Introduction
Body dysmorphic disorder (BDD) is characterized by an intense preoccupation with perceived, or slight, defects in physical appearance, which is typically, accompanied by compulsive behaviors. The insight that the perceived defects or flaws in appearance are within ‘normal’ limits, and in most cases not visible to others, is usually poor but it varies along a continuum from fair insight to delusional beliefs (American Psychiatric Association, 2013). BDD affects some 0.7–2.4% of the adult population, with the disorder being slightly more common in women (Koran et al. Reference Koran, Abujaoude, Large and Serpe2008; Buhlmann et al. Reference Buhlmann, Glaesmer, Mewes, Fama, Wilhelm and Brähler2010; Veale et al. Reference Veale, Gledhill, Christodoulou and Hodsoll2016). Very few studies exist on the prevalence of BDD in young people. In one community sample (age 12–18 years, mean = 14.5) the prevalence of BDD symptoms in adolescence was 1.7%, with no sex differences (Schneider et al. Reference Schneider, Turner, Mond and Hudson2016). In another sample consisting of high school students (age 14–19 years, mean = 15.8), the estimated prevalence was 2.2%, with BDD symptoms being more common in girls with a female-to-male ratio of 1.64 (Mayville et al. Reference Mayville, Katz, Gipson and Cabral1999). Finally, in a non-clinical sample of adolescents and young adults (age 15–21, mean 17.1) the estimated prevalence was 3.6%, with a higher prevalence in girls (Möllmann et al. Reference Möllmann, Dietel, Hunger and Buhlmann2017). However, due to the sample consisting primarily of females, no firm conclusions could be drawn regarding possible sex differences. These studies indicate that BDD symptoms are common already in this age group, but more work is needed in order to elucidate possible sex differences that may occur in young people.
Even though BDD is associated with marked impairment, suffering, and suicidality (Phillips et al. Reference Phillips, Menard, Fay and Weisberg2005; Phillips & Menard, Reference Phillips and Menard2006), it has been historically understudied and research into the etiology of BDD is still in its infancy. Family studies have found evidence of familial transmission of BDD (Bienvenu et al. Reference Bienvenu, Samuels, Riddle, Hoehn-Saric, Liang and Cullen2000; Phillips et al. Reference Phillips, Menard, Fay and Weisberg2005), and recent twin studies have started to disentangle to what degree genetic and environmental factors contribute to the liability of body dysmorphic symptoms. In adults, the heritability of body dysmorphic concerns has been estimated to 42–44%, with non-shared environmental factors accounting for the remaining variance (Monzani et al. Reference Monzani, Rijsdijk, Anson, Iervolino, Cherkas and Spector2011, Reference Monzani, Rijsdijk, Harris and Mataix-Cols2014; López-Solà et al. Reference López-Solà, Fontenelle, Alonso, Cuadras, Foley and Pantelis2014). As previous twin studies have included only adult twins, the heritability of BDD symptoms among young people remains unknown. BDD has a retrospectively reported mean age of onset at 16 (Gunstad & Phillips, Reference Gunstad and Phillips2003; Phillips et al. Reference Phillips, Menard, Fay and Weisberg2005), moreover, it is known that genetic influences of behavioral traits may change with age (Bergen et al. Reference Bergen, Gardner and Kendler2007). Currently, the genetic architecture and risk factors for BDD in adolescence and across the lifespan remain largely unknown.
Despite scant research, findings from studies using convenience sampling suggest that the patterns of comorbidity in adolescents are similar to those observed in adult samples (Phillips et al. Reference Phillips, Didie, Menard, Pagano, Fay and Weisberg2006), but the association between BDD and developmentally relevant conditions such as attention-deficit/hyperactivity disorder (ADHD) or autism spectrum disorder (ASD) has not been studied. Furthermore, although comorbid substance abuse and BDD has been associated with higher rates of lifetime suicide attempts (Kelly et al. Reference Kelly, Simmons, Wang, Kraus, Donahue and Phillips2017), the risk for alcohol abuse in young people with BDD remains largely unknown. It is of clinical importance to establish to what extent BDD symptoms in young people co-occur with other psychiatric symptoms. Indeed, a better understanding of these patterns may have nosological as well as clinical implications for prognosis and treatment.
In this study, we used data from a validated screening tool for BDD in 15 377 twins from the Swedish Twin Registry to estimate the prevalence of clinically significant BDD symptoms in adolescents and young adults. We also report on the contribution of genetic and environmental risk factors to the liability of body dysmorphic concerns at crucial times of its development, and the risk for co-existing psychopathology in cases with clinically significant BDD symptoms.
