Introduction
Chemical control using herbicides is the dominant weed management practice in current agriculture (Beckie Reference Beckie2006; Powles and Yu Reference Powles and Yu2010). Herbicides offer a straightforward approach to managing invasives by reducing tillage operations and providing higher effectiveness and other beneficial factors. Chemical control decisions depend on several factors, such as crop sensitivity, herbicide accessibility, or weed infestation (Radosevich et al. Reference Radosevich, Holt and Ghersa2007; Robbins et al. Reference Robbins, Crafts and Raynor1953). The development and commercialization of transgenic crops genetically engineered for herbicide resistance increased the use of certain herbicides, such as glyphosate (Bonny Reference Bonny2016).
During the past three to four decades, weeds exhibiting herbicide resistance have increased exponentially, from only three unique resistance cases in the early 1970s to 530 cases by 2024. Moreover, the number of weed populations resistant to multiple sites of action increased from 33 in 2000 to 103 in 2020 (Heap Reference Heap2024). The presence of herbicide-resistant weeds increases management challenges and the cost of achieving effective control. Alongside efficacy loss due to weed resistance, herbicide discovery and registrations have decreased in recent years, and only a few new molecular target sites are projected to be introduced in the market after decades of stagnation (Campe et al. Reference Campe, Hollenbach, Kämmerer, Hendriks, Höffken, Kraus, Lerchl, Mietzner, Tresch, Witschel and Hutzler2018; Duke and Dayan Reference Duke and Dayan2022; Kraehmer et al. Reference Kraehmer, Almsick, Beffa, Dietrich, Eckes, Hacker, Hain, Strek, Stuebler and Willms2014; Qu et al. Reference Qu, He, Yang, Lin, Yang, Wu, Li and Yang2020; Selby et al. Reference Selby, Satterfield, Puri, Stevenson, Travis, Campbell, Taggi, Hughes and Bereznak2023; Shino et al. Reference Shino, Hamada, Shigematsu, Hirase and Banba2018; Umetsu and Shirai Reference Umetsu and Shirai2020). Besides the discovery of new protein binding sites and the development of new molecules, research should also focus on ways to reactivate herbicides lost to resistance or reverse herbicide resistance in problematic weeds. Adding metabolic inhibitors or oxidative stress inducers to increase herbicide efficacy or overcome metabolic resistance is one viable solution to the lack of new chemistries. It is well established that adding certain compounds can reverse herbicide tolerance or provide synergistic effects (Dücker et al. Reference Dücker, Parcharidou and Beffa2020; Ezra et al. Reference Ezra, Dekker and Stephenson1985; Takano et al. Reference Takano, Beffa, Preston, Westra and Dayan2020). The inhibition of glutathione S-transferases (GSTs) will likely increase herbicide efficacy, and this review focuses on potential candidates to be used in agricultural scenarios as herbicide synergists or inducers, in the case of crop safeners.
Glutathione S-transferases
The possible mechanisms of herbicide resistance in weeds are divided into target-site (TSR) and non–target site resistance (NTSR). TSR encompasses any modification in the enzyme targeted by the herbicide that will prevent binding or amplify the gene encoding the enzyme requiring more of the herbicide for complete inhibition. NTSR includes any plant mechanism that reduces the amount of herbicide reaching the target site. Herbicide detoxification (Figure 1), an important NTSR mechanism, is a multiphase process that starts with parent molecule transformation into hydrophilic metabolites by cleavage, oxidation, or reduction (Phase I) mediated by cytochrome P450 monooxygenases (P450s or CYPs), carboxylesterases, or other enzymes. Phase I-transformed molecules are then conjugated to a sugar molecule or reduced glutathione (GSH; Phase II) catalyzed by glucosyltransferases or GSTs, and nontoxic metabolites are transported from the cytosol and compartmentalized into the vacuole or cell walls via adenosine triphosphate-binding cassette transporters (Phase III). The conjugation to GSH (Phase II) to inactivate toxic compounds catalyzed by GSTs is a crucial step in cell and tissue protection and, consequently, is one type of metabolic resistance mechanism in weeds and tolerance mechanism in crops (Délye Reference Délye2013; Délye et al. Reference Délye, Jasieniuk and Le Corre2013; Powles and Yu Reference Powles and Yu2010; Rigon et al. Reference Rigon, Gaines, Küpper and Dayan2020; Zhao et al. Reference Zhao, Ye and Fu2023).
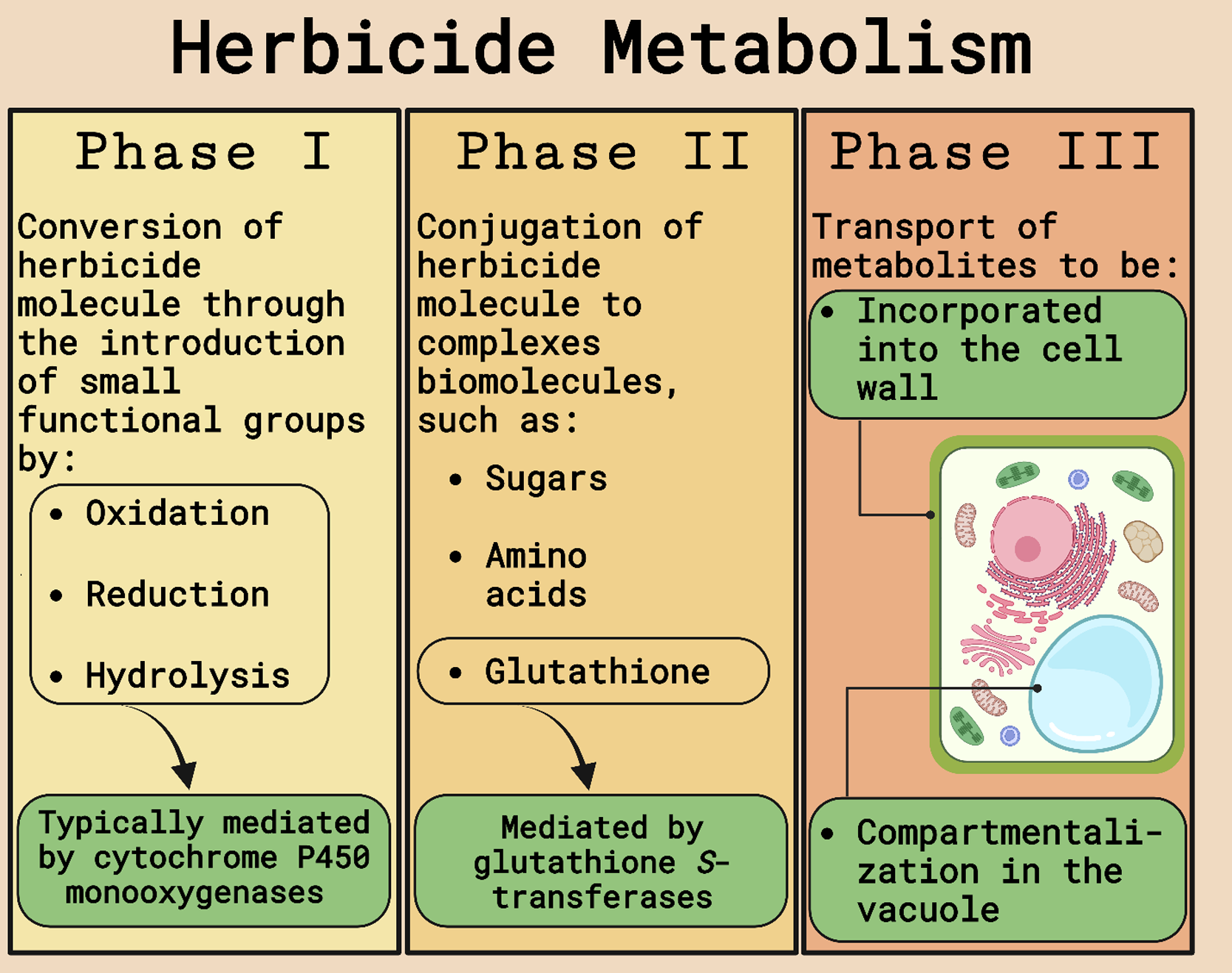
Figure 1. Schematic of herbicide metabolism in plants. Adapted from Gaines et al. (Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020) and Nandula et al. (Reference Nandula, Riechers, Ferhatoglu, Barrett, Duke, Dayan, Goldberg-Cavalleri, Tetard-Jones, Wortley, Onkokesung, Brazier-Hicks, Edwards, Gaines, Iwakami, Jugulam and Ma2019). Figure created with BioRender.com (Science Suite Inc., Toronto, ON, Canada).
