Anaemia during pregnancy is a considerable health problem around the world and Fe deficiency is the most common cause of anaemia( Reference Stevens, Finucane and De-Regil 1 , Reference Black, Victora and Walker 2 ). Globally, the prevalence of anaemia in pregnant women has decreased since 1995, but in 2011 38 % of pregnant women still suffered from anaemia( Reference Stevens, Finucane and De-Regil 1 ). In 2012, the prevalence of anaemia during pregnancy in China was 17·2 %( 3 ); however, the prevalence of anaemia among pregnant women strongly varies by region and women’s gestational age. The China National Nutrition and Health Survey showed that the prevalence of anaemia was 20 % among pregnant women in poor rural areas( Reference Li, Hu and Mao 4 ). A prospective cohort study in south China indicated that about 70 % of pregnant women had Fe-deficiency anaemia( Reference Huang, Purvarshi and Wang 5 ). There is very limited information on anaemia status of Tibetan pregnant women. Our previous hospital-based study in Lhasa showed that Tibetan pregnant women had lower Hb levels than non-Tibetan women throughout the entire pregnancy, averaging 126·6 g/l for Tibetans and 134·6 g/l for non-Tibetans( Reference Xing, Yan and Dang 6 ). The percentage of women with Hb<110 g/l was 18·5 % before correction for altitudes and 71·2 % after correction for altitudes( Reference Xing, Yan and Dang 7 ). Another population-based study showed that the prevalence of anaemia in pregnant women during the first, second and third trimester in rural Tibet was 79·5, 86·1 and 87·3 %, respectively( Reference Kang, Li and Dang 8 ). These findings suggested that Tibetan women were more likely to be anaemic during pregnancy, compared with those in other regions in China.
Anaemia during pregnancy is associated with adverse birth outcomes and has long-term effects. Anaemia in the third trimester of pregnancy increases the incidence of low birth weight (LBW) and preterm birth( Reference Huang, Purvarshi and Wang 5 , Reference Levy, Fraser and Katz 9 ). Hb reduction during pregnancy is associated with small for gestational age (SGA) births, placental weight and placental ratio( Reference Williams, Evans and Newnham 10 – Reference Jwa, Fujiwara and Yamanobe 12 ). LBW babies are at higher risks of morbidity and mortality, and of adverse physical development in childhood, compared with normal birth weight babies( Reference Class, Rickert and Lichtenstein 13 , Reference Zhou, Zeng and Dang 14 ). Anaemia during pregnancy might have long-term adverse effects on younger children, and a study indicated that Fe-deficiency anaemia in the third trimester was associated with worse mental development of rural Chinese young children( Reference Chang, Zeng and Brouwer 15 ).
Tibet is located in the Qinghai-Tibet plateau, where more than 45 % of areas are above the altitude of 4000 m( Reference Zhang 16 ). Hypoxia at high altitude significantly increases Hb( 17 ). However, the relationship between high altitude and Hb is less clear in those adapted to high altitudes, such as Tibetans( Reference Xing, Yan and Dang 7 , Reference Moore 18 , 19 ). Thus, anaemic status and its adverse effects during pregnancy among Tibetans require further investigation. Previous cross-sectional surveys showed that the nutritional status of women and children in Tibet was worse than that in any other regions in China( Reference Xing, Yan and Dang 6 – Reference Kang, Li and Dang 8 , Reference Wang, Dang and Yan 20 , Reference Dang, Yan and Yamamoto 21 ). The LBW rate was 10·5 % in rural Tibet, higher than that in other regions in China (4·6 %)( Reference Bianba, Pubu and Kang 22 , Reference Yu, Zhao and Liu 23 ). However, there is a lack of follow-up studies on anaemia and LBW among the pregnant Tibetan women.