Method
Study population
The study ‘Child and Adolescent Twin Study in Sweden’ (CATSS; Anckarsäter et al. Reference Anckarsäter, Lundström, Kollberg, Kerekes, Palm and Carlström2011) is a prospective longitudinal study of all twins born in Sweden since July 1992. Parents of these twins were interviewed when the twins were 9 or 12 years old (CATSS-9/12) and further information was collected at ages 15 (CATSS-15), and 18 (CATSS-18). At the follow-ups, the twins were asked to complete a battery of questionnaires, including a measure of body dysmorphic concerns. In CATSS-15, body dysmorphic data were available for 6968 twin individuals (response rate = 53% of all Swedish twins in the age group), and in CATSS-18 the sample included 3738 individuals with available body dysmorphic data (response rate = 48%).
The ‘Young Adult Twins in Sweden Study’ (YATSS 20–28) is a separate survey that ran from March 2013 to January 2014 and included all young adult twins born in Sweden from May 1985 to June 1992. There is no overlap between CATSS and YATSS cohorts. The twins were contacted and asked to complete a survey consisting of questions evaluating somatic illnesses and psychiatric disorders, including a measure of body dysmorphic concerns. Detailed information about the YATSS 20–28 cohort can be found in Ivanov et al. (Reference Ivanov, Nordsletten, Mataix-Cols, Serlachius, Lichtenstein and Lundström2017). Data on body dysmorphic symptoms were available for 4671 individual twins (response rate = 29%), the mean age of participants in the YATSS 20–28 cohort was 23.9 years (s.d. = 2.0, median = 24.0, range = 20–28). Demographic characteristics in all samples are displayed in Table 1.
Table 1. Demographic and clinical characteristics of the twins at age 15 (CATSS-15), age 18 (CATSS-18), and ages 20–28 (YATSS)
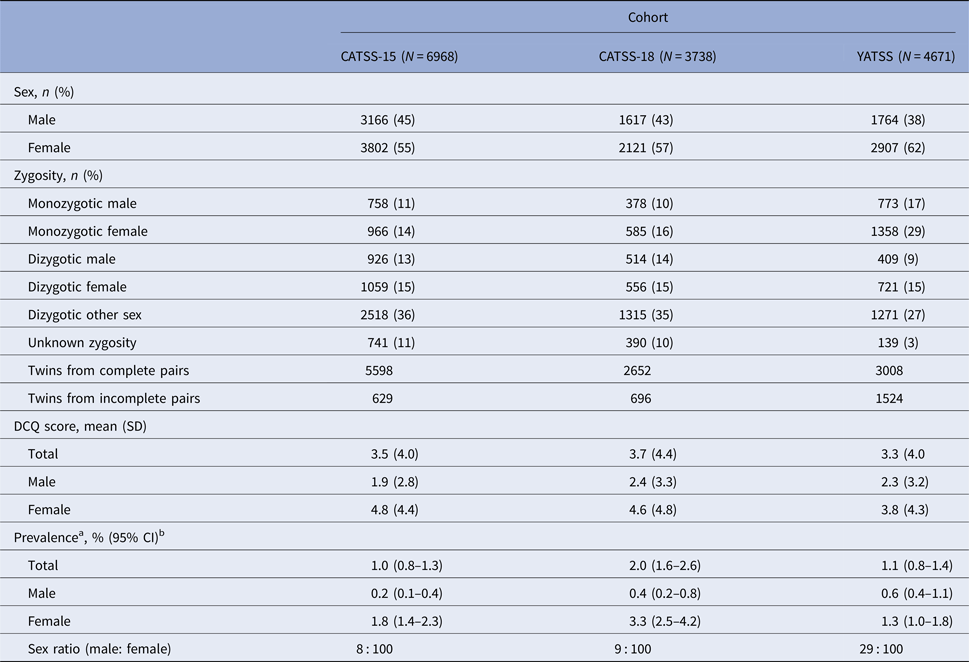
a Clinical Significant Body Dysmorphic Symptoms defined as ⩾17 on the Dysmorphic Concerns Questionnaire (DCQ).
b Confidence intervals adjusted for the clustering of twins within families.
CATSS, Child And Adolescent Twin Study in Sweden; YATSS, Young Adult Twin Study in Sweden; SD, Standard Deviation; CI, Confidence interval.
Zygosity was determined by 49 single-nucleotide polymorphisms (SNPs; Hannelius et al. Reference Hannelius, Gherman, Mäkelä, Lindstedt, Zucchelli and Lagerberg2007). If DNA was unavailable, an algorithm based on twin similarity that correctly classifies 95% of twins compared with DNA testing was used (Magnusson et al. Reference Magnusson, Almqvist, Rahman, Ganna, Viktorin and Walum2013). The Regional Ethics Review Board in Stockholm approved all data collection points and samples included in the study (reference numbers 02–289, 03–672, 2009–739–31/5, 2010/1410–31/1, and 2012/2107–31/3).