GSH, a tripeptide formed by gamma-glutamic acid, cysteine, and glycine, is key to the detoxification of reactive oxygen species (ROS). This tripeptide provides cell defense by detoxifying detrimental substances using varied mechanisms, such as peroxide reduction, electrophilic compound conjugation, and free radical scavenging. However, the existence of an enzyme system able to catalyze the conjugation of this tripeptide to toxins is crucial for plant survival and defense. GST enzymes are essential in detoxifying endogenous or exogenous toxic compounds by catalyzing the conjugation of the nucleophilic thiol group from reduced GSH to co-substrates possessing an electrophilic center. After conjugation, GSH–metabolite conjugates, which usually have low or zero toxicity, are imported into the vacuole and catabolized in Phase IV reactions (Csiszár et al. Reference Csiszár, Hecker, Labrou, Schröder and Riechers2019; Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011; Dostalek and Stark Reference Dostalek, Stark, Anzenbacher and Zanger2012; Edwards et al. Reference Edwards, Dixon and Walbot2000; Grill et al. Reference Grill, Tausz and De Kok2001; Hayes and McLellan Reference Hayes and McLellan1999; Katerova and Miteva Reference Katerova, Miteva, Anjum, Chan and Umar2010). The processing of these GSH–herbicide conjugates likely varies between plant species and tissues (Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011; Tal et al. Reference Tal, Romano, Stephenson, Schwan and Hall1993).
The GST protein family is abundant in plants. Previous studies have identified 61, 85, 90, 101, and 115 GSTs in the genome of Arabidopsis thaliana (L.) Heynh., rice (Oryza sativa L.), tomato (Solanum lycopersicum L.), soybean [Glycine max (L.) Merr.], and blackgrass (Alopecurus myosuroides Huds.), respectively (Casey and Dolan Reference Casey and Dolan2023; Islam et al. Reference Islam, Rahman, Islam and Ghosh2017; Jain et al. Reference Jain, Ghanashyam and Bhattacharjee2010; Parcharidou et al. Reference Parcharidou, Dücker and Beffa2024; Wagner et al. Reference Wagner, Edwards, Dixon and Mauch2002). The GSTs of plants (vascular and nonvascular) are divided into 12 distinct classes: phi (GSTF), tau (GSTU), zeta, EF1Bγ (elongation factor 1B gamma), hemerythrin, iota, lambda, DHARs (GSH-dependent dehydroascorbate reductases), TCHQD (tetrachlorohydroquinone dehalogenase), theta, glutathionyl-hydroquinone reductases, and ureidosuccinate transport 2 prion protein (Casey and Dolan Reference Casey and Dolan2023; Estévez and Hernández Reference Estévez and Hernández2020; Lallement et al. Reference Lallement, Meux, Gualberto, Dumarcay, Favier, Didierjean, Saul, Haouz, Morel-Rouhier, Gelhaye, Rouhier and Hecker2015; Liu et al. Reference Liu, Han, Ren, Yang and Zeng2013). Among these classes, theta and zeta are present in mammals and plants. The phi, tau, DHAR, and lambda classes are only present in plants (Dixon et al. Reference Dixon, Lapthorn and Edwards2002; Estévez and Hernández Reference Estévez and Hernández2020). In plants, oxidative stress induces GST activity, specifically phi and tau classes. These two classes are the most abundant in plants. Phi and tau GSTs are directly involved in catalyzing GSH conjugation with various xenobiotics and pesticides. Because this conjugation detoxifies toxic by-products, levels of cell death are reduced by GST activity (Dixon and Edwards Reference Dixon and Edwards2010; Droog Reference Droog1997; Edwards et al. Reference Edwards, Dixon and Walbot2000; Mauch and Dudler Reference Mauch and Dudler1993; Zheng et al. Reference Zheng, Board, Fei, Sun, Lv, Yan, Liu, Shen and Luo2008).
Most GST isoforms exist as dimers with two identical (homodimeric) or different (heterodimeric) subunits. The isoforms may occur as monomers or oligomers as well (Dixon et al. Reference Dixon, Cole and Edwards1999; Grill et al. Reference Grill, Tausz and De Kok2001). Enzymes from the GST superfamily generally have a catalytic center divided into two functional sites: G-site and H-site. The H-site is the hydrophobic pocket near the G-site that has a high affinity with hydrophobic and electrophilic substrates. Large hydrophobic compounds will likely bind to the H-site of the enzymes. The hydrophilic G-site specifically interacts with GSH; consequently, it is the GSH binding pocket of the enzyme (Dirr et al. Reference Dirr, Reinemer and Huber1994; Frova Reference Frova2003; Thom et al. Reference Thom, Cummins, Dixon, Edwards, Cole and Lapthorn2002). Due to this high GSH specificity, G-site residues are very conserved among all GST classes, unlike those of the H-site (Prade et al. Reference Prade, Huber and Bieseler1998; Ricci et al. Reference Ricci, Maria, Antonini, Turella, Bullo, Stella, Filomeni, Federici and Caccuri2005; Sylvestre-Gonon et al. Reference Sylvestre-Gonon, Law, Schwartz, Robe, Keech, Didierjean, Dubos, Rouhier and Hecker2019). In phi and tau GST enzymes, the active site, characterized by the presence of a conserved serine residue, activates the sulfur atom in the cysteine residue in GSH (i.e., lowers its pKa), forming reactive thiolate species (Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011; Nianiou-Obeidat et al. Reference Nianiou-Obeidat, Madesis, Kissoudis, Voulgari, Chronopoulou, Tsaftaris and Labrou2017). The hydrophobic acceptor of GSTs will be oriented to have its electrophilic center available for nucleophilic reactions (substitution or addition) (Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011). An in-depth review covering the structure of these enzymes and their subunits has been provided by Dixon and Edwards (Reference Dixon and Edwards2010), Sylvestre-Gonon et al. (Reference Sylvestre-Gonon, Law, Schwartz, Robe, Keech, Didierjean, Dubos, Rouhier and Hecker2019), and Vaish et al. (Reference Vaish, Gupta, Mehrotra, Mehrotra and Basantani2020).
The GST enzyme family metabolizes or binds a vast array of xenobiotic compounds, but an extensive literature review supports the involvement of GST enzymes with herbicide detoxification. Regarding the most abundant GSTs in plants, the phi and tau classes have different affinities toward herbicides. When cloned and expressed in Escherichia coli, rice tau class GST enzymes showed higher activity toward fluorodifen (a diphenyl ether; Group 14), while phi class GST enzymes had more specificity toward chloroacetamide herbicides (alachlor, acetochlor, and metolachlor) (Cho and Kong Reference Cho and Kong2007). Like rice, tobacco (Nicotiana tabacum L.) plants overexpressing tau GSTs (CsGSTU1 and CsGSTU2) from sweet orange [Citrus sinensis (L.) Osbeck] or from soybean (GmGSTU4) also showed an increase in tolerance to fluorodifen (Benekos et al. Reference Benekos, Kissoudis, Nianiou-Obeidat, Labrou, Madesis, Kalamaki, Makris and Tsaftaris2010; Cicero et al Reference Cicero, Madesis, Tsaftaris and Piero2015).
The GST enzymes are present in all tissues and throughout different plant stages (Holt et al. Reference Holt, Lay, Clarke, Dinsmore, Jepson, Bright and Greenland1995; Vaish et al. Reference Vaish, Parveen, Gupta and Basantani2022). However, the GST subclass and expression level may vary according to tissue, stage, environmental conditions, stress (abiotic and biotic), and, especially, plant species. Rice tau GST (OsGSTU4) was overexpressed in A. thaliana plants, and transgenic plants showed an increase in oxidative stress tolerance and chlorophyll content retained under stress conditions at different plant stages. These modified plants also showed reduced accumulation of ROS and higher GST activity (Sharma et al. Reference Sharma, Sahoo, Devendran and Jain2014). Herbicide detoxification via GSH conjugation was essential for corn (Zea mays L.) and giant foxtail (Setaria faberi Herrm.) seedlings, but no effect was observed in mature plants (Hatton et al. Reference Hatton, Cole and Edwards1996). Besides the degradation of potentially toxic compounds, phi and tau GSTs are typically induced whenever the plant is stressed, and different stress types (biotic vs. abiotic) induce differential GST expression (Hasan et al. Reference Hasan, Islam, Hasan, Sajib, Ahmed, Islam and Ghosh2020; Marrs Reference Marrs1996; Mauch and Dudler Reference Mauch and Dudler1993; Sappl et al. Reference Sappl, Carroll, Clifton, Lister, Whelan, Millar and Singh2009; Soviguidi et al. Reference Soviguidi, Liu, Pan, Abou-Elwafa, Rao, Abel, Zhang and Yang2022; Ulmasov et al. Reference Ulmasov, Ohmiya, Hagen and Guilfoyle1995). The GST classes and levels can also vary within the same species, which may explain why certain crop cultivars can withstand higher stress levels (Deng and Hatzios Reference Deng and Hatzios2002; Li et al. Reference Li, Gao, Xu, Pang, Liu, Wang and Tan2017; Shimabukuro et al. Reference Shimabukuro, Frear, Swanson and Walsh1971).
Although xenobiotic detoxification by GSH conjugation is the most investigated function of plant GSTs, roles of this enzyme family also include important processes such as targeting transmembrane transport of endogenous substrates, tissue protection against oxidative damage, and nonenzymatic binding (intracellular transport). It has been proposed that oxidative metabolism derivatives such as hydroperoxides serve as natural substrates for GST enzymes (Edwards et al. Reference Edwards, Dixon and Walbot2000; Grill et al. Reference Grill, Tausz and De Kok2001; Mannervik et al. Reference Mannervik and Danielson1988; Masella et al. Reference Masella, Di Benedetto, Varì, Filesi and Giovannini2005). The GST antioxidant response is essential in the natural plant defense system in the presence of stress (Gallé et al. Reference Gallé, Czékus, Bela, Horváth, Ördög, Csiszár and Poór2019; Marrs Reference Marrs1996; Wagner et al. Reference Wagner, Edwards, Dixon and Mauch2002).