Deficiencies in micronutrients during pregnancy are common in developing countries and have been associated with maternal anaemia and adverse birth outcomes. These deficiencies may be exacerbated during pregnancy because of increased requirements for the growing fetus, placenta and maternal tissues( Reference Haider and Bhutta 24 ). Globally, anaemia due to Fe deficiency is one of the most prevalent micronutrient deficiencies. Therefore, preventive measures are needed to reduce and control anaemia during pregnancy. Some preventative measures during pregnancy that are recommended include the fortification of principal foods with Fe, the increase of health and nutritional awareness and the improvement in sanitation. However, the most direct way to improve Fe status and reduce the occurrence of anaemia is suggested to be antenatal supplementation with Fe-containing micronutrients( Reference Chang, Zeng and Brouwer 15 , Reference Haider and Bhutta 24 , 25 ). A systematic review and meta-analysis including forty-eight randomised trials (twenty-seven in high-income countries and twenty-one in low- or middle-income countries; the dosage of Fe ranged from 10 to 240 mg daily and duration of supplementation varied from 7 to 30 weeks during pregnancy) and forty-four cohort studies (twenty-two each from high-income and low- or middle-income countries, respectively) was conducted to assess prenatal Fe supplements( Reference Haider, Olofin and Wang 26 ). The review showed that Fe use during pregnancy increased maternal mean Hb level by 4·59 g/l and in turn reduced the risk of anaemia, Fe deficiency, Fe deficiency anaemia and LBW. These effects were particularly strong among pregnant women with anaemic status at baseline or those from countries with low or middle income( Reference Haider, Olofin and Wang 26 ). Thus, this study suggested that the effects of micronutrient supplements containing Fe during pregnancy might vary with region, population and anaemic status at the beginning of intervention.
Tibet is a very specific setting characterised with high altitudes and hypoxia, and the effects of Fe supplementation during pregnancy at high altitudes on maternal Hb, anaemia and birth outcomes remain unknown. Therefore, we conducted a prospective cohort study in rural Tibet to assess the positive impacts of multiple micronutrients (MMN) supplementation containing Fe during pregnancy on maternal anaemia and adverse birth outcomes such as LBW and preterm delivery.
Methods
Study design and population
A prospective cohort study was conducted in two rural counties (Dazi and Qushui), in Lhasa, located in the middle of Tibet. The mean altitude of the two counties is 3706 m above sea level. Lhasa area is one of the main habitats of Tibetans.
This study was approved by the Ethics Committee in Medical Research, Xi’an Jiaotong University (no 20070712), and registered in the Chinese Clinical Trial Registry (Registration no.: ChiCTR-PNRC-09000597).
The study sample consisted of the pregnant women in the two counties who met the study selection criteria (Fig. 1). The women having ≤24 weeks of gestation were invited to participate in the study and had to provide signed informed consent. The women were excluded from the study if they already took Fe, folic acid (FA) or other micronutrient supplements for more than two weeks, or had other serious illnesses. The eligible pregnant women from Dazi took MMN, whereas those from Qushui took FA. Randomisation was not implemented for the reasons of cultural and religious sensitivity in the study population. FA is the standard prenatal supplementation recommended by the National Health and Family Planning Commission of China, so FA was used as the control( 27 ). All participants were required to take the supplements from enrolment to delivery. The sample size considerations were based on a survey in rural Lhasa areas. The anaemia prevalence of pregnant women in the third trimester in rural Lhasa is 75·9 % (adjusted by altitude)( Reference Xing, Yan and Dang 6 ). Taking MMN supplements during pregnancy was assumed to reduce anaemia prevalence by 11 % compared with FA( Reference Osrin, Vaidya and Shrestha 28 ). Using α=0·05 and power=80 %, 920 subjects were required. A total of 1100 pregnant women were required after accounting for about 15 % dropout/loss to follow-up.

Fig. 1 Flow chart depicting participant selection process. FA, folic acid; MMN, multiple micronutrients.
Enrolment and pregnancy surveillance procedures
At the outset of this study, the trained village doctors and social workers conducted a survey of all women of reproductive age living in the village to identify those who were likely to become pregnant. The village doctors visited these women every month and asked them the date of their last menstrual period (LMP). The women whose periods were delayed by more than 5 d were given urine pregnancy tests, and confirmed pregnancies were reported to the maternal and child health care (MCH) workers in the township hospitals of the studied counties. The informed consent was obtained from all participants.
Newly identified pregnant women were interviewed to record their socio-demographic status and menstrual, reproductive and medical histories. Recruited pregnant women received three free antenatal care checks at enrolment, 28 and 32 weeks of gestation, respectively, at which they were given physical examination and asked about pregnancy complications. All the information collected during the pregnancy until the sixth week after delivery follow-up visit was recorded in the Pregnancy Care Record Book, which served as both a clinical record and a data capture instrument.
