the next 5 min, 15 : 85 in the next 15 min, 40 : 60 in the subsequent 20 min, and 95 : 5 in the next 10 min. The ratio was then brought down to the initial level of 5 : 95 in the last 10 min. Serial dilutions of standards (Sigma-Aldrich, USA) of the phenolic compounds were prepared in methanol and used to obtain standard curves. Concentrations (ppm) of individual phenolics in the samples were estimated by comparing the retention time and peak area of sample chromatograms with those of standards.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was applied to field data (2014–2016) with six hybrids as the levels of between-group factor and three study years as the levels of within-subject repeated measure factor. One-way ANOVA was used to analyze data from the pot culture experiment with six hybrids under controlled conditions, laboratory oviposition preference test with three hybrids under free-choice situation, fecundity data of females reared on shoots, RRR and RSR; data were subjected to square root or arcsin transformation wherever needed. Repeated measures ANOVA was used for arcsin-transformed mortality rates of the borer reared on three hybrids with hybrids as the levels of between-group factor and cumulative mortality in different weeks after setup as the levels of within-subject repeated measure factor. Two-way factorial ANOVA was applied to laboratory life cycle parameters with hybrid (three) and sex (male and female) serving as factors. Tukey HSD test for equal or unequal replications was employed for multiple comparisons of treatments in all ANOVA. Simple correlation coefficients were computed between infestation parameters and quantitative plant morphological characters of different hybrids. To assess the effect of qualitative plant characters on infestation parameters, Mann–Whitney U test was applied for characters with two categories, and Kruskal–Wallis ANOVA by ranks and multiple comparison tests were used for characters with more than two categories. Bar diagrams were used for graphical depiction and mean comparison of field attack rates, RRR, OPI on leaf basis, fecundity, and RSR. The analyses were performed using StatSoft, Inc. (2004).
Results
Chilo sacchariphagus indicus infestation levels among hybrids
Screening of 535 hybrids through the process of elimination of susceptible hybrids in four successive years (2013–2016) led to the selection of four hybrids, namely Co 293, Co 389, Co 62019 and Co 62213 by the end of 2016. In the first trial (2013) at ICAR-SBIRC, Kannur, attack rates on cane basis (percent incidence) were lower in these four hybrids (4.55–15.15%) than in the susceptible checks, i.e., Co 86032 (29.17%) and Co 1060 (30.43%) (Table S4). However, attack rates on internode basis (percent intensity) (3.85–11.76%) did not show clear-cut trend among the hybrids.
In the three trials (2014–2016) conducted at RSCL, Mundiyampakkam, injury levels were generally higher (Table 1) than those observed in 2013 at Kannur (Table S4). Besides, injury levels varied widely among the three years with percent incidence and infestation index showing far higher values in 2016. Repeated measures ANOVA of percent incidence was significant for hybrids (F5, 12=60.89; p < 0.01) and years (F2, 24=51.75; p < 0.01) but not their interaction (F10, 24=2.25; p > 0.05). Mean percent incidence was significantly lowest in Co 293 (10.04 ± 2.56) and highest in the standards Co 1060 (65.59 ± 2.56) and Co 86032 (46.30 ± 2.56); the differences were not significant among the remaining three hybrids. Among the years, the mean percent incidence did not differ between 2014 and 2015 but was significantly highest in 2016. Repeated measures ANOVA of percent intensity was not significant for hybrids (F5, 12=2.22; p > 0.05) but significant for years (F2, 24=17.41; p < 0.01) and their interaction (F10, 24=3.52; p < 0.01); it was significantly lower in 2015. With repeated measures ANOVA of infestation index being significant for hybrids (F5, 12=46.07; p < 0.01), years (F2, 24=91.18; p < 0.01), and their interaction (F10, 24=4.79; p < 0.01), the parameter followed the same trend as percent incidence showing a slight decrease in 2015.
Table 1. Comparative levels of injury by Chilo sacchariphagus indicus among six sugarcane hybrids screened in the field at Mundiyampakkam, Tamil Nadu State, India, during 2014–2016

@ Means ± SE followed by the same lower case letter in the column and upper case letter in the row are not significantly different (p > 0.05) by repeated-measures ANOVA and Tukey HSD test on 1 arcsin and 2 square root transformed values.