Metabolic Resistance to Herbicides via GSH Conjugation Catalyzed by GST Enzymes
Enhanced GST activity was previously observed in weeds and crops showing metabolic resistance to various herbicides, such as atrazine and chlorimuron-ethyl (Alla and Hassan Reference Alla and Hassan2006; Evans et al. Reference Evans, O’Brien, Ma, Hager, Riggins, Lambert and Riechers2017; Lamoureux et al. Reference Lamoureux, Rusness and Tanaka1991). Interestingly, GST conjugation with herbicides usually occurs more rapidly in crops than in weeds (Busi et al. Reference Busi, Porri, Gaines and Powles2018; Dücker et al. Reference Dücker, Parcharidou and Beffa2020; Edwards et al. Reference Edwards, Dixon and Walbot2000; Nakka et al. Reference Nakka, Godar, Thompson, Peterson and Jugulam2017). The first report of GSH conjugation conferring herbicide tolerance (atrazine) in plants was in 1970 with corn and grain sorghum [Sorghum bicolor (L.) Moench]. The leaf tissue of these two species had a high amount of GST activity with atrazine (photosystem II inhibitor; Group 5) as substrate, while no enzyme activity was observed in sensitive species (Frear and Swanson Reference Frear and Swanson1970).
Multiple herbicide resistance (MHR) in some weeds is also linked to increased detoxification ability, leading to protection against multiple xenobiotics (Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards, Hughes, Kaundun, Hutchings, Steel and Edwards2013). Studies with multiple herbicide-resistant A. myosuroides showed the ability to reduce oxidative injury with a phi class GST (AmGSTF1) was induced by herbicides, such as paraquat, fluorodifen, and chlorotoluron. The AmGSTF1 enzyme showed high activity as a GSH peroxidase, which reduces organic hydroperoxides, protecting cells from the toxicity caused by ROS (Cummins et al. Reference Cummins, Cole and Edwards1999; Hayes and McLellan Reference Hayes and McLellan1999). A different study expressed the phi AmGSTF1 from A. myosuroides in A. thaliana. Like herbicide-resistant A. myosuroides, modified A. thaliana plants showed resistance to multiple herbicides (alachlor, atrazine, and chlorotoluron). The insertion of phi-GST induced changes in the A. thaliana metabolism led to an accumulation of protective compounds. Resistance was reversed by adding the synthetic GST inhibitor, 4-chloro-7-nitrobenzofurazan (NBD-Cl), in modified plants and a resistant A. myosuroides population (Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards, Hughes, Kaundun, Hutchings, Steel and Edwards2013). When tau GST from tomato was expressed in yeast, resistance to hydrogen peroxide–induced stress was improved (Kampranis et al. Reference Kampranis, Damianova, Atallah, Toby, Kondi, Tsichlis and Makris2000). Similarly to MHR in weeds, multiple drug resistance in humans is also connected to GST enzymes. The overexpression in cancer cells of a GST class only present in humans and animals (pi; GSTP1-1) is linked to multiple drug resistance in humans by detoxification and immune system signaling functions (Ricci et al. Reference Ricci, Maria, Antonini, Turella, Bullo, Stella, Filomeni, Federici and Caccuri2005). These results further show the involvement of GSTs in detoxifying exogenous and endogenous compounds.
Due to their crucial role in abiotic and biotic stress tolerance, plant GSTs are an attractive target for overcoming herbicide resistance and increasing pesticide efficacy on target pests (Nianiou-Obeidat et al. Reference Nianiou-Obeidat, Madesis, Kissoudis, Voulgari, Chronopoulou, Tsaftaris and Labrou2017). By inhibiting the GSTs, was describing herbicide efficacy. Also, if direct GSH conjugation of herbicides or antioxidant plant defense mechanisms is inactivated or reduced. The GST inhibitors can be natural or synthetic, as described in the following sections.
Natural GST Inhibitors
Phenolic compounds are secondary plant metabolites that include an aromatic ring with one or more hydroxyl substituents. Some plant secondary metabolites are highly phytotoxic, with great potential as new herbicide modes of action (Duke et al. Reference Duke, Romagni and Dayan2000). Interestingly, phenolic compounds are both natural GST inducers and inhibitors in plants. These compounds are divided into phenolic acids, flavonoids, tannins, stilbenes, lignans, and lignins (Grill et al. Reference Grill, Tausz and De Kok2001; Harborne Reference Harborne1973; Lin et al. Reference Lin, Xiao, Zhao, Li, Xing, Li, Kong, Li, Zhang, Liu, Chen, Qin, Wu and Chen2016). Studies have shown that flavonoids and phenolic acids have high potential as GST inhibitors in different organisms.
Flavonoids are large polyphenolic compounds mostly known as natural pigments (anthocyanins) present in plant tissues and possess strong antioxidant properties that reduce free radical formation. Additionally, some flavonoids inhibit the enzymes responsible for superoxide anion production (Panche et al. Reference Panche, Diwan and Chandra2016; Pietta Reference Pietta2000; Procházková et al. Reference Procházková, Boušová and Wilhelmová2011). Previous research identified that flavonoids bind with high affinity to a phi GST (AmGSTF1) in multiple herbicide–resistant A. myosuroides; pendimethalin (microtubule assembly inhibitor; Group 3) resistance reversal in this species was linked with the high binding affinity of the flavonoids to the GST active site (Schwarz et al. Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021).
Georgakis et al. (Reference Georgakis, Poudel, Vlachakis, Papageorgiou and Labrou2021) used the phi GSTs from rigid ryegrass (Lolium rigidum Gaudin) (LrGSTF), A. myosuroides, barley (Hordeum vulgare L.) (HvGSTF), and wheat (Triticum aestivum L.) (TaGSTF) to analyze the inhibition potency of selected pesticides and natural products in vitro. The flavonoids quercetin (Figure 2A) and ellagic acid (Figure 2B) displayed enzyme inhibition greater than 70% with all phi GSTs tested. Curcumin (Figure 2C) showed relatively weak inhibition (less than 40%). Several herbicides were included in this work, and only butachlor (very-long-chain fatty-acid elongase inhibitor; Group 15) showed significant GST inhibition in all species studied. Butachlor exhibited 44%, 52%, 78%, and 70% phi GST inhibition potency for HvGSTF, TaGSTF, LrGSTF, and AmGSTF, respectively. In different studies, quercetin showed a moderate inhibition activity in wheat, while transgenic tobacco overexpressing a tau GST from A. thaliana (AtGSTU19) showed affinity and specific interactions with GSH derivatives from flavonoids, including quercetin (Cummins et al. Reference Cummins, O’Hagan, Jablonkai, Cole, Hehn, Werck-Reichhart and Edwards2003; Dixon and Edwards Reference Dixon and Edwards2018). Schwarz et al. (Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021) used the quercetin structure to generate several derivatives. Among the products, compound 55, which combined a quercetin nucleus with a C-5 long-chain hydroxycarboxylate, showed promising in vitro inhibition levels of A. myosuroides (AmGSTF1) phi GSTs with enough water solubility to be applied in small greenhouse trials. The application of this GST inhibitor 24 h before herbicide treatment partially reversed pendimethalin resistance in A. myosuroides in a greenhouse trial. The application of compound 55 before herbicide treatment decreased normal A. myosuroides shoot growth by 34%. However, this compound was highly selective. Compared with a phi class GST from A. thaliana (AtGSTF8), the fold inhibition with AmGSTF1 with compound 55 was reduced, indicating this GST inhibitor is highly species selective, which would be undesirable in a large-scale agricultural scenario. Conversely, pure curcumin inhibited growth at the same levels as glyphosate in sourgrass [Digitaria insularis (L.) Mez ex Ekman], spreading liverseed grass [Urochloa decumbens (Stapf) R. Webster], and wild radish (Raphanus raphanistrum L.) (Garrido Reference Garrido2018).