Micronutrient supplements
In this study, two kinds of supplements were used: MMN and FA. The MMN supplement (Materna/tablet; Wyeth Pharmaceutical Co., Ltd) consisted of the following components (per tablet): 450 µg of vitamin A, 900 µg of β-carotene, 6·25 µg of vitamin D, 30 mg of vitamin E, 3 mg of vitamin B1, 3·4 mg of vitamin B2, 10 mg of vitamin B6, 12 µg of vitamin B12, 100 mg of vitamin C, 30 mcg of biotin, 1 mg of FA, 20 mg of nicotinamide, 10 mg of pantothenic acid, 150 µg of iodine, 25 µg of Mo, 250 mg of Ca, 25 mg of Zn, 60 mg of Fe, 2·0 mg of Cu, 25·0 µg of Cr, 5 mg of Mn, 50 mg of Mg and 25·0 µg of Se. The folate-only supplement (Scrianen/tablet; Peking University Pharmaceutical Co., Ltd) contained 0·4 mg of FA/tablet. All micronutrient supplements were commercially available at the time of the study.
At enrolment, each woman received thirty tablets with the instructions to take one tablet daily, preferably after meals and at the same time every day. Either the township MCH workers or the village doctors visited the women every month to provide supplements and to retrieve the used empty bottles and record the number of remaining tablets.
Data collection
Maternal Hb during pregnancy was tested from the capillary blood collected at enrolment (≤24 weeks of gestation), 28 and 32 weeks of gestation, respectively. HemoCue portable spectrophotometers were used to assay Hb levels. The method of American Centers for Disease Control and Prevention (CDC) was used in this study to adjust Hb levels based on the altitude for estimating the anaemia prevalence. The adjusted formula is expressed as ΔHb=−0·032×(Alt×3·3)+0·022×(Alt×3·3)2, where Alt refers to the altitude (km) of the village where the participant lived( 17 ). Participants whose adjusted Hb level was <110 g/l were considered anaemic( 25 , Reference McLean, Cogswell and Egli 29 ).
Birth weight and length were measured by the hospital nurses within 1 h of a child’s delivery. Birth weight was measured using an electronic scale (Type BD-585; TANITA Corporation, Ltd) with precision to the nearest 10 g. For home births (11 % of all births), the township MCH workers visited the women at home within 72 h of delivery to measure the birth anthropometry and collect the delivery information. LBW was defined as birth weight <2500 g( Reference Steer, Alam and Wadsworth 30 ). Birth length was measured to the nearest 1 mm using a portable measuring board with a fixed head piece.
Gestational age at birth was calculated as completed days based on the 1st day of the LMP, which was obtained from the baseline interview. Spontaneous abortion was defined as the spontaneous end of confirmed pregnancy before 28 weeks of gestational age. Preterm delivery was defined as delivery at <37 weeks of gestational age. A stillbirth is defined as the spontaneous death of a fetus after 28 weeks of gestation but before delivery of the baby’s head. Neonatal deaths were defined as deaths amongst live-born infants occurring within 28 d of delivery. Early neonatal death was defined as the death within 7 d after birth. Late neonatal death was referred to as death between 8 and 28 d after birth. We also reported perinatal mortality, which included both stillbirths and early neonatal deaths.
Statistical analysis
A wealth index was constructed from an inventory of six household assets (including the following family belongings: motorcycles, automobiles, cattle, goats, chickens or ducks, televisions) using a principal component analysis method( Reference Zeng, Yan and Chen 31 ). The compliance rate was determined by dividing the number of actual supplements consumed by the number of supplements expected to be consumed. In the analysis, we only included singleton pregnancy to avoid the possible contamination effect on anaemia and birth outcomes because the multiple pregnancies are more vulnerable to those outcomes.
To address the possible imbalance in the participants’ characteristics between the two treatment groups owing to the non-randomised design of this study, a propensity score approach was used. We calculated the propensity score (a predicted probability of woman being in Dazi county) for each woman using a logistic regression model based on fourteen prespecified baseline characteristics (except Hb level adjusted by altitude) as listed in Table 2 and used this score as a control variable in the covariate-adjusted analysis of the outcome variables( Reference Yue 32 ).
The primary outcome was analysed using a generalised estimating equation (GEE) model with treatment, time at measurement, interaction between treatment and time as fixed effects, and the propensity score and Hb level (adjusted by altitude) at baseline as covariates. OR, adjusted OR and its 95 % CI for having anaemia during prenatal care between two treatment groups were derived from the GEE model. Other outcome variables were analysed similarly using a GEE model. For the GEE model analysis of the continuous outcome such as Hb level, weeks of gestation at birth and birth weight, normal distribution and identity link functions were used. For the GEE analysis of the binary outcomes such as anaemia, preterm delivery, LBW and mortality outcomes, binomial distribution and logit link functions were used. An exchangeable covariance structure was used for all GEE models. In addition, the two-sample t test or Wilcoxon’s rank test was used to assess statistical differences in continuous variables between the two supplementation groups. P<0·05 was considered statistically significant. All analyses were performed using SAS version 9.3 (SAS Institute).
