The increase of global mean surface temperature by the end of the 21st century relative to around the millenium is likely to be 0·3 to 4·8 °C according to the last assessment report of the Intergovernmental Panel on Climate Change (IPCC, 2014), with greater warming in the Northern Hemisphere. Under high environmental temperature and humidity, the ability of animals to dissipate heat is reduced, causing increases in their body temperature and heat stress (HS). It is expected that farm animals will face heat waves at a higher frequency, intensity and duration, which will negatively affect their health, welfare and performance (Salama et al. Reference Salama, Caja, Hamzaoui, Such, Albanell, Badaoui, Loor, Villalba and Manteca2016). Furthermore, warmer and wetter weather (i.e., warmer winters) encourages the survival of disease vectors, which would make animals more exposed to diseases. The correlation between HS and susceptibility of dairy animals to both noninfectious and infectious diseases further complicates the issue.
Cases of lameness associated with an increase of total standing time per day due to HS in lactating dairy cows were reported in summer (Cook et al. Reference Cook, Mentink, Bennett and Burgi2007). There is also an increment of intra-mammary infections in dairy cattle (Waage et al. Reference Waage, Sviland and Odegaard1998) and sheep (Sevi & Caroprese, Reference Sevi and Caroprese2012) during summer, leading to drastic economic losses. Thompson et al. (Reference Thompson, Tao, Monteiro, Jeong and Dahl2014) showed that HS in the dry period negatively affected the immune response later in lactation when cows received a Staphylococcus aureus challenge; compared with cooled cows, the non-cooled cows had lower neutrophil counts and milk somatic cell count (SCC). They also found that non-cooled cows during the dry period had higher incidence of mastitis after parturition. In dairy goats, Love (Reference Love2015) also observed that the increase in SCC in milk after a LPS challenge was delayed until 4 h compared to only 2 h in thermal neutral (TN), and SCC in TN recovered faster.
Understanding the molecular mechanisms of the immune-dysfunction observed under HS conditions could uncover targets for development of new strategies to improve animal immunity under HS conditions. Our hypothesis was that heat stress would negatively affect milk production and the functionality of immune cells, which could explain deteriorated performance and the greater incidence of diseases in dairy animals under high ambient temperatures. Therefore, the aim of the present study was to evaluate the effect of heat stress for 5 weeks on milk yield, milk composition, and blood transcriptomics in dairy goats.
Materials and methods
Animal care conditions and management practices agreed with the procedures stated by the Ethical Committee of Animal and Human Experimentation of the Universitat Autonoma de Barcelona (UAB) and the codes of recommendations for the welfare of livestock of the Ministry of Agriculture, Food and Environment of Spain.
Animals, treatments, and management conditions
Eight multiparous Murciano-Granadina lactating dairy goats (43·3 ± 1·6 kg BW; 81 ± 3 d of lactation; 2·00 ± 0·04 L/d milk), with healthy and symmetrical udders from the herd of the SGCE (Servei de Granges i Camps Experimentals) of the UAB were used. Goats were kept in individual metabolic cages (1·5 m × 0·50 m) throughout the experiment. Goats were divided into 2 balanced groups (n = 4 each) according to body weight, milk yield, and milk composition recorded before the experiment.
Goat groups were submitted to 2 different environmental conditions for 35 d. Treatments and temperature–humidity index (THI) were thermal neutral (TN; 15 to 20 °C and 45 ± 5% relative humidity; THI = 59 to 65) and heat stress (HS; 37 ± 0·5 °C during the day, and 30 ± 0·5 °C during the night and 40 ± 5% relative humidity; THI = 75 to 83). The THI values were calculated according to NRC (1971) as follows:
where Tdb is the dry bulb temperature (°C) and RH is the relative humidity (%). Values of THI greater than 80 are considered to cause moderate HS in dairy goats (Silanikove & Koluman, Reference Silanikove and Koluman2015).
Throughout the experiment (mid-December to mid-February), the TN goats were kept indoors and the temperature was maintained at 15 to 20 °C with the help of electric heather equipped with a thermostat (3·5 kW; General Electric, Barcelona, Spain). Temperature and relative humidity averaged 16·7 ± 0·3 °C and 45 ± 5% (THI = 61) for the TN goats. The HS goats were in a 4 × 6 × 2·3 m isolated chamber (Euroshield, ETS Lindgren-Euroshield Oy, Eura, Finland) provided with a temperature and humidity controlling system (Carel Controls Ibérica, S.L., Barcelona, Spain). A continuous 90 m3/h air turnover was maintained throughout the experiment.