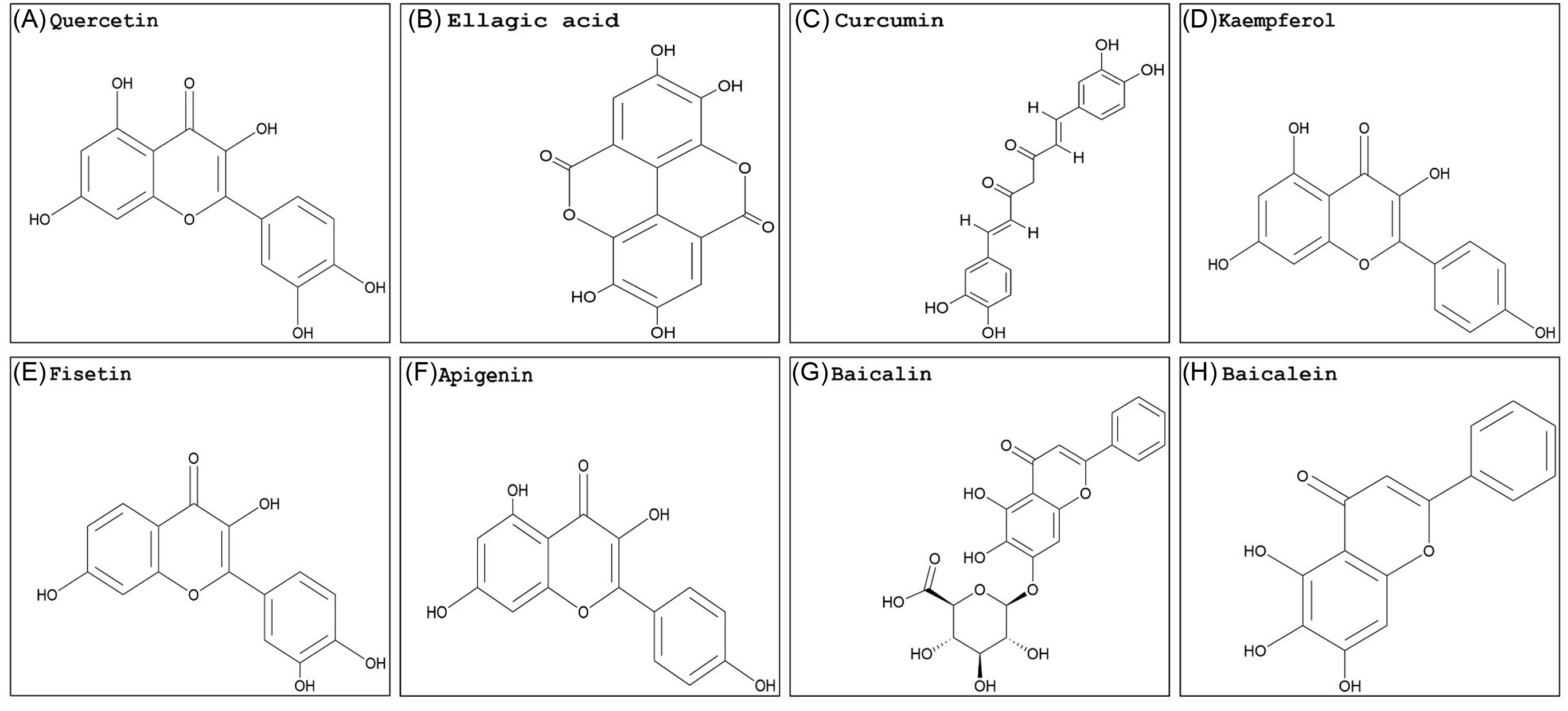
Figure 2. Naturally occurring flavonoid structures: (A) quercetin, (B) ellagic acid, (C) curcumin, (D) kaempferol, (E) fisetin, (F) apigenin, (G) baicalin, and (H) baicalein. The PubChem CID information was provided earlier in the review. Chemical structures were generated using ChemDraw Professional v. 22.2 (PerkinElmer, Waltham, MA, USA).
Quercetin and ellagic acid are found in many fruits and vegetables, while curcumin is mostly from turmeric roots (Curcuma longa L.). Literature on plant GST inhibitors is scarce and underdeveloped compared with what is available for animal cells. Besides having the ability to strongly inhibit GSTs, many flavonoids are highly recognized for their anticarcinogenic, anti-inflammatory, antioxidant, and signaling properties (Das et al. Reference Das, Bickers and Mukhtar1984; Sturm et al. Reference Sturm, Hu, Zimmermann, Fritz-Wolf, Wittlin, Rahlfs and Becker2009; Vattem and Shetty Reference Vattem and Shetty2005). Albeit in different systems, research conducted on animal cells is valuable in providing insights into the interaction of GSTs and inhibitors on a cellular level that may be applied to future investigations with plants. Overall, results varied when animal GSTs were used. In different studies, the inhibition of GSTs by quercetin, ellagic acid, and curcumin varied greatly, indicating that the product inhibitory effect will likely change by species and cell type (Boušová et al. Reference Boušová, Hájek, Dršata and Skálová2012; Breinholt et al. Reference Breinholt, Lauridsen and Dragsted1999; Hayeshi et al. Reference Hayeshi, Mutingwende, Mavengere, Masiyanise and Mukanganyama2007; Iio et al. Reference Iio, Kawaguchi, Sakota, Otonari and Nitahara1993; Kurata et al. Reference Kurata, Suzuki and Takeda1992). Additionally, quercetin reduced the nuclear content of GSH and induced a pro-oxidant response that was not observed in plant cells (Sahu and Gray Reference Sahu and Gray1996). Because phi and tau GSTs are the most abundant classes in plants, the results described above indicate variable inhibition by natural compounds per GST class should be expected in animals versus plant systems.
Kaempferol (Figure 2D) and fisetin (Figure 2E) are also examples of flavonoids investigated for GST inhibition properties in animal cells, specifically in tumor or cancer research. Fisetin has shown a strong inhibition ability of human GSTs, negatively impacting protein expression (Alqarni et al. Reference Alqarni, Foudah, Muharram and Labrou2021; Iio et al. Reference Iio, Kawaguchi, Sakota, Otonari and Nitahara1993). Fisetin decreased overall GST activity in cancer cell lines in a dose-dependent manner, which resulted in growth inhibition and apoptosis (programmed cell death) (Youns and Hegazy Reference Youns and Hegazy2017). Kaempferol decreased the GST activity of rat liver nuclei, which compromised the nuclear antioxidant response (Sahu and Gray Reference Sahu and Gray1996). The literature supports the ability of kaempferol and fisetin to inhibit GST activity in animal cells, but the herbicidal potential of these two compounds has not yet been investigated. Even though the GSTs have a similar and somewhat conserved catalytic core structure, their protein sequences can differ significantly (Dixon and Edwards Reference Dixon and Edwards2010; Vaish et al. Reference Vaish, Gupta, Mehrotra, Mehrotra and Basantani2020). Additionally, members from the phi, tau, theta, and zeta classes possess a conserved serine in their respective N-terminal active sites. The serinyl-GSTs catalyze GSH conjugation and also have some level of peroxidase activity, which are crucial activities for overall stress tolerance and herbicide detoxification (Axarli et al., Reference Axarli, Dhavala, Papageorgiou and Labrou2009; Sylvestre-Gonon et al. Reference Sylvestre-Gonon, Law, Schwartz, Robe, Keech, Didierjean, Dubos, Rouhier and Hecker2019). Therefore, the inhibition effect of fisetin and kaempferol is likely to change.
The flavonoid apigenin (Figure 2F) is found in chamomile (Matricaria chamomilla L.), which successfully reversed weed resistance to herbicides in A. myosuroides. Control of resistant plants with pinoxaden, an acetyl CoA carboxylase (Group 1) inhibitor, was achieved when the flavonoid apigenin 1 was added to the herbicide solution. In addition, a ligand cocktail with several small molecules was prepared to evaluate the binding affinity of the A. myosuroides phi GST enzyme (AmGSTF1), in which the enzyme bound to apigenin 1 instead of other molecules (Schwarz et al. Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021). Likewise, in plants, protoapigenone, a natural derivative of apigenin 1, significantly inhibited human GSTP1-1 in vivo and in vitro experiments. Besides inhibition, cells treated with protoapigenone had increased ROS levels, which impacted apoptosis (Chen et al. Reference Chen, Hsieh, Tsai, Kang, Chang, Wu and Wu2011b). Due to the differences between animal and plant cells and the GSTs expressed in each organism, enhanced ROS levels and induced apoptosis by the flavonoid cannot be assumed. In rat GSTs, apigenin 1 induced enzyme activity in heart cells but not the colon or liver (Breinholt et al. Reference Breinholt, Lauridsen and Dragsted1999). Therefore, it is likely that apigenin might show different levels of activity in distinct plant tissues as well.
Some flavonoids are synthesized in a tissue-specific manner. The flavonoid baicalin (Figure 2G) is one example. Baicalin or baicalein (Figure 2H), the flavone without the sugar moiety, are two of the main flavonoids present in Chinese skullcap (Scutellaria baicalensis Georgi), both having a strong ability to inhibit human GSTs (Aksoy & Küfrevioglu, Reference Aksoy and Küfrevioglu2018; Cho et al. Reference Cho, Lee, Ahn, Kim, Kim, Lee, Lee, Park and Yang2008). In plants, the effect of co-crystallizing baicalein with the herbicide metamitron, a triazinone pertaining to photosystem II (Group 5) inhibitors, was evaluated. After simulated rainfall, Kentucky bluegrass (Poa pratensis L.) control was significantly higher (65%) when the crystalline form (including the herbicide and baicalein) was used compared with the herbicide metamitron alone (3%). The authors attribute this increase to the higher leaching potential associated with metamitron alone. However, this study did not measure GST activity (Xiao et al. Reference Xiao, Wu, Zhou, Yin and Yang2022). Therefore, a synergistic interaction between baicalein and metamitron might have happened without detection. Additionally, a mixture of baicalin with glufosinate increased Palmer amaranth (Amaranthus palmeri S. Watson) control by 24% without causing injury to glufosinate-resistant soybean (Carvalho-Moore et al. Reference Carvalho-Moore, Norsworthy, Bonilha Piveta, Castner, Woolard and Arnold2022). Previously, upregulation of GST genes was observed in treated A. palmeri plants showing tolerance to glufosinate (Salas-Perez et al. Reference Salas-Perez, Saski, Noorai, Srivastava, Lawton-Rauh, Nichols and Roma-Burgos2018). This finding indicates the involvement of GST enzymes in reducing glufosinate sensitivity in A. palmeri, possibly through antioxidant activities, which could explain the increase in control when baicalin was added to the herbicide solution. However, there is no research demonstrating the capacity of GST enzymes to conjugate and detoxify glufosinate. Glufosinate and metamitron are classified as herbicides with a mode of action involving the rapid, light-activated accumulation of ROS (HRAC 2024; Takano et al. Reference Takano, Beffa, Preston, Westra and Dayan2020; Traxler et al. Reference Traxler, Gaines, Küpper, Luemmen and Dayan2023). The tripeptide GSH and GST enzymes are tightly connected to antioxidation signaling, cell protection, and regeneration of other antioxidants (Dhindsa Reference Dhindsa1991; Roxas et al. Reference Roxas, Smith, Allen and Allen1997; Vanacker et al. Reference Vanacker, Carver and Foyer2000). By inhibiting this enzyme family, it is possible that baicalin or baicalein might increase herbicide efficacy through reducing the antioxidative activity provided by the GSTs. However, this flavonoid has been associated with increased oxidative stress response in human cells, an undesirable characteristic in potential herbicide synergists (Du et al. Reference Du, Han, Zhang, Lin, Wu, Wang, Ji, Lu, Yu and Liang2010; Wen et al. Reference Wen, Zhao, Bhadauria and Nirala2013).