Results
Study sample and characteristics
Fig. 1 shows the flow chart of participants in this study. The enrolment began on 26 October 2007, and continued until 4 May 2011. The last baby was born on 12 December 2011, and all follow-up stopped on 6 March 2012. Over a 4·5-year period, there were 2821 confirmed pregnant women who lived in the two rural counties of Qushui and Dazi in Lhasa. Among them, 568 pregnant women refused to participate and 1104 women were excluded from the study as per the exclusion criteria. Thus, in total, 1149 pregnant women were enrolled in the study. There were 1013 singleton pregnant women in the third trimester available for analysis. The birth weights were not measured for thirteen stillbirths and the birth weight data for five live births were missed. Therefore, the birth weights of 1021 singleton live births were available for analysis.
The two counties, Qushui and Dazi, are almost identical in terms of geographical features and social status, such as population size, economic situation, medical service level and the number of women of childbearing age (Table 1). Characteristics of the pregnant women at enrolment are shown in Table 2. All participants were Tibetan. Most were educated, but only 45·6 % had completed an elementary school education. In all, 17·4 % had low BMI (BMI<18·5 kg/m2) and 499 participants (43·4 %) were in the first pregnancies.
Table 1 Baseline status of two counties in 2006
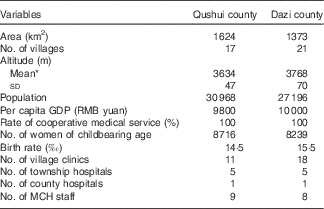
MCH, maternal and child health care.
* t Test was used to examine statistical difference in means, t=38·80, P<0·001.
Table 2 Baseline household and participant characteristics at enrolment (Mean values and standard deviations; medians and interquartile ranges (IQR))

FA, folic acid; MMN, multiple micronutrients.
* t Test or Wilcoxon rank test was used to examine statistical differences in continuous variables.
Compliance with supplementation
The supplementation began, on average, at 15·0 (sd 5·0) weeks of gestation for both groups. The mean dosage number of supplements consumed per woman during pregnancy was 151 (sd 35) in the FA group and 148 (sd 37) in the MMN group (P=0·141). The compliance with supplementation during pregnancy was 88·2 % (91·3 % in the FA group and 85·7 % in the MMN group; P<0·001) (Table 3). There were a few self-reported side effects, such as nausea, vomiting, headache and dizziness. The prevalence of such side effects was below 2·3 % and comparable between the two treatment groups.
Table 3 Number of doses and compliance of supplements consumed by supplementation groupsFootnote * (Numbers and percentages; mean values and standard deviations)
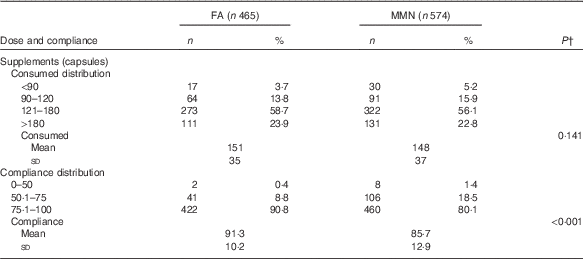
FA, folic acid; MMN, multiple micronutrients.
* Compliance calculated by number of actual supplements consumed divided by number of supplements expected to be consumed.
† t Test was used to examine statistical differences in means.
Comparison of maternal anaemia between the supplementation groups
Blood Hb samples were available for 1037 women at enrolment, 1031 in the second prenatal care and 1013 in the third prenatal care. The blood samples were taken at a mean gestation of 15·0 (sd 5·0), 28·3 (sd 1·1) and 32·2 (sd 1·2) weeks for the first, second and third prenatal care, respectively. There was a significant increase in the Hb with MMN supplement compared with FA supplement in the third trimester (adjusted mean difference: 5·18; 95 % CI 2·98, 7·37 g/l; P<0·001), resulting in significant reduced odds of anaemia in the third trimester for these women (AOR: 0·63; 95 % CI 0·45, 0·88; P=0·007) (Table 4).