Goats had a 2-wk pre-experimental period under TN conditions for the adaptation to the diet and to metabolic cages. Photoperiod was maintained constant at 12–12 h light–dark (09·00 to 21·00) and data of environmental temperature and humidity were recorded every 10 min using 2 data loggers (Opus 10, Lufft, Fellbach, Germany).
Feed was offered ad libitum at 0930 h (120% intake of the previous day) and consisted of a total mixed ration (alfalfa hay, 70%; ground barley grain, 14·4%; corn flour, 8·4%; soybean meal, 2·5%; soybean hulls, 4·3%; molasses, 0·3%; salt, 0·01%; sodium bicarbonate, 0·03%; carbonate, 0·02%; dicalcium phosphate, 0·01%; calcium carbonate, 0·01%; and CVM for goats, 0·02%). The ration contained (on DM basis) 17·5% CP, 43·8% NDF, 27·0% ADF, and 1·41 Mcal NEL. Additionally, mineral and vitamin blocks were freely available (Na, 16%; Ca, 12%; bicarbonate and seaweed, 12%; P, 5·5%; Mg, 2·2%; Zinc oxide, 2000 mg/kg; manganese sulfate, 1000 mg/kg; potassium iodide, 60 mg/kg; cobalt, 40 mg/kg; iron sulfate, 40 mg/kg; sodium selenite, 15 mg/kg; yeasts and S. cerevisiae, 10 mg/kg; vitamin A, 120 000 IU/kg; vitamin D3, 32 000 IU/kg; vitamin E, 120 mg/kg).
Goats were milked once daily (0800 h) with a portable milking machine (Westfalia-separator Ibérica, Granollers, Spain). Milking was conducted at a vacuum pressure of 42 kPa, a pulsation rate of 90 pulses/min, and a pulsation ratio of 66%. The milking routine included cluster attachment without udder preparation or teat cleaning, machine milking, machine stripping before cluster removal, and teat dipping in an iodine solution (P3-ioshield, Ecolab Hispano-Portuguesa, Barcelona, Spain).
Measurements and analyses
Rectal temperatures (RT) and respiratory rates (RR) were recorded daily at 0800, 1200, and 1700 h. The RT was measured by a digital clinical thermometer (ICO Technology, Barcelona, Spain; range, 32 to 43·9 °C; accuracy, ±0·1 °C). The RR was calculated as the number of flank movements during 60 s.
Feed intake was recorded daily by an electronic scale (model Fv-60 K; A&D Mercury PTY, Thebarthon, Australia; accuracy, ±20 g) and water consumption was daily measured by an electronic scale (model JC30; JC Compact, Cobos Precision, Barcelona, Spain; accuracy, ±10 g). Trays with saw dust were put below the drinking troughs and weighed once daily to take into account water wastes.
Milk yield (kg/d) of individual goats was recorded daily throughout the experiment by the electronic scale used for water consumption measurement. Milk composition was evaluated weekly. A milk sample of approximately 100 ml was collected and preserved with an antimicrobial tablet (Bronopol, Broad Spectrum Microtabs II, D&F Control Systems, San Ramon, CA) at 4 °C until analysis. Refrigerated milk samples were sent to the Laboratori Interprofessional Lleter de Catalunya (Allic, Cabrils, Barcelona, Spain) for the analyses of total solids (TS), fat, protein (N × 6·38), lactose, and SCC using Milkoscan (MilkoScan FT2 – infrared milk analyzer, Foss 260, DK-3400 Hillerød, Denmark) and an automatic cell counter (Fossomatic 5000, Foss Electric, Hillerød, Denmark) previously calibrated for goat milk.
Blood sampling and microarrays
At d 35, blood samples were collected in 10-ml vacutainers containing EDTA (BD Diagnostics, Franklin Lakes, NJ, USA) and kept on ice. The RNA was extracted from the whole blood immediately using the RiboPure-Blood Kit (Thermo Fisher Scientific, Madrid, Spain). The integrity of the total RNA was assessed by Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), and the RIN averaged 8·51 ± 0·23. The extracted RNA was frozen at −80 °C until analysis.
For microarrays, the 24 000 transcript Affymetrix GeneChip Bovine Genome Array and the 3’ IVT Express Kit (Affymetrix, Santa Clara, CA) were used. Five μg of total RNA from each sample were first reverse-transcribed to the single-stranded cDNAs using a T7 promoter-oligo(dT) primer. The double-stranded cDNA was then synthesized using DNA polymerase and RNase H, and used as templates for the in vitro transcription to generate multiple copies of biotin-modified aRNA. The full-length biotinylated aRNA was fragmented into 35- to 200-base fragments and then hybridized to GeneChip Bovine Genome Arrays for 16 h at 45 °C in a rotating Affymetrix GeneChip Hybridization Oven 320. After hybridization, arrays were washed and stained in an automated Affymetrix GeneChip Fluidic Station F450. The arrays were scanned with an Affymetrix GeneChip Scanner 3000 and the images quantified using Affymetrix GeneChip Operating Software.