Compared with flavonoids, phenolic acids and xanthones are much smaller groups, and only a few compounds within these groups display promising GST-inhibiting activity. The advantage of these compounds is their relatively smaller size (i.e., molecular weight) and likely water solubility. Studies evaluating the potential use of phenolic acids or xanthones to enhance herbicide efficacy have yet to be conducted. However, some inferences can be made based on the research available on animal cells. Two phenolic acids, caffeic acid (Figure 3A) and chlorogenic acid (Figure 3C), demonstrated a dose-dependent rat liver GST inhibition in vitro (Das et al. Reference Das, Bickers and Mukhtar1984). The carrot (Daucus carota L.) extract, rich in chlorogenic acid, also showed potent GST inhibition (Atalar et al. Reference Atalar, Aras, Türkan, Barlak, Yildiko, Karatas and Alma2021). Caffeic acid had nearly no effect on recombinant cattle tick [Rhipicephalus (Boophilus) annulatus] GST activity, while a plant extract with high levels of gallic acid (Figure 3B) strongly inhibited GST activity (Guneidy et al. Reference Guneidy, Shahein, Abouelella, Zaki and Hamed2014). Results obtained from investigations with extracts containing high amounts of the phenolic compound gallic acid are promising, because gallic acid exhibited strong potential for GST inhibition in vitro (Boušová et al. Reference Boušová, Hájek, Dršata and Skálová2012). Tumbleweed (Gundelia tournefortii L.) seed extract, which is rich in gallic acid, demonstrated effective cytosolic GST inhibition in sheep liver extracts (Coruh et al. Reference Coruh, Celep, Özgökçe and İşcan2007b). Additionally, a high degree of inhibition of GST was correlated to high gallic acid content in extracts from three different species from the Apiaceae family (Coruh et al. Reference Coruh, Celep and Özgökçe2007a). Xanthones are natural compounds encountered in a limited number of species, including some plant families (mainly Gentianaceae and Guttiferae), fungi, and lichens (Badiali et al. Reference Badiali, Petruccelli, Brasili and Pasqua2023; Jensen et al., Reference Jensen, Schripsema, Struwe and Albert2002). Xanthones (Figure 4A) are effective antioxidants with heterocyclic structures (Martínez et al. Reference Martínez, Galano and Vargas2011, Reference Martínez, Hernandez-Marin and Galano2012; Thong et al. Reference Thong, Quang, Bui, Dao and Nam2015). Only a few studies investigated the potential of xanthones on GST inhibition, and none were conducted in plant systems. In these studies, natural xanthone and xanthone derivatives were analyzed and found to be potent inhibitors of human GSTP1-1, greater than 85% (Mukanganyama et al. Reference Mukanganyama, Bezabih, Robert, Ngadjui, Kapche, Ngandeu and Abegaz2011; Zoi et al. Reference Zoi, Thireou, Rinotas, Tsoungas, Eliopoulos, Douni, Labrou and Clonis2013).
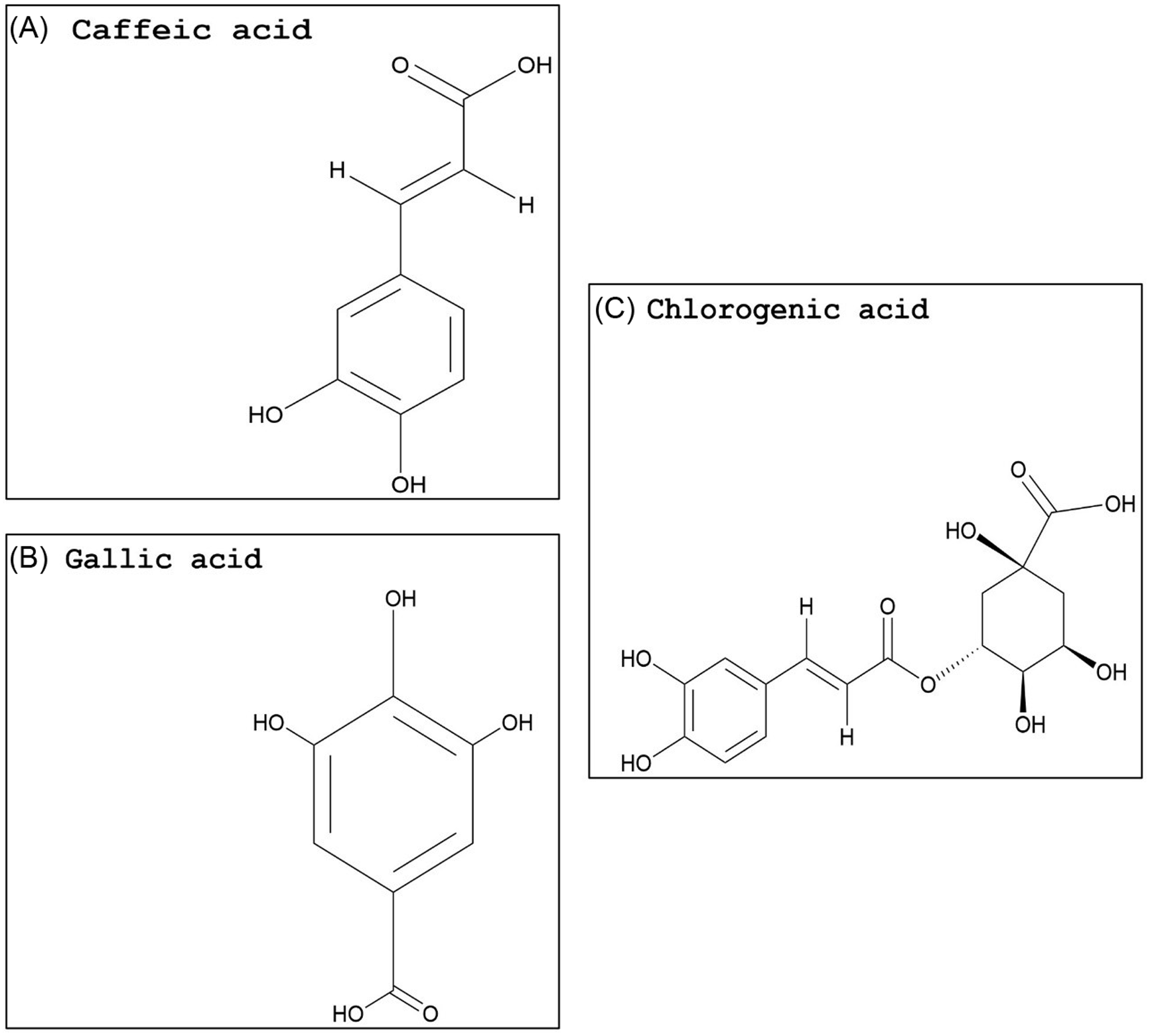
Figure 3. Naturally occurring phenolic acid structures: (A) caffeic acid, (B) gallic acid, and (C) chlorogenic acid. The PubChem CID information was provided earlier in the review. Chemical structures were generated using ChemDraw Professional v. 22.2 (PerkinElmer, Waltham, MA, USA).