Measures
Body dysmorphic disorder
The Dysmorphic Concern Questionnaire (DCQ; Oosthuizen et al. Reference Oosthuizen, Lambert and Castle1998) is a seven-item, self-report measure that assesses concern with one's own physical appearance and bodily function. Responders rate their concern about their physical appearance relative to others on a four-point scale ranging from 0 (none) to 3 (much more than most people), yielding a total score between 0 and 21. The DCQ has good psychometric properties; it discriminates BDD from clinical controls and appears to be a useful screening instrument for BDD in non-clinical samples (Oosthuizen et al. Reference Oosthuizen, Lambert and Castle1998; Jorgensen et al. Reference Jorgensen, Castle, Roberts and Groth-Marnat2001; Stangier et al. Reference Stangier, Connell, Adam-Schwebe, Berger and Wolter2003; Mancuso et al. Reference Mancuso, Knoesen and Castle2010). As concerns with physical appearance are not limited to BDD, we slightly modified the DCQ instructions to state that the responder should not include concerns related to ‘weight or being too fat’ when answering the questions. This was done to better capture the BDD phenotype and to exclude answers from participants whose primary concerns were dissatisfaction with body size or appearance concerns due to being overweight. In an attempt to identify participants whose concerns were due to an objective disfigurement caused by a medical condition or injury, the following question was added to the DCQ: ‘Are your appearance concerns due to an injury or medical condition that has disfigured you or significantly changed your appearance? If yes, please specify’ Participants who answered yes and provided clear and recognizable causes for disfigurement (e.g. amputations, cleft lip, vitiligo, scoliosis) were excluded from the analysis.
Different cut-off points have been proposed for the DCQ, but here we only report prevalence estimates for the highest proposed cut-off ⩾17 in order to differentiate BDD symptoms from eating disorder symptomatology (Stangier et al. Reference Stangier, Connell, Adam-Schwebe, Berger and Wolter2003; Mancuso et al. Reference Mancuso, Knoesen and Castle2010; Monzani et al. Reference Monzani, Rijsdijk, Anson, Iervolino, Cherkas and Spector2011). Furthermore, we validated the DCQ using data from the current sample combined with cases fulfilling DSM-5 diagnostic criteria for BDD ascertained from three clinical samples (Monzani et al. Reference Monzani, Krebs, Anson, Veale and Mataix-Cols2013; Enander et al. Reference Enander, Ivanov, Andersson, Mataix-Cols, Ljótsson and Rück2014, Reference Enander, Andersson, Mataix-Cols, Lichtenstein, Alström and Andersson2016). The area under the curve was 0.98 (95% CI = 0.97–0.99) when comparing patients diagnosed with BDD and non-BDD participants from the twin samples. Using the cut-off ⩾17 correctly identified 96% of diagnosed patients with a sensitivity of 56% and a specificity of 99% (details of the psychometric evaluation of the DCQ are available from the first author upon request). The DCQ displayed good internal consistency across all cohorts (Cronbach's α = 0.85–0.89), and a principal component analysis revealed a single factor structure explaining 54–58% of the variance. Due to positive skewness in total DCQ scores in all three cohorts (CATSS-15 skewness = 1.51; CATSS-18 skewness = 1.61; YATSS 20–28 skewness = 1.61), a logarithmical transformation using the natural logarithm was performed, resulting in reduced skewness (CATSS-15 skewness = 0.02; CATSS-18 skewness = 0.09; YATSS 20–28 skewness = 0.18).
Neuropsychiatric disorders
In CATSS-9/12, parents of twins were interviewed with the Autism-Tics, ADHD, and other co-morbidities inventory (A-TAC; Hansson et al. Reference Hansson, Svanström Röjvall, Råstam, Gillberg, Gillberg and Anckarsäter2005) A-TAC is a validated telephone interview with questions regarding lifetime symptoms of ADHD, and ASD closely mapped after the corresponding DSM-IV criteria. A cutoff ⩾6 was used for ADHD, and a cutoff ⩾4.5 for ASD. These cutoffs have been validated as broad screening cutoffs to identify cases, with a higher sensitivity than specificity and have a good interrater agreement (Larson et al. Reference Larson, Anckarsäter, Gillberg, Ståhlberg, Carlström and Kadesjö2010; Reference Larson, Kerekes, Selinus, Lichtenstein, Gumpert and Anckarsäter2014).