Statistical analyses
Physiological and performance data
Data were analyzed by the PROC MIXED for repeated measurements of SAS v. 9·1·3 (SAS Inst. Inc., Cary, NC, USA). The statistical mixed model contained the fixed effect of environmental treatment (TN vs. HS), measuring day (1 to 35), and the random effect of the animal (1 to 8), the interaction (treatment × day), and the residual error. Differences between least squares means were determined with the PDIFF option of SAS.
Microarray gene expression data analysis
Computational and statistical analyses were carried out using Bioconductor (http://www.bioconductor.org/) packages of R software (version 3.0.3). The gene expression profiles (CEL format) of the 8 chips were converted into expression values using the Microarray Suite 5·0 (MAS5) function of the Affy package of R. The raw data were background corrected and normalized using log2-transformation. To filter out uninformative data, the Affy Absent/Present algorithm was run and non-expressed transcripts were excluded. The selection of differentially expressed transcripts was based on Student's t-test for comparison of means for each transcript between HS and TN groups. The false discovery rate for differentially expressed transcripts was controlled according to the Benjamini-Hochberg procedure (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) with an adjusted P < 0·05.
Functional bioinformatics analysis using the dynamic impact approach
The Affy probeset IDs were transformed to Entrez Gene IDs using the bovine.db package of Bioconductor and DAVID program (https://david.ncifcrf.gov). The obtained Entrez Gene IDs were submitted for the functional analysis of DEG by the Dynamic Impact Approach (DIA; Bionaz et al. Reference Bionaz, Periasamy, Rodriguez-Zas, Hurley and Loor2012), which relies on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database. The DIA calculates the ‘impact’ and the ‘flux’ (i.e. direction of the impact, increase or decrease) using the entire list of DEG mapped to the corresponding biological pathways in the KEGG database. Thus, the change in flux of a metabolic or signaling pathway is determined by the significant change (i.e. P value) and the magnitude of the change (i.e. by including the fold change) for proteins involved in the pathway. For the current analysis, the standard settings were used: at least 4 genes or 20% of the genes (whichever bigger) of each pathway were represented on the array.
Results and discussion
Effects of heat stress on performance
The effects of HS on RT and RR during the experimental period are shown in Fig. 1. Goats exposed to HS had the maximum values of RT and RR during the first 3 d and then decreased (P < 0·05), but remained greater (P < 0·001) than the TN throughout the experiment. The greatest values of RT and RR were observed in the HS goats at 17·00, the increases being 1 °C and 3·3-fold (P < 0·001) on average when compared to TN at 17·00 throughout the experiment. Pugh & Baird (Reference Pugh and Baird2012) reported that reference RR for adult goat ranges between 15 and 30 breaths/min. The RR values in our HS goats were greater that these reference values at all day time points (08·00, 12·00, and 17·00) throughout the 35 experimental days.

Fig. 1. Rectal temperature and respiration rate throughout the day (08·00, 12·00, and 17·00) in Murciano-Granadina dairy goats under thermal neutral (TN; n = 4) and heat stress (HS; n = 4) conditions in mid-lactation. 13·5% FCM = kg of milk yield × [0·432 + 0·162 × (fat %)].
On average, DM intake decreased by 29% in HS (P < 0·001) when compared to TN goats (Table 1). In contrast, water consumption increased 69% under HS conditions. Obtained results agree with those reported for the same breed of dairy goats in late-lactation under similar HS conditions (Hamzaoui et al. Reference Hamzaoui, Salama, Albanell, Such and Caja2013) and heat-stressed dairy cows (Wheelock et al. Reference Wheelock, Rhoads, VanBaale, Sanders and Baumgard2010; Gao et al. Reference Gao, Guo, Quan, Nan, Sanz-Fernández, Baumgard and Bu2017).