Screening of hybrids for resistance
Under natural conditions
Of the 535 Indian hybrids screened for their relative degree of resistance in the field, four hybrids remained resistant for three consecutive years. In the final confirmatory screening studies (2016), when the four resistant hybrids were evaluated along with two susceptible hybrids, only one hybrid (Co 293) emerged as resistant (Table S3) on the basis of highest incidence among the four years (14.55%), whereas the remaining five were found to be susceptible (Figure 2a) with an incidence range of 46.97–74.01%.

Figure 2. Relative resistance status of six sugarcane hybrids against Chilo sacchariphagus indicus: (a) categorization on the basis of highest infestation rate among four years (2013–2016); R – resistant; S – susceptible, (b) relative resistance ratio (RRR) in natural conditions, and (c) RRR under artificial inoculation. Vertical bars denote mean ± SE; bars with same lower case letter are not significantly different (p > 0.05) for RRR.
Under artificial conditions
Under artificial infestation, all hybrids sustained higher levels of damage (Table 2) than in natural conditions. Percent of incidence differed significantly among the six hybrids (F5, 12=8.70; p < 0.01) with the lowest value in Co 293 (26.10%). However, the higher levels of incidence in the remaining five hybrids did not differ among them. On the other hand, percent of intensity (F5, 12=1.08; p > 0.05) and infestation index did not differ (F5, 12=1.98; p > 0.05) among different hybrids.
Table 2. Resistance status of six sugarcane hybrids against Chilo sacchariphagus indicus under artificial infestation in pot culture

! Means ± SE followed by the same letter are not significantly different (p > 0.05) by one-way ANOVA and Tukey’s HSD test on 1 arcsin and 2 (x+0.5)0.5transformed values.
@ On the basis of categorization followed for field screening.
Relative Resistance Ratio (RRR)
RRR computed from the field data of the hybrids was significantly (F5, 12=9.37; p < 0.01) lowest in Co 293 (0.167), whereas it was uniformly higher in the remaining five hybrids (Figure 2b). On the other hand, RRR for the hybrids tested under artificial conditions showed nonsignificant differences (F5, 12=1.06; p > 0.05) though the value was the lowest for Co 293 (0.208) and uniformly higher for the remaining five hybrids (Figure 2c).
Oviposition behavior
The three hybrids showed significant differences in all three oviposition parameters examined for C. sacchariphagus indicus under free-choice situation in the laboratory. At the end of the 7-day observation period, percent of leaf bits oviposited differed significantly (F2, 63= 12.48; p < 0.01) among the three hybrids (Table 3). It was significantly lowest in Co 293, whereas it did not differ between the other two hybrids. The percent of egg masses deposited on the resistant hybrid (Co 293) was significantly (F2, 63= 17.03; p < 0.01) lowest among the three hybrids; the susceptible Co 86032 and Co 1060 showed similar percent of egg masses. Percent of egg numbers deposited also varied significantly (F2, 63= 29.35; p < 0.01) among the hybrids with Co 293 receiving significantly lowest percent of eggs while the other two recording about four times higher, yet statistically similar, percent of egg numbers. Egg number/mass did not differ significantly (F2, 136= 1.14; p > 0.05) among the three hybrids.
Table 3. Oviposition behavior of Chilo sacchariphagus indicus on three sugarcane hybrids under free-choice situation

1Mean of 22 replications; 2 n = 17 – 73 egg masses.
! Means ± SE followed by the same letter in a column are not significantly different (p > 0.05) by one-way ANOVA and Tukey’s HSD test for equal or unequal replications on (x+0.5)0.5 transformed values.
Oviposition preference index
OPI on per leaf bit basis for the three hybrids differed significantly for egg mass number (F2, 63= 15.81; p < 0.01) and egg number (F2, 63= 29.66; p < 0.01) (Figure 3a). Both parameters were significantly lowest on Co 293, whereas they did not differ between Co 1060 and Co 86032. When computed on the basis of weight of leaf bits provided as food, OPI did not differ among the three hybrids for both egg mass number and egg number (Table 4). Similarly, OPI calculated on the basis of area of leaf bit did not differ for egg mass number. However, it was significantly lowest in Co 293 and similar for the other two hybrids for egg number.

Figure 3. Behavioral and biological suitability of three sugarcane hybrids to Chilo sacchariphagus indicus: (a) per leaf basis oviposition preference index for egg mass number and egg number, (b) mean fecundity on shoots, and (c) relative suitability ratio on shoots. Vertical bars denote mean ± SE; bars with same upper or lower case letter are not significantly different (p > 0.05).