Table 4 Comparison of anaemia and Hb (g/l) of Tibetan pregnant women between supplementation groups (Mean values and standard deviations; odds ratios, adjusted odds ratios (AOR) or differences and 95 % confidence intervals)
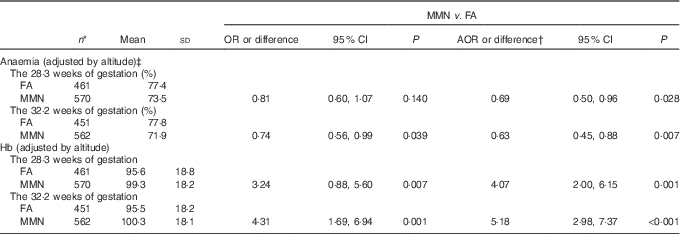
FA, folic acid; MMN, multiple micronutrients.
* Data missing on the 28·3 weeks of gestation for 8 women, and the 32·2 weeks of gestation for twenty-six women.
† Adjusted for the propensity scores and Hb levels (adjusted by altitude) at enrolment in generalised estimating equation model.
‡ Anaemia defined as Hb<110 g/l.
Comparison of birth outcomes between the supplementation groups
Birth weight and length samples were available for 1021 and 995 single live births, respectively. After the supplementation, the mean birth weight was 2981·9 (sd 475·7) g in the FA group and 3006·9 (sd 387·2) g in the MMN group (adjusted mean difference: 36·78; 95 % CI −19·42, 92·98 g; P=0·200). The effect of supplementation on birth length was not statistically significant (adjusted mean difference: 0·02; 95 % CI −0·43, 0·47 cm; P=0·939). MMN resulted in a significantly longer gestation compared with that of FA (adjusted mean difference: 0·56; 95 % CI 0·33, 0·79 weeks; P<0·001). There were significant decreases in the odds of LBW and preterm delivery in the MMN group compared with the FA group (AOR: 0·58; 95 % CI 0·36, 0·91; P=0·019; AOR: 0·31; 95 % CI 0·15, 0·61; P=0·001; respectively) (Table 5).
Table 5 Comparison of outcomes of child health between supplementation groups (Mean values and standard deviations; odds ratios, adjusted odds ratios (AOR) or differences and 95 % confidence intervals)

FA, folic acid; MMN, multiple micronutrients.
* Adjusted for the propensity scores in generalised estimating equation model.
† Data analysis only includes single live births.
‡ Data analysis only includes single births.
MMN supplementation was associated with decreased perinatal mortality compared with FA supplementation (AOR: 0·46; 95 % CI 0·23, 0·94; P=0·034), but it showed no effects on the rates of neonatal mortality, early neonatal mortality and stillbirth, although more stillbirths and neonatal death mortality were reported in the FA group (Table 6).
Table 6 Comparison of neonatal deaths and fetal loss between supplementation groups (Odds ratios, adjusted odds ratios (AOR) and 95 % confidence intervals)
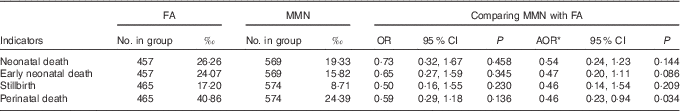
FA, folic acid; MMN, multiple micronutrients.
* Adjusted for the propensity scores in generalised estimating equation model.
Discussion
To the best of our knowledge, this is the first intervention study on prenatal MMN supplementation on maternal anaemia and adverse birth outcomes among Tibetan women living at higher altitudes. The present study found that the prenatal MMN supplementation increased Hb levels and reduced anaemia significantly during the third trimester compared with the FA supplementation. Furthermore, MMN tended to increase gestational duration and decrease the risks of LBW and preterm delivery. This study provides some important evidence indicating that the MMN supplementation should be required in formulating nutrition policy on nutrient supplementation for pregnant women at high-altitude areas.
Improvement of maternal anaemia
In the present study, MMN or FA was administered to two groups of Tibetan pregnant women. The Hb of the MMN group increased during the third trimester by 5·18 g/l at 32 weeks of gestation, significantly higher compared with the FA group. Accordingly, the supplementation with MMN decreased the risk of maternal anaemia by 37 % after controlling for the potential confounders compared with FA. These results further confirmed the possibility that the supplementation with Fe-containing micronutrients during pregnancy improved maternal anaemia, which would benefit both the mother and the child( Reference Haider, Olofin and Wang 26 ). However, the supplementation with Fe-containing micronutrients for pregnant Tibetan women might be even more necessary because rural Tibetan mothers had fairly monotonous dietary patterns with traditional Tibetan foods( Reference Wang, Dang and Yan 20 ). As anaemia prevalence was high among pregnant women in rural Tibet( Reference Xing, Yan and Dang 6 , Reference Kang, Li and Dang 8 ), the routine supplementation of Fe-containing micronutrients during pregnancy should be included in antenatal care among Tibetan women at higher altitudes.