Obsessive-compulsive and related disorders
In CATSS-9/12, parents of twins were interviewed using two A-TAC items addressing obsessions and compulsions, mapped after the DSM-IV criteria for obsessive-compulsive disorder (OCD; Hansson et al. Reference Hansson, Svanström Röjvall, Råstam, Gillberg, Gillberg and Anckarsäter2005). In YATSS 20–28, the presence of probable OCD was assessed with the Obsessive-Compulsive Inventory Revised (OCI-R) using a validated cut-off ⩾21 (Foa et al. Reference Foa, Huppert, Leiberg, Langner, Kichic and Hajcak2002). The OCI-R was updated to reflect DSM-5 criteria for OCD, and thus the items that assess hoarding symptoms were removed. Hoarding symptoms were assessed with the Hoarding Rating Scale-Self Report (HRS-SR; Tolin et al. Reference Tolin, Frost and Steketee2010). The presence of clinically significant hoarding symptoms was defined as scoring at least moderate severity on items measuring clutter and difficulties discarding and on at least one of the items measuring distress and impairment. This procedure has previously been described as criteria for clinically significant hoarding and closely resembles the DSM-5 criteria (Frost & Hristova, Reference Frost and Hristova2011).
Eating disorders
The Eating Disorder Inventory-2 (EDI-2; Garner et al. Reference Garner, Olmstead and Polivy1983) is a validated instrument that can discriminate between patients with eating disorders, other psychiatric disorders, and non-affected individuals (Nevonen & Broberg, Reference Nevonen and Broberg2001; Nevonen et al. Reference Nevonen, Clinton and Norring2009). In CATSS, a validated cutoff ⩾21 on the EDI-2 symptom index (the total sum score of the drive for thinness, bulimia, and body dissatisfaction subscales) was used as the threshold to identify cases with clinically significant eating disorder symptomatology. In YATSS 20–28, EDI-2 data were not available, instead two dichotomously scored items were used to capture self-reported lifetime occurrence of an eating disorder: ‘Have you ever had bulimia nervosa/anorexia nervosa?’
Alcohol abuse
In CATSS-18, the Alcohol Use Disorders Identification Test (AUDIT) was used to capture hazardous or harmful alcohol use, with a score of ⩾8 to identify cases (Saunders et al. Reference Saunders, Aasland, Babor, la Fuente de and Grant1993).
Statistical analysis
We estimated the prevalence of BDD in the different age samples, both combined and separately by sex. The association between BDD and psychiatric comorbidity was examined using logistic regression models. Odds ratios were adjusted for zygosity and age (when appropriate). Confidence intervals were calculated using a cluster-robust sandwich estimator accounting for correlations within twin pairs.
Twin methodology relies on the comparison of monozygotic twins who are assumed to be genetically identical, and dizygotic twins who, on average, share 50% of their segregating alleles. Further, the equal-environment assumption states that monozygotic and dizygotic twins are equally correlated in their exposure to environmental events of etiological importance, and has shown strong empirical support in the study of psychiatric disorders (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1993). If genes influence a trait, there will be a more pronounced similarity within monozygotic twins, compared with within dizygotic twins. Twin pair correlations allow for estimation of the variation in a phenotype attributed to additive genetic (A), shared environmental (C), and non-shared environmental (E) factors (including measurement error).
Only twins with known zygosity were included in the model-fitting analyses (CATSS-15: n = 6227; CATSS-18: n = 3348; YATSS 20–28: n = 4532). All pairs where at least one twin had available data were included in analyses. We estimated intraclass correlations (i.e. the correlation between twins in a pair) for the DCQ total scores in monozygotic and dizygotic twins. To assess the appropriateness of data for heritability analyses we performed assumptions tests separately for each age sample. In a first step, a fully saturated model was fitted to estimate the means and covariance matrices that provided the best fit for our data. In the YATSS 20–28 sample, we additionally adjusted means for age. We then proceeded to equate the means and variances across twin order, zygosity, and sex to test whether any of these restrictions produced a worse fit, using likelihood ratio tests. The assumption testing showed that the data were appropriate for heritability analyses, with the exception that males had significantly lower mean scores in all three age groups on the DCQ (p < 0.05 in all samples). We, therefore, allowed the means for males and females to be different in the model fitting analysis. Maximum-likelihood model fitting was performed for each age sample separately (Neale & Cardon, Reference Neale and Cardon2013). As the objective is to quantify the nature and magnitude in genetic and environmental risk factors for dysmorphic concerns, we chose to present measures of accuracy of the estimates (A, C, and E) and avoid hypothesis testing. This is in line with the recommendations based on simulations, which show that in such situations parameter estimates from the full model are typically more accurate than those from submodels even if the latter provides a better model fit (Sullivan & Eaves, Reference Sullivan and Eaves2002). Basic statistical analysis and data preparation were performed using SAS 9.3. Twin analyses were performed using the OpenMx package (openmx.psyc.virgina.edu) in R (cran.r-project.org).