Table 4. Oviposition preference index (OPI) for Chilo sacchariphagus indicus attack on three sugarcane hybrids

! Means ± SE followed by the same letter in a column are not significantly different (p > 0.05) by one-way ANOVA and Tukey’s HSD test.
Larval mortality rates on resistant/susceptible hybrids
Larval survival examined for C. sacchariphagus indicus reared on shoots showed significantly (F2, 42= 9.13; p < 0.01) highest mean mortality rate in the hybrid Co 293 (58.22%) (Table 5). In contrast, larval mortality rates were lower and similar when reared on the susceptible hybrids Co 86032 and Co 1060. Mean mortality rates of larvae increased progressively over the period of observation with significant differences (F5, 210 = 223.87; p < 0.01) among different weeks. Neonate larval mortality in Co 293 was 10-fold greater than that in Co 1060 and five-fold greater than that in Co 86032 in the first week of observation. Thereafter, mortality gradually increased in all the hybrids. The hybrid x week interaction was also significant (F10, 210 = 2.41; p < 0.01).
Table 5. Cumulative larval mortality rates (%) of Chilo sacchariphagus indicus reared on three sugarcane hybrids in the laboratory

! Means ± SE followed by the same lower case letter in the column and upper case letter in the row are not significantly different (p > 0.05) by repeated-measures ANOVA and Tukey HSD test on arcsin transformed values.
Biology of C. sacchariphagus indicus on three hybrids
Life cycle in shoots
Larval developmental parameters did not vary considerably when reared on shoot bits of different hybrids in the laboratory. Larval and pupal periods were the highest on Co 293 but did not differ significantly either among the hybrids or between sexes (Table 6). However, pupal weight differed significantly among hybrids and sexes: mean pupal weight was the highest in the susceptible Co 86032, while it was similar in the other two hybrids; it was significantly higher in female than in male. Just as larval and pupal periods, adult longevity did not differ either among hybrids or sexes. Sex ratio of emerging adults ranged from 1.2: 1.0 (♂ :♀) in Co 293 and Co 86032 to 1.4: 1.0 (♂ :♀) in Co 1060 resulting in a slightly higher percent of males (58.8%) in Co 1060 than in Co 293 and Co 86032 (54.5%). Fecundity of females emerging from rearings on the three hybrids differed significantly (F2, 38=6.21; p < 0.01); it was significantly lower in Co 293 and Co 1060 than in Co 86032 (Figure 3b).
Table 6. Life cycle of Chilo sacchariphagus indicus reared on shoots of three sugarcane hybrids in the laboratory
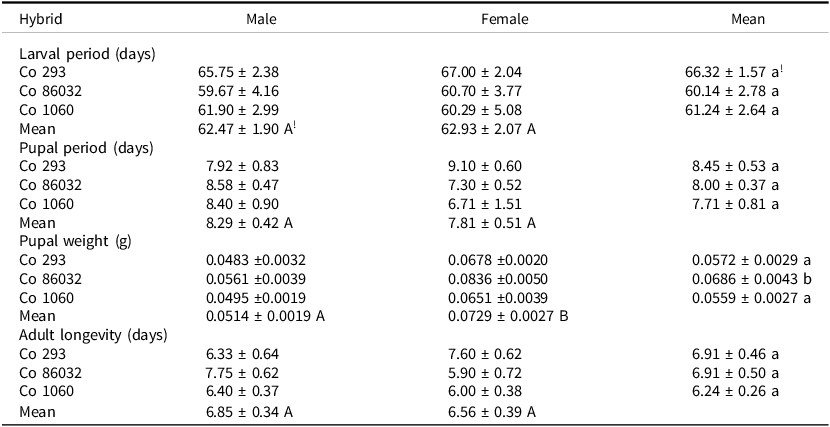
! Means ± SE followed by the same lower case letter in the column and upper case letter in rows for each parameter are not significantly different (p > 0.05) by factorial ANOVA and Tukey unequal N HSD test.
Relative suitability ratio (RSR)
RSR computed from ranks based on larval and pupal durations in shoots differed significantly among the three hybrids (F2, 48= 4.585; p < 0.05). Co 293 emerged as the most unsuitable host with significantly lowest RSR of 0.583 (Figure 3c), whereas Co 86032 was the most suitable for larval development with the highest RSR of 0.765; Co 1060 showed intermediate response (RSR=0.652).