Table 1. Lactational performance of Murciano-Granadina dairy goats under thermal neutral (TN; n = 4) and heat stress (HS; n = 4) conditions in mid-lactation

1: Fat corrected milk
Compared to TN, milk yield, fat-corrected milk, milk fat, and milk protein of HS goats decreased (P < 0·001) by 8, 15, 12, and 13%, respectively (Table 1). Goats in the current experiment were in mid lactation and milk production losses were greater than those observed by Hamzaoui et al. (Reference Hamzaoui, Salama, Albanell, Such and Caja2013) in late-lactating goats. The negative effects of HS on the lactational performance of dairy cows, goats, and sheep are well known and are usually attributed to the decline in feed intake and direct effect on mammary gland (Baumgard & Rhoads, Reference Baumgard and Rhoads2013; Salama et al. Reference Salama, Caja, Hamzaoui, Such, Albanell, Badaoui, Loor, Villalba and Manteca2016).
By d 35, HS goats were still having greater (P < 0·001) rectal temperature and respiration rate (+0·69 °C and 1·5 fold, respectively) and were producing less amounts of milk. This clearly indicates that at d 35, goats were under significant HS when blood samples were collected for microarray analyses.
Identification of differentially expressed genes in response to HS
Gene expression was evaluated in the total blood cells, but the signal intensity of globin genes was low and did not affect the detection of gene expression. Among the 24 128 probes contained in the GeneChip, 14 316 probes were filtered out due to absent or very low expression levels. The remaining 9,812 transcripts were subjected to a Student's t-test for comparison of expression means. Benjamini-Hochberg corrected transcripts (P < 0·05) were used for further analysis. After conversion of transcripts to their related corresponding genes, the statistical analysis revealed that HS resulted in 143 differentially expressed genes (DEG; 55 upregulated and 88 downregulated). Furthermore, the fold change (FC) among DEG was modest (only 42 genes had FC ≥1, Tables 2 and 3). This could be due to the fact that blood samples were collected at d 35 of HS, and it could be possible that more changes in gene expression would have been detected if samples were collected at the first 3–4 d of HS. As shown in Fig. 1, HS goats experienced the highest RR and RT values during the first few days of HS and partially recovered thereafter, but differences remained significant until day 35. So, transcriptomics evaluation at day 35 reflects the chronic effect of heat stress.
Table 2. Top upregulated genes of blood cells in heat-stressed Murciano-Granadina dairy goats for 35 d compared with thermal neutral counterparts

Table 3. Top downregulated genes of blood cells in heat-stressed Murciano-Granadina dairy goats for 35 d compared with thermal neutral counterparts

Functional bioinformatics analysis of differentially expressed genes