A cluster-randomised trial from rural western China showed that the supplementation with MMN during pregnancy could decrease the risk of maternal anaemia by 28 % compared with supplementation with FA( Reference Zeng, Dibley and Cheng 33 ). MMN in the present study decreased the risk of maternal anaemia by 37 %, which was a significant improvement. Although the observed difference should not be overstated because of the non-randomised nature of this study, the difference might also be due to higher dose of Fe in MMN supplements of the present study (60 mg Fe) than that of the previous study (30 mg)( Reference Zeng, Dibley and Cheng 33 ), and the higher vitamin C in our MMN supplements that might enhance the absorption of Fe. Moreover, a meta-analysis also indicated that the risk of maternal anaemia in the third trimester could be decreased by an average of 34 % after the supplementation with Fe-containing micronutrients during pregnancy( Reference Haider, Olofin and Wang 26 ). Dose–response analysis showed a linear decrease in maternal anaemia with higher doses of Fe, up to 66 mg/d( Reference Haider, Olofin and Wang 26 ).
The chemical composition of Hb suggests that a deficiency of amino acids or Fe in the maternal diet might cause anaemia( Reference Wu 34 , Reference Zimmermann 35 ). Increased Fe intake during pregnancy is necessary to meet the Fe requirements of the mother and the developing fetus, as well as the haemodilution, which occurs in the second and third trimesters( Reference Wu, Imhoff-Kunsch and Girard 36 ). It may suggest that the high dose of Fe in micronutrients might be a key factor. Moreover, a deficiency of vitamins (vitamin B6, vitamin B12 and folate) and minerals (cobalt, Mg, Zn and Cu) may also indirectly result in anaemia because they participate in one-carbon-unit metabolism and DNA synthesis( Reference McNulty and Scott 37 , Reference Said 38 ). Therefore, the reduction of pregnancy anaemia by the MMN supplementation might be attributed partly to such vitamins and minerals. In this sense, the MMN supplements could be a priority policy recommendation during pregnancy to prevent and control maternal anaemia.
About 70 % of the women in the MMN group remained anaemic in the third trimester after the adjustment of altitudes by the CDC method. A trial in rural northern China found that prenatal Fe supplementation (300 mg ferrous sulfate) reduced anaemia, but most women still had Fe deficiency( Reference Zhao, Xu and Zhou 39 ). Another cluster-randomised trial conducted in rural western China found that in both Fe-FA and MMN groups more than 40 % of the pregnant women were still anaemic in the third trimester( Reference Zeng, Dibley and Cheng 33 ). Blood volume expansion during the third trimester partly accounts for the high prevalence of anaemia( 25 , Reference Scholl 40 ). Moreover, the absorption of Fe during pregnancy is a complex physiological process, in which the Fe absorption decreases during the first trimester of pregnancy with a progressive increase in the remaining two trimesters of pregnancy because of the greater requirements for Fe in later pregnancy. However, the amounts that can be absorbed from the diet are less than the Fe requirements in later pregnancy( Reference Bothwell 41 ).
In addition, we found that although the percentage of anaemic Tibetan women during pregnancy was quite high in the present study, about half of anaemic women did not exhibit typical clinical symptoms of anaemia such as fatigue, reduced physiological endurance, shortness of breath and headache especially on exercise, paleness of skin or a rapid heartbeat( Reference Krayenbuehl, Naumann and Kaser 42 – Reference Brunner and Wuillemin 44 ). Our previous study also found that prevalence of maternal anaemia varied with differing correction methods for altitudes( Reference Xing, Yan and Dang 6 ), suggesting that current available methods were probably not suitable for adjusting for Hb level according to the altitude in pregnant women living in the highlands of Tibet. Better adaptation to high altitudes for Tibetans might account for this( Reference Moore 18 , 19 ), besides the unbalanced diets and poor nutritional education( Reference Wang, Dang and Yan 20 ).