Plant characters vs. borer injury
Role of plant morphology
Among the selected quantitative plant morphological characters, leaf length and cane thickness were positively and significantly correlated with borer incidence for the six hybrids that entered the fourth field trial; however, only leaf length showed significant positive correlation with intensity (Table 7). On the other hand, morphological characters were not significantly correlated with infestation parameters for the 52 hybrids selected at the end of three field trials. Nonparametric analysis of qualitative characters vis-à-vis borer attack rates showed nonsignificant relationship for rind color, pith, sheath-clasp, and leaf hairiness (Table 8). For sheath-clasp, despite nonsignificant ANOVA, percent infestation was significantly (z=2.672) lowest in medium class (mean rank=21.23) and highest in loose class (mean rank=33.28).
Table 7. Correlations between plant characters and Chilo sacchariphagus indicus attack rates on sugarcane hybrids screened under natural conditions

*p < 0.05; **p < 0.01; rest not significant.
Table 8. Relationship of stem and leaf characteristics with attack rates of Chilo sacchariphagus indicus in 52 sugarcane hybrids

a Kruskal–Wallis ANOVA
b Mann-Whitney U test.
ns p > 0.05.
Phenolics vs. borer incidence
Among the phenolics determined in shoots of the three hybrids, rutin, vanillic acid, ellagic acid, ferulic acid, coumarin, flavone, catechin, and orcinol showed a clear trend of highest quantity in Co 293 followed by Co 1060 and Co 86032 in lower amounts (Table S5). The two phenolics gallic acid and phloroglucinol were totally absent in Co 293. While gallic acid was present in very high quantities in the remaining two hybrids, pholoroglucinol was observed in far smaller quantities. Caffeic acid was the lowest in Co 293 and highest in Co 1060; syringic acid did not show clear-cut trend.
Discussion
The first trial with 535 hybrids was conducted at ICAR-SBIRC to avoid the movement of germplasm out of the center. The process of elimination of susceptible hybrids adopted in the study ensured that a manageable number of hybrids was carried forward for subsequent replicated trials in the pest endemic area. In three trials (2014–2016) conducted in the borer endemic area, incidence was the lowest in 2014 but continued to increase reaching its peak in 2016, the highest being in the susceptible hybrids in all three years. Such increased levels of incidence, which could partly be due to variation in weather factors during the study period, ensured repeated screening of possible escapes. Consequently, the number of resistant hybrids decreased progressively over the study period ultimately leaving only Co 293 as resistant at the end of the final field trial.
Under intense borer attack facilitated by artificial inoculation, however, Co 293 turned out to be moderately resistant, whereas the remaining three hybrids maintained their susceptible status. The categorization protocol of Mukunthan (Reference Mukunthan, Sreenivasan, Amalraj and William Jebadas2001) and Mahesh et al., (Reference Mahesh, Srikanth, Chandran and Singaravelu2018) for C. sacchariphagus indicus followed in the present study demarcated lower ranges of infestation in each category than those proposed by Radadia and Shinde (Reference Radadia and Shinde2013) in their system of classification. Despite following such more stringent classification, the hybrid Co 293 maintained its resistance status.
To evaluate sugarcane cultivars against D. saccharalis, Bessin et al., (Reference Bessin, Reagan and Martin1990) developed the parameter of relative survival as the number of adult emergence holes relative to the number of bored internodes which was later adopted by Reay-Jones et al., (Reference Reay-Jones, Showler, Reagan, Legendre, Way and Moser2005). Subsequently, Wilson et al., (Reference Wilson, Van Weelden, Beuzelin, Reagan, Way, White, Wilson and Showler2015) developed a RRR for E. loftini based on rankings given to proportions of bored internodes and relative survival to accommodate the discrepancies observed in the levels of resistance based on these two considered individually. In contrast, canes affected by C. sacchariphagus indicus do not exhibit adult emergence holes as the larvae pupate in leaf axils after exiting the bored internode (David Reference David, David, Easwaramoorthy and Jayanthi1986). Hence, we used rankings based on percent of incidence (cane-based damage) and percent of intensity (internode-based damage) to modify RRR. The lowest RRR recorded for Co 293, though significantly different from that of the remaining hybrids in the field trial alone, clearly indicated its resistance status.