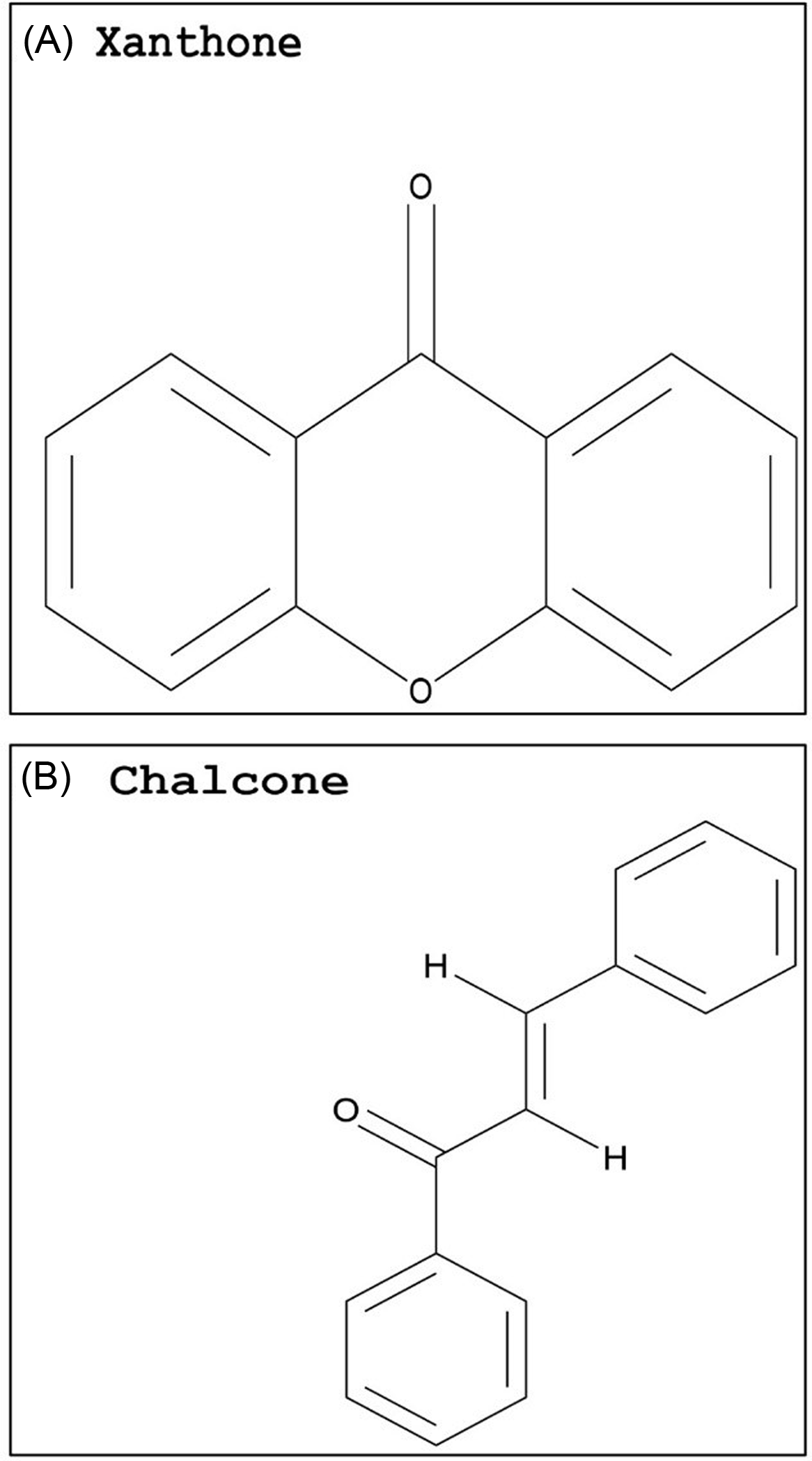
Figure 4. Basic skeleton of a xanthone (A) and chalcone (B). The PubChem CID information was provided earlier in the review. Chemical structures were generated using ChemDraw Professional v. 22.2 (PerkinElmer, Waltham, MA, USA).
The chalcone class is another natural compound reported to inhibit GST activity (Figure 4B). Chalcone is the substrate for the enzyme chalcone isomerase, which is essential in the biosynthesis of secondary metabolites, such as flavonoids, tannins, and flavonols (Shirley Reference Shirley1996). Phytotoxicity caused by this compound has been observed in a variety of plant species through in vivo and in vitro studies (Bittencourt et al. Reference Bittencourt, Santos and Souza2007; Chen et al. Reference Chen, Yun, Deng and Yogo2004, Reference Chen, Yun, Deng and Yogo2011a; Díaz-Tielas et al. Reference Díaz-Tielas, Grana, Sotelo, Reigosa and Sanchez-Moreiras2012; Garrido Reference Garrido2018). When evaluating the effect of diverse chalcones on the control of various plant species, 3,4-dimetoxichalcone resulted in higher growth inhibition than glyphosate in D. insularis, U. decumbens, R. raphanistrum, and hairy beggarticks (Bidens pilosa L.) (Garrido Reference Garrido2018). However, the study did not explore the potential inhibition of GSTs, thus the growth inhibition observed cannot be directly linked to GST-inhibiting effects of chalcones without additional research.
Synthetic GST Inhibitors
Previous reports have shown the potential of GST inhibitors to overcome herbicide resistance by reducing detoxification rates, including resistance to fenoxaprop (acetyl CoA carboxylase inhibitor; Group 1), alachlor (very-long-chain fatty-acid elongase inhibitor; Group 15), atrazine, and flufenacet (Group 15) (Pelon et al. Reference Pelon, Abdollahi, Gaines and Dayan2023). Several previously studied inhibitors were first synthesized under laboratory conditions and used in human research, specifically multiple drug and tumor-cell resistance (Georgakis et al. Reference Georgakis, Poudel, Vlachakis, Papageorgiou and Labrou2021; Ricci et al. Reference Ricci, Maria, Antonini, Turella, Bullo, Stella, Filomeni, Federici and Caccuri2005; Schwarz et al. Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021; Turella et al. Reference Turella, Filomeni, Dupuis, Ciriolo, Molinari, Maria, Tombesi, Cianfriglia, Federici, Ricci and Caccuri2006). Resistant A. myosuroides was treated with a mixture of the phenylurea herbicide chlorotoluron (photosystem II inhibitor; Group 5) plus the GST inhibitor 4-chloro-7-nitrobenzofurazan (NBD-Cl; Figure 5A). Treatment with this GST inhibitor 48 h before herbicide treatment successfully reversed resistance to postemergence application of chlorotoluron in A. myosuroides by inhibiting a phi-class GST, AmGSTF1 (Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards, Hughes, Kaundun, Hutchings, Steel and Edwards2013). Treatment with NBD-Cl reversed resistance to three herbicides in A. myosuroides and multiple drug resistance function in human GSTs (Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011). This compound is a competitive inhibitor to these enzymes by limiting the active site access and reducing substrate binding in the hydrophobic domain (Schwarz et al. Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021). The efficacy of the preemergence Group 15 herbicide S-metolachlor in a resistant A. palmeri population was regained by adding NBD-Cl (Brabham et al. Reference Brabham, Norsworthy, Houston, Varanasi and Barber2019). Similar results were obtained with another Amaranthus species, waterhemp [Amaranthus tuberculatus (Moq.) Sauer] (Strom et al. Reference Strom, Hager, Seiter, Davis and Riechers2020). However, S-metolachlor metabolism rates decreased and resistance in resistant populations was partially reversed by adding a P450 inhibitor, malathion, indicating that diverse detoxification pathways were present in A. tuberculatus (Kerr et al. Reference Kerr, Concepcion, Strom and Riechers2023; Strom et al. Reference Strom, Hager, Concepcion, Davis, Seiter, Morris, Kaundun and Riechers2021).
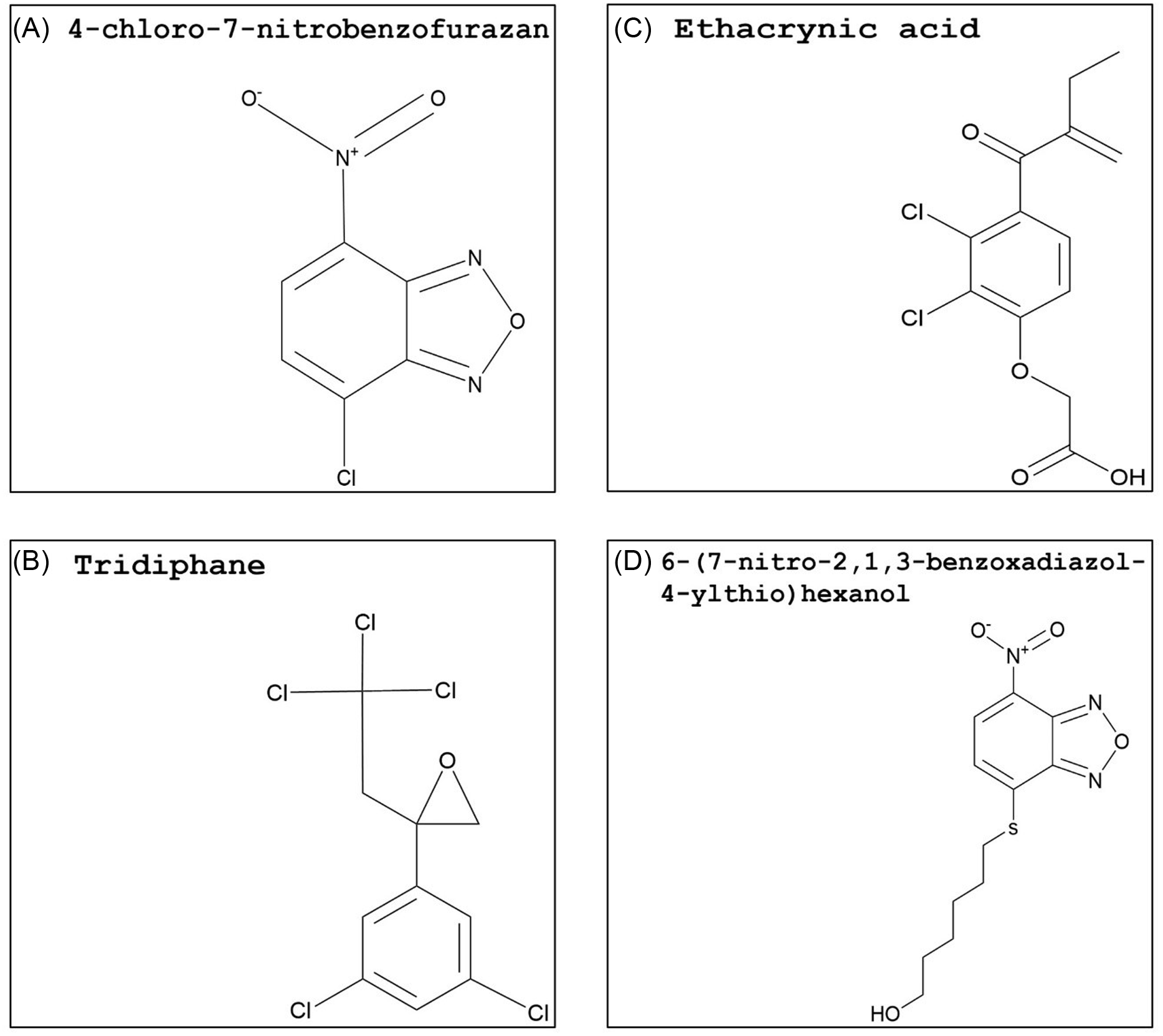
Figure 5. Synthetic glutathione S-transferase inhibitors: (A) 4-chloro-7-nitrobenzofurazan (NBD-Cl), (B) tridiphane, (C) ethacrynic acid, and (D) 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX). The PubChem CID information was provided earlier in the review. Chemical structures were generated using ChemDraw Professional v. 22.2 (PerkinElmer, Waltham, MA, USA).