Results
Prevalence
The prevalence of clinically significant BDD symptoms, and a likely diagnosis of BDD (a total score ⩾17 on the DCQ) in the twin samples was 1.0% (95% CI 0.8–1.3) in CATSS-15, 2.0% (95% CI 1.6–2.6) in CATSS-18, and 1.1% (95% CI 0.8–1.4) in YATSS 20–28. Across all cohorts, the prevalence was significantly higher in females than in males (CATSS-15,χ2 = 43.48 [p < 0.001]; CATSS-18, χ2 = 38.76 [p < 0.001]; YATSS 20–28 χ2 = 4.94 [p = 0.03]). The prevalence in males increased significantly with age (χ2 = 6.17 [p = 0.01]; Table 1).
Heritability
Table 2 provides intraclass correlations for MZ and DZ twins. Across all three cohorts, the intraclass correlations were higher for MZ than for DZ twins, indicating genetic influences on body dysmorphic concerns, however, the MZ correlations were <1.0, also indicating environmental effects on the phenotype.
Table 2. Univariate heritability estimates of body dysmorphic concerns in age 15 (CATSS-15, n = 6227), 18 (CATSS-18, n = 3348), and 20–28 years (YATSS, n = 4532)
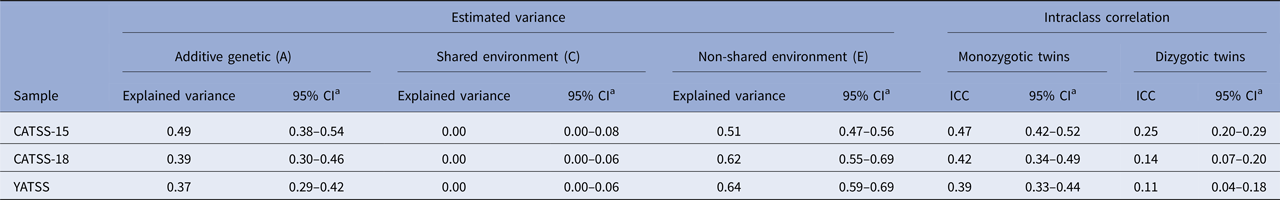
a Profile likelihood confidence intervals.
CATSS, Child And Adolescent Twin Study in Sweden; YATSS 20–28, Young Adult Twin Study in Sweden; CI, Confidence Interval; ICC, Intraclass correlation
In all three cohorts, the ACE-model provided an adequate fit to the data compared with the saturated model (p:s = 0.26–0.43). The heritability of dysmorphic concerns was estimated to be 49% (95% CI 38–54%) at age 15, 39% (95% CI 30–46%) at age 18, and 37% (95% CI 29–42%) in young adults ages 20–28. Shared environmental factors were estimated to be 0% in all cohorts, with the remaining variance being accounted for by non-shared environmental factors, estimated to be 51% (95% CI 47–56%) at age 15, 61% (95% CI 54–68%) at age 18, and 63% (95% CI 58–69%) at ages 20–28 (Table 2).
Co-existence of psychiatric symptomatology
The number of males who screened positive for clinically significant BDD symptoms was low, and as a result, the odds ratios (ORs) could not be calculated for this group due to zero values. Because of this, only results in females are presented. Overall, the co-existence of any psychiatric symptomatology in cases with clinically significant BDD symptoms was common in all three age groups. Sixty-five percent (95% CI 54–76%) of BDD cases in CATSS-15, 61% (95% CI 50–73%) in CATSS-18, and 45% (95% CI 31–59%) in YATSS 20–28 screened positive for at least one comorbid disorder. The ORs for specific co-existing psychiatric symptoms in females with clinically significant BDD symptoms compared with individuals without clinically significant BDD symptoms are shown in Table 3. In CATSS-15, females with BDD were twice as likely as unaffected individuals to endorse comorbid ADHD and showed a fivefold risk for ASD. The risk for co-occurring obsessive-compulsive and related disorders (OCD or hoarding disorder) showed a five to sevenfold increase at age 18, and ages 20–28 years, but no increased risk was observed at age 15. Clinically significant eating disorder symptomatology (measured with EDI-2) in individuals with BDD was high at both ages 15, and 18, with a 13- and 7-fold increased risk, respectively. At age 20–28, women who screened positive for BDD were four times more likely to endorse a lifetime occurrence of bulimia nervosa, but no increased risk for the co-occurrence of anorexia nervosa was observed. Furthermore, of those with BDD, 43% reported problematic drinking, with a threefold increased risk for alcohol abuse.