Eoreuma loftini oviposits exclusively on dry leaves, dry tips of leaves, dry leaf sheaths, between leaf sheath and stalk, and in concealed sites on dried sugarcane leaves located on the lower part of the plant (reviewed in Reay-Jones et al., Reference Reay-Jones, Wilson, Showler, Reagan and Way2007). Hence, Wilson et al., (Reference Wilson, Van Weelden, Beuzelin, Reagan, Way, White, Wilson and Showler2015) measured host preference in terms of early instar larval injury recorded as percentage of bored internodes which reflects not only establishment of early instar larvae but also oviposition preference. However, C. sacchariphagus indicus oviposits only on fresh leaves, showing preferences between adaxial and abaxial surfaces; upper, middle, and lower portions; and lamina, midrib, and leaf margin (Anonymous 2018). First instar larvae feed on inner side of top leaf sheath for a week and only second instar tunnels into the top internode (David Reference David, David, Easwaramoorthy and Jayanthi1986). In view of the gap between oviposition and larval tunneling, proportion of internodes tunneled cannot taken as an index of oviposition preference. Hence, oviposition preference was examined in the laboratory on excised leaves, giving due consideration to the preferences exhibited by borer females for egg laying (Anonymous 2018).
In studies on E. loftini, a sugarcane cultivar with greater volume of dry leaves was more attractive than another cultivar for oviposition and this decreased egg laying in the latter was suggested as antixenosis conferring resistance (Reay-Jones et al., Reference Reay-Jones, Wilson, Showler, Reagan and Way2007). Further, complete lack of oviposition by D. saccharalis on two cultivars was suggested to be due to nonpreference by ovipositing females (Salgado et al., Reference Salgado, Wilson, Villegas, Richard and Penn2022b). In the present study, that the moths of C. sacchariphagus indicus experienced antixenosis on leaf bits of Co 293 was evident from the significantly lowest proportions of leaf bits oviposited, egg masses deposited and egg numbers deposited in this hybrid in free-choice tests. However, the slightly yet nonsignificantly smaller egg number per mass in this hybrid indicated that Co 293 did not induce behavioral change suggesting lack of complete immunity.
The significantly lowest OPI on per leaf bit basis in Co 293 for both egg mass number and egg number was suggestive of antixenosis. Since the mean weight and area of the ≈ 17 cm L leaf bits of the three hybrids provisioned in the studies showed considerable variation, which indicated a gradation in biomass, OPI was computed on the basis of weight or area of leaf bit. Such standardization also distinguished the hybrids, though significant for egg number on area basis alone. Since volatile production is related to leaf size (Agelopoulos et al., Reference Agelopoulos, Chamberlain and Pickett2000) and leaf size is smallest in Co 293 among the three hybrids, probably due to the presence of S. spontaneum in its lineage (Balasundaram et al., Reference Balasundaram, Pramachandran, Chandran and Natarajan2005), the antixenosis in this resistant hybrid could be due to lower quantities of attractive volatiles and higher quantities of repellents emitted, besides the possible influence of variable free amino acids (Reay-Jones et al., Reference Reay-Jones, Wilson, Showler, Reagan and Way2007) and headspace volatiles produced by sugarcane (Salin et al., Reference Salin, Srikanth, Singaravelu and Nirmala2021). In addition, the presence of S. spontaneum in the lineage may have conferred borer resistance in the form of nonpreference for oviposition known for the species (David Reference David, David, Easwaramoorthy and Jayanthi1986).
An earlier study with D. saccharalis on sugarcane cultivars suggested that reduced frequency of establishment of young larvae in the stalk would be a major component of resistance to the borer (White Reference White1993). Significantly lowest survival of the borer in laboratory rearing tests established Co 293 as the most unsuitable among the three hybrids. Further, 5-10 fold higher levels of initial mortality of neonate larvae in Co 293 than in the other two hybrids suggested possibility of this hybrid showing early resistance against the borer probably mediated by the high quantities of the eight phenolics detected in HPLC studies. When reared on shoots, larval development of C. sacchariphagus indicus was not affected unlike in earlier studies wherein prolongation of larval duration was observed in resistant sugarcane cultivars for Chilo infuscatellus Snellen (Lepidoptera: Crambidae) (Bhavani et al., Reference Bhavani, Reddy, Rao and Lakshmi2012) and D. saccharalis (Salgado et al., Reference Salgado, Wilson, Villegas, Richard and Penn2022b). However, pupal weight was significantly lower for C. sacchariphagus indicus in Co 293 than in the susceptible Co 86032. Apparently, minor nutritional or biochemical differences, including phenolics, in the hybrids did not affect larval duration upon sustained feeding but reduced metabolism and conversion of food into biomass resulting in lower pupal weight with females showing greater tolerance. Lower fecundity in the resistant Co 293, an apparent effect of reduced pupal weight, could probably be due to nutritional or biochemical factors. Examination of amount of food consumed and digested in relation to biomass accumulated would throw more light on the role of nutritional biochemistry of the hybrids on borer biology and possible antibiosis effect. RSR computed using the ranks of larval and pupal durations in shoots accommodated the variations in these parameters and exhibited a definite trend, once again establishing the unsuitability of Co 293.