A different study with A. tuberculatus from Illinois further investigated the response of atrazine-resistant populations (Ma et al. Reference Ma, Kaundun, Tranel, Riggins, McGinness, Hager, Hawkes, McIndoe and Riechers2013) by the addition of NBD-Cl. In one of the A. tuberculatus populations, NBD-Cl applied 2 d before atrazine preemergence or postemergence treatment significantly increased control of resistant plants compared with atrazine alone, which is indicative of herbicide metabolism via GST activity (Ma et al. Reference Ma, Evans and Riechers2016). This population overexpressed AtuGSTF2, a phi-class GST gene strongly linked to herbicide detoxification in metabolic atrazine-resistant A. tuberculatus populations (Evans et al. Reference Evans, O’Brien, Ma, Hager, Riggins, Lambert and Riechers2017). Atrazine resistance in velvetleaf (Abutilon theophrasti Medik.) and A. palmeri is also linked to higher GST activity (Anderson and Gronwald Reference Anderson and Gronwald1991; Nakka et al. Reference Nakka, Godar, Thompson, Peterson and Jugulam2017). The mixing of GST inhibitors with this herbicide might be an option for overcoming or delaying atrazine resistance. However, the tolerance to atrazine in corn is due to rapid herbicide metabolism catalyzed by high GST activity (Timmerman Reference Timmerman1989), and herbicide safety in cereal crops will need to be investigated. Although NBD-Cl is a strong candidate for use as a herbicide synergist, this compound is not deemed safe for humans (NCBI 2024). Nonetheless, its structure might be used as a backbone to synthesize safer, selective, and more effective molecules.
Tridiphane (Figure 5B), a competitive inhibitor of GSH conjugation with respect to certain herbicides, has been extensively studied as a herbicide synergist. Both tridiphane and ethacrynic acid reversed flufenacet resistance in A. myosuroides (Dücker et al. Reference Dücker, Parcharidou and Beffa2020). Additionally, five A. myosuroides GSTs (GSTU1, GSTU2, GSTU8, GSTF4, and GSTF5) upregulated in flufenacet-resistant plants were expressed in E. coli, and slow to moderate rates of herbicide detoxification were identified with all expressed GSTs (Parcharidou et al. Reference Parcharidou, Dücker, Zöllner, Ries, Orru and Beffa2023). Tridiphane acted as a herbicide synergist when added to atrazine, alachlor, or EPTC and increased proso millet (Panicum miliaceum L.) control (Ezra et al. Reference Ezra, Dekker and Stephenson1985; Lamoureux and Rusness Reference Lamoureux and Rusness1986). This GST inhibitor was also successful in reversing metribuzin resistance in narrow-leafed lupin (Lupinus angustifolius L.) and increasing atrazine postemergence control of S. faberi (Boydston and Slife Reference Boydston and Slife1986; Pan et al. Reference Pan, Si, Yu, Tu and Powles2012). Both atrazine and metribuzin are photosystem II–inhibiting herbicides (Group 5) but are categorized in different subfamilies: atrazine is a triazine, while metribuzin is a triazinone (HRAC 2024). Hence, tridiphane may have a high affinity with the GSTs involved in detoxifying xenobiotics containing nitro groups in a benzene ring structure. In multiple herbicide-resistant A. myosuroides, tridiphane was an ineffective inhibitor of AmGSTF1 (Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards, Hughes, Kaundun, Hutchings, Steel and Edwards2013).
In contrast with tridiphane, ethacrynic acid (Figure 5C) and 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX; Figure 5D) are primarily used in cancer and multiple drug resistance research with a limited number of studies on plants GSTs. Investigations with cancer drug–resistant cell lines showed that ethacrynic acid was a potent GST inhibitor, and its addition enhanced drug toxicity in resistant cell lines (Oakley et al. Reference Oakley, Bello, Mazzetti, Federici and Parker1997; O’Dwyer et al. Reference O’Dwyer, LaCreta, Nash, Tinsley, Schilder, Clapper, Tew, Panting, Litwin, Comis and Ozols1991; Tew et al. Reference Tew, Bomber and Hoffman1988). In plants, response to ethacrynic acid strongly varied depending on species and GST class. A zeta GST from wheat (TaGSTZ1) and tau and zeta GST isoforms from A. thaliana (AtGSTU19 and AtGSTZ1, respectively) showed low or no activity toward ethacrynic acid (DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002; Dixon et al. Reference Dixon, Cole and Edwards2000). On the other hand, flufenacet plus ethacrynic acid partially reversed A. myosuroides resistance (Dücker et al. Reference Dücker, Parcharidou and Beffa2020). Additionally, the addition of this GST inhibitor to metolachlor applications reduced the amount of herbicide being detoxified in a tolerant corn cultivar (Li et al. Reference Li, Gao, Xu, Pang, Liu, Wang and Tan2017). Investigating a strong competitive inhibitor for human GSTP1-1 enzyme, Ricci et al. (Reference Ricci, Maria, Antonini, Turella, Bullo, Stella, Filomeni, Federici and Caccuri2005) concluded that NBDHEX triggers apoptosis (programmed cell death) in human tumor cell lines by binding to the hydrophobic domain of the GST. In a different study, seven GST inhibitors were generated that target the human GSTP1-1 isoform, an important target in cancer therapy. These inhibitors were based on the structure of a common, synthetic GST substrate, 1-chloro-2,4-dinitrobenzene (Habig et al. Reference Habig, Pabst and Jakoby1974). Derivatives were produced to have inhibition rates comparable to the effective control (ethacrynic acid), high cell permeability, and the ability to target the G-site specifically. Among the inhibitors, two of the derivatives showed an inhibitory effect comparable to the control with ethacrynic acid. Both compounds showed covalent bonds and irreversible GST inhibition and possess sulfonyl fluoride in their structure, which makes the molecule highly electrophilic (Shishido et al. Reference Shishido, Tomoike, Kuwata, Fujikawa, Sekido, Murakami-Tonami, Kameda, Abe, Kimura, Shuto and Abe2019).
Manipulating GSTs for Crop Safety
Using safeners is a sound approach to protect plants from the detrimental effects caused by herbicides. Although safeners induce the expression of plant detoxification genes and enzyme activities, the detailed mechanism of action on how these compounds shield and avoid adverse outcomes remains to be completely elucidated (Riechers et al. Reference Riechers, Kreuz and Zhang2010). As mentioned earlier, GST enzymes have a crucial role in stress tolerance and plant defense by detoxifying xenobiotic compounds, including herbicides (Baek et al. Reference Baek, Goodrich, Brown, James, Moose, Lambert and Riechers2019; Cummins et al. Reference Cummins, Dixon, Freitag-Pohl, Skipsey and Edwards2011; DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002; Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011; Grill et al. Reference Grill, Tausz and De Kok2001; Riechers et al. Reference Riechers, Kreuz and Zhang2010). Extensive research conducted on the use of safeners enhancing GST activity has collectively demonstrated that enhancement of this enzyme activity promotes rapid herbicide metabolism, achieving crop protection against selected herbicides (Deng and Hatzios Reference Deng and Hatzios2002; Edwards et al. Reference Edwards, Buono, Fordham, Skipsey, Brazier, Dixon and Cummins2005; Galon et al. Reference Galon, Maciel, Agostinetto, Concenço and Moraes2011; Riechers et al. Reference Riechers, Kreuz and Zhang2010).