Improvement of birth outcomes
This study pointed to the possibility that the MMN supplementation during pregnancy in Tibetan women might improve certain birth outcomes. Compared with FA, the MMN supplement group had an (albeit statistically non-significant) increase in birth weight and significantly decreased the occurrence of LBW by 42 %. This result implied that the MMN supplementation might positively affect Tibetan babies with LBW. We also found that the MMN supplements could extend the duration of gestation by 0·56 weeks and decreased preterm birth by 69 % compared with FA. However, a significant difference was not found in the prevalence of the infant with SGA between MMN and FA (35·2 v. 30·1 %. SGA was defined as birth weight less than 10th percentile of the gestational age-sex specific US reference for fetal growth( Reference Alexander, Himes and Kaufman 45 )). Thus, it was possible that MMN reduced LBW through prolonging the gestation period and thus decreasing preterm birth.
The beneficial effects of the MMN supplementation on fetal growth could be addressed through several biological mechanisms. Deficiencies of B vitamins might cause elevated homocysteine levels, which can lead to endothelial cell dysfunction and affect placental function( Reference Furness, Fenech and Dekker 46 , Reference Qureshi, Ahmad and Qureshi 47 ). Although we did not collect the data on the status of B vitamins during pregnancy in this study, our previous study found that Tibetan women with a child aged <24 months suffered from poor dietary intake of protein and B vitamins( Reference Wang, Dang and Yan 20 ). This suggested that the MMN supplements could help maintain normal homocysteine levels during pregnancy. Deficiency of some vitamins and minerals may also bring about negative effects on gene regulation and cellular metabolism( Reference Furness, Fenech and Dekker 46 , Reference Ashworth and Antipatis 48 ). Moreover, the MMN supplementation could improve the nutritional and Hb status of Tibetan women because they require more micronutrients including vitamins and minerals during pregnancy( Reference Haider and Bhutta 24 ). Furthermore, MMN was found to reduce the risk of perinatal mortality by 54 %, which might be a clinically important finding. The reduction of perinatal mortality could be attributed to the reduction of LBW and preterm births, which was supported by evidence of the important role of antenatal Fe supplements on the reduction of LBW and preterm delivery in the previous studies( Reference Haider, Olofin and Wang 26 , Reference Siega-Riz, Hartzema and Turnbull 49 ). However, these results should be interpreted cautiously because the present study was not powered to address this issue. These results, therefore, suggested that the MMN supplements might play a positive role in the improvement of birth outcomes of Tibetan babies.
The response to the micronutrient supplementation during pregnancy varied by circumstances and population. A trial in China observed that the MMN supplements containing 30 mg of Fe significantly increased birth weight, but there was no significant reduction in LBW and early neonatal mortality. Fe-FA supplements containing 60 mg of Fe decreased neonatal mortality, which could be attributed to high dose of Fe in the supplements( Reference Zeng, Dibley and Cheng 33 ). In the present study, the MMN supplements contained 60 mg of Fe, and no reduction of neonatal mortality in Tibetan babies was observed, but a reduction of perinatal mortality was still observed. We thought that a smaller sample size could account for this variation, but a different physiological response to MMN for Tibetan women could not be ruled out. Similar improvement of birth outcomes by MMN supplementation was also observed in a trial in Nepal. A 77 g increase in average birth weight and a decrease in the incidence of LBW by 25 % in the MMN group (fifteen vitamins and minerals, contained 30 mg of Fe) was observed compared with the Fe-FA group (contained 60 mg of Fe). However, no difference was recorded for the duration of gestation or infant length( Reference Osrin, Vaidya and Shrestha 28 ). On the basis of the present study, the positive effect of the MMN supplementation during pregnancy on birth weight could possibly be related to the addition of Fe.