Table 3. Risk for co-existing psychopathology in females with clinically significant body dysmorphic symptomsa in CATSS-15, CATSS-18, YATSS 20–28 and when compared with females without symptoms
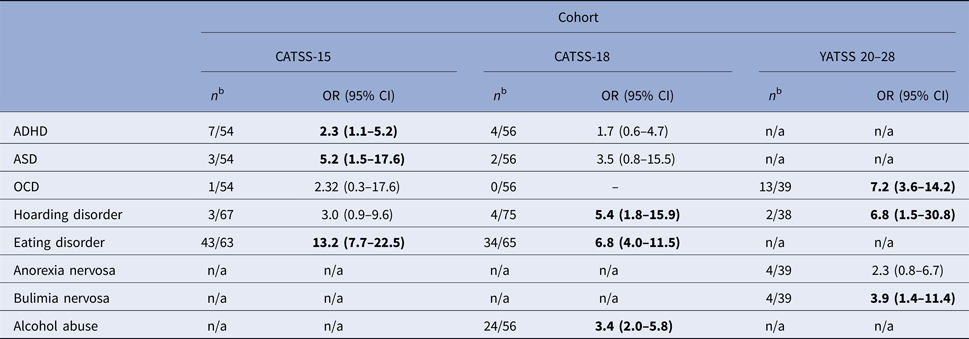
Bold indicates p < 0.05.
–, Values for OR and 95% CI could not be calculated due to zero values.
n/a, not available.
a Clinically Significant Body Dysmorphic Symptoms defined as ⩾17 on the Dysmorphic Concerns Questionnaire (DCQ).
b Number of twins with clinically significant BDD symptoms whom also scored above cut-off on measures of psychiatric psychopathology. The denominator varies as not all cases had complete data on measurements of psychopathology.
CATSS, Child And Adolescent Twin Study in Sweden; YATSS 20–28, Young Adult Twin Study in Sweden; OR, Odds ratio; CI, Confidence Interval; ADHD, Attention deficit/hyperactivity disorder; ASD, Autism spectrum disorder; OCD, Obsessive-compulsive disorder.
Discussion
To our knowledge, this is the first genetic epidemiological study of body dysmorphic concerns in a large population-based sample of adolescents and young adults (N = 15 377). The occurrence of cases with clinically significant BDD symptoms and a likely diagnosis of BDD was estimated to be around 1–2%, with the prevalence being significantly higher in females across all cohorts. The estimated heritability of body dysmorphic concerns was moderate across the age groups, with the remaining variance explained by non-shared environmental effects. The highest heritability was observed in age 15 (49%), it then slightly diminished with age and was estimated at 39% in 18-year olds, and at 37% in ages 20–28. Our findings also show that individuals with clinically significant BDD symptoms had an increased risk for co-existing neuropsychiatric, OCD spectrum and alcohol-related psychiatric symptoms measured in childhood and adulthood.
Our nation-wide sample shows that clinically significant BDD symptoms are relatively common in these age groups, and confirms the retrospective reports of age at onset described by adults diagnosed with BDD (Gunstad & Phillips, Reference Gunstad and Phillips2003; Phillips et al. Reference Phillips, Menard, Fay and Weisberg2005). The occurrence is also in line with the suggested prevalence of clinically significant BDD symptoms reported in community samples of adolescents (Mayville et al. Reference Mayville, Katz, Gipson and Cabral1999; Schneider et al. Reference Schneider, Turner, Mond and Hudson2016). In adults, the sex ratio of BDD is about equal (Veale et al. Reference Veale, Gledhill, Christodoulou and Hodsoll2016), but there has been uncertainty with regards to sex differences in the occurrence of BDD symptoms in young people. One study has indicated that there are no sex differences (Schneider et al. Reference Schneider, Turner, Mond and Hudson2016), whilst another two studies have indicated that females are more likely to endorse symptoms of BDD (Mayville et al. Reference Mayville, Katz, Gipson and Cabral1999; Möllmann et al. Reference Möllmann, Dietel, Hunger and Buhlmann2017). Our population-based sample shows that BDD symptoms are significantly elevated in females compared to males, but also suggest that the occurrence of BDD symptoms in males increases with age. This may indicate sex differences in age of onset, where females experience symptoms earlier in life. Sex differences in age of onset could explain why the sex ratio differs in young people but is reported to be about equal in adults. Furthermore, this is corroborated by anecdotal evidence that boys with BDD less frequently present themselves at the child and adolescent psychiatric clinics, and by the low participation of boys in clinical trials of BDD (Mataix-Cols et al. Reference Mataix-Cols, Fernández de la Cruz, Isomura, Anson, Turner and Monzani2015). An additional explanation for the observed sex differences is that these may be due to hormonal activation that takes place during pubertal development. In other internalizing disorders (such as depression, anxiety) the sex ratio is equal before puberty, but the symptoms become more prevalent in girls after puberty (Hayward & Sanborn, Reference Hayward and Sanborn2002; Angold & Costello, Reference Angold and Costello2006). Specifically, more advanced Tanner stages of pubertal development in girls predict the sex differences in the occurrence of internalizing disorders better than age (Hayward & Sanborn, Reference Hayward and Sanborn2002), suggesting that sex hormones play a part in the pathophysiology of these disorders. Furthermore, preliminary evidence suggests that estradiol levels moderate the magnitude of genetic effects for disordered eating during puberty (Klump et al. Reference Klump, Keel, Sisk and Burt2010). Sex-specific hormonal activation such as estrogen and testosterone during puberty may possibly account for at least part of the sex differences in clinically significant BDD symptoms amongst youth observed in this and other studies. Nonetheless, it should be noted that the association between hormones and BDD has not yet been studied and we do not have access to blood/saliva samples or Tanner stages in our data to directly address this hypothesis; this warrants further investigation.