The safener fenclorim provides tolerance to the very-long-chain fatty-acid elongase (Group 15) inhibitors pretilachlor and acetochlor in rice (Avent et al. Reference Avent, Norsworthy, Butts, Roberts and Bateman2023; Ebert and Gerber Reference Ebert, Gerber, Hatzios and Hoagland1989; Wu et al. Reference Wu, Omokawa and Hatzios1996). Without safeners, rice shows high sensitivity to chloroacetamide herbicides (Fogleman et al. Reference Fogleman, Norsworthy, Barber and Gbur2019; Godwin et al. Reference Godwin, Norsworthy and Scott2018). Interestingly, fenclorim is highly selective toward several plant species by herbicide combinations. Besides rice and pretilachlor or acetochlor, a seed treatment with fenclorim reduced imazamox or bicyclopyrone injury in tomato (Castro et al. Reference Castro, Pucci, Duarte, Burgos and Tseng2020). In both studies, seeds were treated with fenclorim, and an increase in GST activity was identified in young root and shoot tissues treated with safeners, including fenclorim (Deng and Hatzios Reference Deng and Hatzios2002; DeRidder and Goldsbrough Reference DeRidder and Goldsbrough2006; Hu et al. Reference Hu, Yao, Cai, Pan, Liu and Bai2020; Riechers et al. Reference Riechers, Zhang, Xu and Vaughn2003; Scarponi et al. Reference Scarponi, Del Buono and Vischetti2005). One study determined that treating rice shoots with pretilachlor and fenclorim reduced the persistence of the herbicide by 48 h (Scarponi et al. Reference Scarponi, Del Buono and Vischetti2005). Conversely, fenclorim accumulated in rice shoots when co-applied with pretilachlor, suggesting that fenclorim may potentiate pretilachlor via metabolic pathways, and based on previous research, the GSTs upregulated by fenclorim likely have a higher affinity for the herbicide over the safener (Deng and Hatzios Reference Deng and Hatzios2002). Similar results were observed by Hu et al. (Reference Hu, Yao, Cai, Pan, Liu and Bai2020), who identified 14 metabolic genes upregulated by fenclorim in rice, with the primary detoxification pathway of pretilachlor being mediated by GSTs (OsGSTU16 and OsGSTF5). Previously, Hatton et al. (Reference Hatton, Cole and Edwards1996) observed that rapid herbicide detoxification via GSH conjugation catalyzed by GSTs was crucial in the tolerance of corn seedlings to atrazine, alachlor, and metolachlor.
In contrast to these results, A. thaliana plants grown from seeds treated with different safeners (benoxacor, fenclorim, or fluxofenim) were severely injured in the presence of chloroacetamide herbicides. Even though injury was observed, GST expression and activity, GSH content, and expression of other detoxification enzymes were enhanced in seedlings treated with safeners (DeRidder et al. Reference DeRidder, Dixon, Beussman, Edwards and Goldsbrough2002; DeRidder and Goldsbrough Reference DeRidder and Goldsbrough2006). Besides fenclorim, additional safeners have been correlated to tolerance of plant species to chloroacetamides via enhanced GST activity. Tolerance to butachlor in wheat can be achieved by adding cloquintocet-mexyl, fenchlorazole-ethyl, or fluxofenim (Scarponi et al. Reference Scarponi, Quagliarini and Del Buono2006). Fluxofenim also protected wheat from S-metolachor, dimethenamid-P, and pyroxasulfone damage with increased GST activity (Raiyemo et al. Reference Raiyemo, Price, Rauch, Campbell, Xiao, Ma, Gross and Prather2021). Grain sorghum and corn tolerance to metolachlor is enhanced due to treatment with fluxofenim and benoxacor, respectively (Irzyk and Fuerst Reference Irzyk and Fuerst1993; Silva et al. Reference Silva, Martins, Silva and Martins2014). Grain sorghum seedlings treated with fluxofenim had increased transcript levels of two phi-class GSTs, SbGSTF1 and SbGSTF2 (Baek et al. Reference Baek, Goodrich, Brown, James, Moose, Lambert and Riechers2019), as well as several other genes associated with detoxification and stress responses.
Isoxadifen-ethyl is a safener mixed with fenoxaprop-p-ethyl to provide rice tolerance and protection of corn plants to nicosulfuron, foramsulfuron, and tembotrione via regulation of several stress response genes (including GSTs), which accelerates herbicide detoxification rates (Bunting et al. Reference Bunting, Sprague and Riechers2004; Schulte and Kocher Reference Schulte and Kocher2009; Shen et al. Reference Shen, Tang, Zeng, Xu, Su and Wu2017; Sun et al. Reference Sun, Wu, Su, Gao and Lu2017, Reference Sun, Xu, Su, Xue, An, Lu and Wu2018; Zhao et al. Reference Zhao, Li, Sun, Xu, Su, Xue, Wu and Lu2022). One important caveat to using safeners is the potential increase in GST expression and activity in nontarget plants, specifically weeds. For instance, fenoxaprop-p-ethyl resistance in one barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] population is strongly associated with a safener (isoxadifen-ethyl) included with the commercial formulation. Compared with treatment with fenoxaprop-p-ethyl alone, resistant plants survived doses 32 times higher when the safener was present in the formulation. When a GST inhibitor (NBD-Cl) was sprayed 48 h before the herbicide, resistance was partially reversed in this biotype. Additionally, GST genes (GST1 and GSTF1) were upregulated in the resistant population (Cutti et al. Reference Cutti, Rigon, Girelli, Angonese, Ulguim and Merotto2022).
Another important consideration is the application method and timing of the safener. As previously mentioned, the herbicide solution mixture of isoxadifen-ethyl with fenoxaprop-p-ethyl had adverse effects on nontarget, weedy plants (Cutti et al. Reference Cutti, Rigon, Girelli, Angonese, Ulguim and Merotto2022), but in a comparison of GST activity for both fenclorim and pretilachlor, early watergrass [Echinochloa oryzoides (Ard.) Fritsch] exhibited no change in enzymatic activity when treated with either fenclorim, pretilachlor, or the combination of the two at the roots (Usui et al., Reference Usui, Deng, Nagao and Shim2001). Therefore, placement and timing of the safener is a critical consideration to prevent undesirable herbicidal effects. The interaction for safening potential is also likely species and herbicide dependent or, within a species, population dependent from metabolic herbicide resistance in weeds.
Final Considerations
Currently, studies investigating new GST inhibitors focus on finding potent compounds that will effectively bind to these enzymes. Previous research has shown that enzyme affinity to the inhibitor is directly correlated with the increase in chain length of the n-alkyl group (Flatgaard et al. Reference Flatgaard, Bauer and Kauvar1993; Mannervik et al. Reference Mannervik and Danielson1988). Additionally, increased inhibition and viability were associated with nitro group or aromatic rings as substituents (Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards, Hughes, Kaundun, Hutchings, Steel and Edwards2013; Schwarz et al. Reference Schwarz, Eno, Freitag-Pohl, Coxon, Straker, Wortley, Hughes, Mitchell, Moore, Cummins and Onkokesung2021). Commercially, this synergistic class is already limited in its adoption and use. Large compounds bearing aromatic rings are hydrophobic and have low solubility in water. Herbicide applications involve large quantities of water, and the ideal scenario is that any additives are compatible and easily blended in the spray mix. Herbicide formulations with the compound already added or spray adjuvants to modify compound solubility will likely be the best approach to overcome this issue.
Mammalian toxicity and price are also two challenging obstacles. Several synthetic GST inhibitors are toxic to humans, animals, or pollinators, and a few are classified as environmental hazards. Toxicity is not a concern with natural polyphenols, as they are already present in several plants and are often consumed by mammals. Future efforts should focus on the natural GST structures to design effective analogues. However, with analogues, the final product price will increase, which might make this product less favorable for farmers, unless synthetic pathways can be optimized.
Based on the literature reviewed, selecting an effective GST inhibitor will likely be specific to the herbicide and weed combinations. Because most experiments were performed in vitro, field performance may differ significantly when added to the spray solution, where biokinetic factors such as uptake and metabolism by the plant may modify their inhibitory activity. Although in vitro experiments offer a fast result, it is impossible to predict plant efficacy based solely on outcomes obtained under controlled conditions and with cells or extracts. Besides the physiological barriers, such as cuticles and cell membranes, these compounds may react negatively when exposed to diverse environmental conditions, such as photodegradation. Farm operations occur during the day, so UV degradation of the inhibitor would be a substantial limitation on the use of any product.
Using naturally available products will likely mitigate challenges related to animal or environmental toxicity and the final price. Therefore, the focus for weed management should remain on investigating possible synergistic interactions between natural GST-inhibiting compounds and herbicides. It is important to emphasize that water solubility and plant uptake are essential barriers to overcome before their commercialization and use. Developing derivatives from these structures might lead to new potent compounds that are soluble enough to be mixed with herbicides. Surfactants and other spray adjuvants added to the herbicide:natural compound mixture might affect plant uptake and need to be investigated as well.
Acknowledgments
The authors thank the University of Arkansas Division of Agriculture for its support.
Funding
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.
Competing interests
The authors declare no competing interests.