A systematic review and meta-analysis of prenatal Fe use confirmed that daily prenatal use of Fe substantially improved birth weight in a linear dose–response manner, probably leading to a reduction in the risk of LBW. However, the Fe supplementation was not related to gestational age, preterm birth and birth length( Reference Haider, Olofin and Wang 26 ). As the MMN supplements for pregnant women are generally a complex of MMN with vitamins and minerals including Fe, FA and other B vitamins, other micronutrients besides Fe in the MMN supplements could contribute to the improvement of birth outcomes( Reference Haider and Bhutta 24 ). As for the effect of the MMN supplements on the mortality of babies, the results varied by study. Our previous study( Reference Zeng, Dibley and Cheng 33 ) did not find the reduction of neonatal mortality by the MMN supplements and the present study also found that the MMN supplements did not reduce neonatal mortality other than the reduction of perinatal death. In another randomised controlled trial in northern China( Reference Liu, Mei and Ye 50 ), the supplementation with Fe-FA (containing 30 mg of Fe) with or without other micronutrients (fifteen vitamins and minerals, contained 30 mg of Fe) did not affect the perinatal mortality rate compared with daily prenatal FA, except that relative risks for the third-trimester maternal anaemia were reduced. However, a large trial in Indonesia showed that the prenatal MMN supplementation (fifteen vitamins and minerals, contained 30 mg of Fe), as compared with Fe-FA (contained 30 mg of Fe), reduced early infant mortality (deaths until 90 d postpartum), especially in the undernourished and anaemic women( Reference Shankar, Jahari and Sebayang 51 ). The results from the present study suggested that, for Tibetan women, the supplementation with Fe-containing MMN during pregnancy tended not only to reduce the prevalence of maternal anaemia but also improve birth outcomes to some extent. However, the observed effects of the MMN supplements on mortality of Tibetan babies should be interpreted with caution.
Strengths and limitations
The lack of scientific research represented a gap in the knowledge on prenatal micronutrients supplementation and its effect on Tibetan mothers and their babies, who live in the highest altitude areas in the world. The present study is the first intervention study assessing the impact of the MMN supplements during pregnancy, compared with FA, on maternal anaemia and birth outcomes in the Tibetan population. In the study, the compliance with supplementation in both arms was higher than predicted, as reflected by a mean maternal compliance rate of 88·2 %. To address the possible imbalance in the participants’ characteristics between two treatment groups owing to the non-randomised design of this study in the analysis, the propensity score was established and used in the GEE model for controlling for potential confounders. Therefore, the present study provides valuable evidence for Tibetan MCH care policy that both Tibetan women and their babies could benefit from the MMN supplementation during pregnancy.
We recognise several limitations in the present study. First, the randomisation allocation was not performed owing to the difficulty of implementation because of cultural and religious sensitivity. Although we have tried to use the propensity score approach to control for any possible imbalance between the two groups, there still might be unobserved heterogeneities. As a result, the observed differences in the maternal anaemia and birth outcomes may be subject to possible unknown confounding factors. Second, we used FA as the control group because it was the standard prenatal supplement recommended by the Chinese Government during the period of this study. Third, the date of LMP may be inaccurate in a small part of the pregnant women and the gestational weeks were identified based on the clinical experiences of obstetricians. Consequently, the study may underestimate the preterm rate in Tibet.
Conclusions
This prospective cohort study has provided some evidence that the MMN supplements have important positive health effects for pregnant women and newborn infants, with the potential to not only reduce the prevalence of maternal anaemia during pregnancy but also improve birth outcomes to some extent in rural Tibet in high altitudes. Further randomised control trials are required to confirm such effects of this MMN formula.
Acknowledgements
The authors thank the following members of the Tibet study team that helped in the implementation of the study: ZhuomaDuoji, CangPu, Yan Xing, Xiaoyan Zhou, Leilei Pei, Chao Zhang, Guodong Wang, Chao Li and Jiangping Li. Zhenjie Wang performed computer programming and data management; GaZhuo, CuoBai and Sang Qu were responsible for computer programming and data management; and Ga Lang was the field supervisor. Yue Cheng provided useful comments about the study design and field procedures. The authors also thank the area coordinators of the Primary Health and Maternal and Child Health Division of Health Bureau of Tibet Autonomous Region, Lhasa Municipal Health Bureau and Health Bureaus in the study counties of Tibet Autonomous Region. Finally, the authors thank Emma Yu Wang for proofreading and editing the paper.
This work was supported by the China Medical Board, USA (grant no. CMB-#02-778), and the National Natural Science Foundation of China (grant no. 30771835).
Y. K. and S. D. developed the study protocol, questionnaires and field procedures, directed the field operations, held routine meetings with community leaders and health staff, contributed to the quality control, data cleaning, data analysis, data interpretation and wrote the first draft of the paper; L. Z. contributed to the preparation of the manual of operations, and helped in training field staff and quality control; D. W. carried out the initial analyses, and reviewed and revised the manuscript; Q. L. helped in the study design, data analysis and the writing of the manuscript; J. W. and L. O. directed the field implementation, community preparation and advocacy; H. Y. was the principal investigator, designed the study hypothesis and protocol, directed the implementation of the study, supervised the field operations, data analysis and data interpretation, edited the paper and acted as the guarantor. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
The authors declare that there are no conflicts of interest.