Two studies have previously estimated the heritability of dysmorphic concerns in adults using the same instrument as administered in this study (DCQ). One study consisting of 3544 adult female twins (mean age of 57) recruited from the TwinsUK registry estimated the heritability at 44% (95% CI 37–50%). In the other twin study (N = 2495), consisting of twins of both sexes with a mean age of 35, recruited from the Australian Twin Registry, the heritability was 42% (95% 30–52%). Our heritability estimates were largest at age 15, and then tended to non-significantly decrease with age but, in general, the heritability estimates in the current study are in line with those previously reported in adults (Monzani et al. Reference Monzani, Rijsdijk, Anson, Iervolino, Cherkas and Spector2011; López-Solà et al. Reference López-Solà, Fontenelle, Alonso, Cuadras, Foley and Pantelis2014). Further longitudinal studies of the development of BDD symptoms across the lifespan are needed in order to evaluate whether the relative genetic and environmental factors on BDD symptoms vary over time, as suggested in other disorders such as OCD (Krebs et al. Reference Krebs, Waszczuk, Zavos, Bolton and Eley2014; Zilhão et al. Reference Zilhão, Smit, Braber den, Dolan, Willemsen and Boomsma2015). The results confirm that BDD has a genetic basis and should encourage researchers to initiate collaborations to gather sufficient DNA samples for study of associated genetic markers; given the suspected shared etiology with other disorders in the OCD spectrum (Monzani et al. Reference Monzani, Rijsdijk, Harris and Mataix-Cols2014), recent advances in OCD genetics (Mattheisen et al. Reference Mattheisen, Samuels, Wang, Greenberg, Fyer and McCracken2015; Noh et al. Reference Noh, Tang, Flannick, O'Dushlaine, Swofford and Howrigan2017) may help accelerate the discovery of BDD-related genes as well.
Across the cohorts, the shared environment was negligible. Instead, unique environmental factors contributed to the risk of developing body dysmorphic concerns. No environmental risk factors for BDD have yet been identified, although, some research suggests that young people with BDD report more appearance-related teasing (Buhlmann et al. Reference Buhlmann, Cook, Fama and Wilhelm2007). Since non-shared environment constitutes a substantial proportion of the variance in the phenotypic expression, future population-based research is needed to identify the nature of these risk factors. This has recently been done in OCD, where for example, smoking during pregnancy or a range of obstetric complications were associated with a higher risk of developing OCD while controlling for familial confounders (Brander et al. Reference Brander, Rydell, Kuja-Halkola, Fernández de la Cruz, Lichtenstein and Serlachius2016).
To our knowledge, this is the first study to report on the co-occurrence of neuropsychiatric symptoms in adolescents with BDD symptoms. At age 15, twins with BDD symptoms showed an increased risk of ADHD and ASD symptoms compared with non-BDD subjects. In BDD, impaired executive functioning has been reported (Dunai et al. Reference Dunai, Labuschagne, Castle, Kyrios and Rossell2009), and the association between ADHD/ASD and BDD in adolescents warrants further investigation in clinically ascertained samples; whether these neuropsychiatric comorbidities interfere with the treatment of BDD is a particularly relevant question. In DSM-5, BDD is classified in the obsessive-compulsive and related disorders chapter and has shown to share a genetic risk factor, particularly with OCD and HD (Monzani et al. Reference Monzani, Rijsdijk, Harris and Mataix-Cols2014). The observed risk for the co-occurrence of these symptom clusters in twins with BDD symptoms in the general population gives further support for a link between these disorders. The risks for clinical significant eating disorder symptoms and lifetime bulimia nervosa was elevated in twins with BDD symptoms, and the pattern of co-occurrence in this representative sample of young people is similar to that observed in clinical samples of adults diagnosed with BDD (Phillips, Reference Phillips1991; Gunstad & Phillips, Reference Gunstad and Phillips2003). A feature common in both BDD and eating disorders is the concern about one's appearance and a negative body image that may constitute an underlying liability, and potentially shared genetic factors between the disorders, which warrant further investigation.