The weak and nonsignificant correlations between morphological characters and infestation parameters of C. sacchariphagus indicus for the 52 hybrids at the end of third field trial could be due to the presence of large number of susceptible entries. On the other hand, among the six hybrids evaluated in the final year, correlations were generally high, though only a few were significant, probably due to the smaller number and minimum variability. Nevertheless, these trends indicated a possible role for plant morphological characters: positive influence of leaf length and width in terms of site selection for oviposition; positive relationship with cane length and thickness suggested the selection of long and thick canes that offer greater quantity of food; negative correlation with fiber content in the cane was suggestive of reduced larval feeding. Cane length, and length and girth of vulnerable portions are known to positively influence borer infestation in genotypes (Asha et al., Reference Asha, Patel, Sugeetha, Pankaja, Mahdev and Kamaraddi2019). In our earlier study with Saccharum spp. clones, cane thickness positively influenced borer incidence in S. officinarum alone (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). Internode hardness, fiber, and pith did not show strong relationship with borer incidence in the present study though in an earlier study with progeny from a cross, internode rind hardness and fiber were more closely associated with resistance than pith (White et al., Reference White, Tew and Richard2006). Higher infestation in loose sheath-clasp varieties suggested their suitability since tight leaf sheath is known to act as an adverse barrier for initial establishment of neonate larvae (Easwaramoorthy and Nandagopal Reference Easwaramoorthy and Nandagopal1986), and it may also render leaf axils inaccessible for pupation. Such a trend was noticed earlier with the borer even in S. barberi clones (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018).
Higher quantities of eight phenolics in Co 293 indicated their possible role as resistant factors. On the other hand, two phenolics absent in Co 293 but present in high quantities in the other two hybrids suggested that they could be acting as possible phagostimulant factors conferring susceptibility. Total phenols in the cane were shown to be negatively related to incidence levels of the borer in selected genotypes earlier (Asha et al., Reference Asha, Patel, Sugeetha, Pankaja, Mahdev and Kamaraddi2019). The limited number of hybrids examined for phenolic composition in the present study disallowed computation of such correlations. Establishment of such relationships and examination of borer biology on phenolic-incorporated diet would establish their role as resistant or phagostimulant factors. Besides, significant impact of constitutive phenolics in sugarcane hybrids on the abundance of woolly aphid Ceratovacuna lanigera Zehntner (Hemiptera: Aphididae) (Srikanth et al., Reference Srikanth, Salin, Kurup, Karthikeyan, Mukunthan and Singaravelu2009) indicated their role in imparting resistance against not only borers but also sucking pests.
In the present study, 535 sugarcane hybrids were selected from the Indian hybrid collection based on regularity of flowering to facilitate easy introgression in breeding programs. However, the four-year elaborate screening process in the field and controlled conditions, supported by laboratory tests on borer biology, culminated in the identification of only one resistant hybrid. Such low levels of resistance in hybrids could be due to the general lack of borer resistance in their predominant progenitor S. officinarum (Mahesh et al., Reference Mahesh, Srikanth, Chandran and Singaravelu2018). Nevertheless, the resistance observed in Co 293 can be exploited in breeding programs for borer resistance by deploying it as a parent.
Acknowledgements
We thank the personnel of M/s Rajshree Sugars and Chemicals Ltd., Mundiyampakkam, Tamil Nadu State, India, for facilitating field experiments and data collection in their research farm. We thank our Director for academic encouragement. We are grateful to Dr. P. Govindaraj, Principal Scientist, Division of Crop Improvement, for arranging rind hardness test and fiber analysis. The help rendered by Ms. M. Dhanalakshmi in maintaining insect cultures and recording observations in the laboratory is gratefully acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0014479724000024