Forty-three percent of females aged 18 with clinical significant BDD symptoms screened positive for problematic drinking, with a three-fold increase in risk when compared with non-BDD subjects. In adults diagnosed with BDD, the lifetime occurrence of an alcohol-use disorder has been estimated to 29–43%, with a majority of patients reporting that BDD contributed to their alcohol use, and that they drank in order to alleviate negative affect and to cope with distress due to body image concerns (Grant et al. Reference Grant, Menard, Pagano, Fay and Phillips2005; Kelly et al. Reference Kelly, Simmons, Wang, Kraus, Donahue and Phillips2017). Furthermore, drinking as a way to cope with negative effect in BDD has been shown to be associated with lifetime rates of attempted suicide (Kelly et al. Reference Kelly, Simmons, Wang, Kraus, Donahue and Phillips2017). This suggests that young patients with BDD and co-occurring alcohol misuse should be more carefully monitored for suicidal ideation. Moreover, clinicians should routinely screen adolescent BDD patients for alcohol use disorder as the risk for problematic drinking is elevated among adolescents with clinically significant BDD symptoms.
Strengths of this study include a nationwide population-based sample of 15 377 adolescents and young adults, and the use of a validated instrument for assessing body dysmorphic concerns and a probable diagnosis of BDD. However, this study also has several limitations. We validated the DCQ in the twin samples using data from patients diagnosed with BDD, and excluded cases with real disfigurements; however, we cannot be certain that all the twin cases that screened positive for clinically significant BDD symptoms on the DCQ would fulfill a diagnosis of BDD if assessed by a clinician. Response rates in the different age groups varied from 29 to 53%. Overall, non-responders in the CATSS cohort at ages 9 and 12 are more likely than responders to be socially disadvantaged and present with different types of health problems but the differences are so small that, with the possible exception of learning disorders, the results are likely generalizable to the total population (Anckarsäter et al. Reference Anckarsäter, Lundström, Kollberg, Kerekes, Palm and Carlström2011). However, a similar study would be required at ages 15, 18 and 20–28 to draw any firm conclusions about the representativeness of our cohorts. Although modeled after DSM-IV criteria, comorbid OCD was not determined using a validated measure and was only based on a parental report at ages 9/12 for the CATSS-twins (Hansson et al. Reference Hansson, Svanström Röjvall, Råstam, Gillberg, Gillberg and Anckarsäter2005). This, taken together with evidence suggesting that females are more likely to have a late onset of OCD (Taylor, Reference Taylor2011) may have led to an underestimation of the occurrence of OCD in the younger age groups, and thus the risk for co-occurring OCD in these age groups should be interpreted with caution. Furthermore, due to the low prevalence of clinically significant BDD symptoms in males, we could only assess the risk of co-existing psychopathology in females. The comorbidity estimates should also be interpreted with caution due to the small number of twins that screened positive for BDD and other mental disorders. It should also be noted that due to positive skewness in total DCQ scores, a logarithmical transformation was performed, which should be taken into account when interpreting the results. Lastly, the prevalence estimates and patterns of psychiatric co-occurrence may have been affected by the lower response rate in the older age groups, as it is plausible that the most extreme cases may be more likely to be non-responders.
Conclusion
In conclusion, we report the largest twin study of body dysmorphic concerns to date, including estimates of the occurrence of clinically significant BDD symptoms and the risk for co-existing psychiatric symptoms in a representative sample of adolescents and young adults. The prevalence clinically significant BDD symptoms and a likely diagnosis of BDD was 1–2%, with the occurrence of BDD in males being rare in ages 15 and 18, but increased significantly in ages 20–28. This may indicate sex differences in age of onset, where males may develop clinically significant BDD symptoms later in life. Overall, the heritability of body dysmorphic concerns in young people are moderate and broadly comparable to that of adults. Our data also show that the co-existence of psychopathology is common in young people with clinically significant BDD symptoms. Moreover, the risk for problematic drinking is elevated; indicating that patients should routinely be screened for alcohol use disorder as comorbid substance abuse and BDD has been associated with higher rates of lifetime suicide attempts.
Acknowledgements
The authors would like to thank Camilla Palm, Barbro Sandin, and Isabelle Kizling at the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet for their assistance in data management and preparation. Financial support was provided through the regional agreement on medical training and clinical research (ALF) between the Stockholm County Council and Karolinska Institutet, the Swedish Research Council, the Swedish Research Council for Health, Working Life and Welfare, and the Swedish Society of Medicine (Söderströmska Königska sjukhemmet, grant number: SLS3B4451) provided funding for this study.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