The functional analyses using the 143 DEG revealed that 31 biological pathways (3 upregulated and 28 downregulated) were impacted by HS (Fig. 2). The upregulated pathways were involved in apoptosis and cell death (i.e. pyrimidine metabolism, purine metabolism, drug metabolism – cytochrome P450 and RNA transport pathways). Some of the 28 downregulated pathways were mainly related to immune cell proliferation and migration (i.e. leukocyte transendothelial migration, cell adhesion molecules, RNA transport, hematopoietic cell lineage and ECM-receptor interaction), lipid metabolism (i.e. adipocyte signaling pathway and PPAR signaling pathways), and tissue repair (i.e. PPAR signaling pathway, arginine and proline metabolism and phagosome).
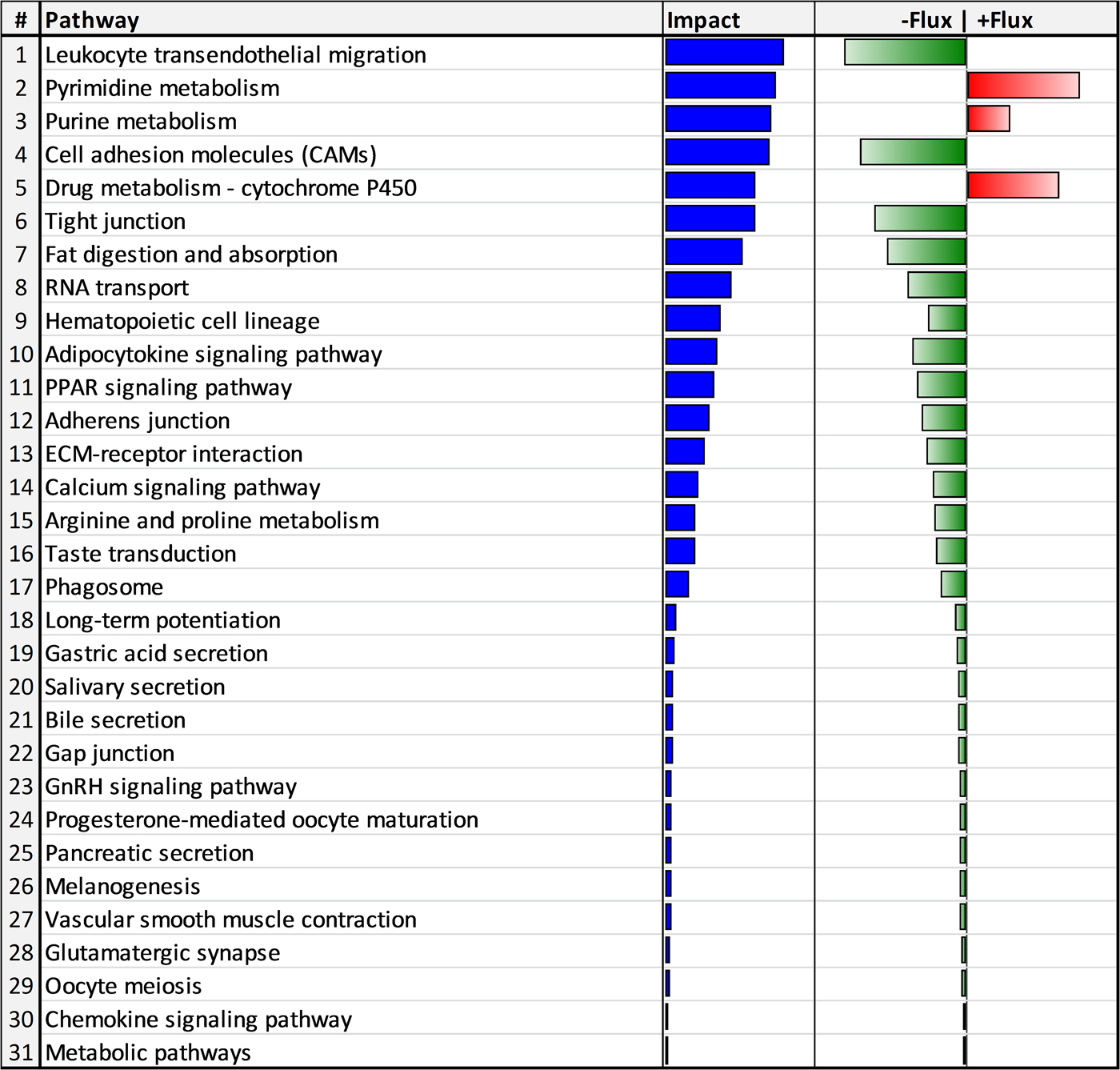
Fig. 2. Biological pathways impacted by heat stress in blood cells of Murciano-Granadina dairy goats in mid lactation. Blue bars denote the impact, whereas red and green bars denote the direction of the impact (red = increase; green = decrease).
Transcriptional activity and cell death
A strong activation of pyrimidine and purine pathways (pathways 2 and 3) was observed jointly with xenobiotic degradation via cytochrome p450 (pathway 5) and a downregulation of RNA transport (pathway 8) in HS goats (Fig. 2). The activation of pyrimidine and purine pathways in the current study is consistent with the upregulation of these pathways by HS in other studies involving plasma metabolomics (Ippolito et al. Reference Ippolito, Lewis, Yu, Leon and Stallings2014), transcriptomics of different tissues (liver, heart, kidney and lung) in rats (Stallings et al. Reference Stallings, Ippolito, Rakesh, Baer, Dennis, Helwig, Jackson, Leon, Lewis and Reifman2014), and transcriptomics of blood in zebu cattle (Kolli et al. Reference Kolli, Upadhyay and Singh2014). The final end-product of the up-regulation of pyrimidine and purine pathways is urea, which has been reported to increase in both blood and urine of heat-stressed dairy cows (Wheelock et al. Reference Wheelock, Rhoads, VanBaale, Sanders and Baumgard2010; Gao et al. Reference Gao, Guo, Quan, Nan, Sanz-Fernández, Baumgard and Bu2017).
Heat stress has been shown to increase cell oxidative stress and the accumulation of free radicals and peroxides, causing protein unfolding and cellular damage in different animal models, including sheep (Chauhan et al. Reference Chauhan, Celi, Leury, Clarke and Dunshea2014) and goats (Di Trana et al. Reference Di Trana, Celi, Claps, Fedele and Rubino2006). The activation of cytochrome p450 pathway by HS in the current study could be related to the fact that cytochrome p450 enzymes are necessary for the cell to get rid of the free radicals and peroxides.
Although there was no change in the expression of heat-shock protein genes at d 35 of HS, we observed an upregulation in the NUP88 (Nucleoporin 88) protein coding gene, which is related to the cellular response to heat stress pathway and regulates the HSF-1-mediated heat shock response. Additionally, several pro-apoptotic protein-coding genes such as GAS2, SCYL2, RNF149 and caspases were upregulated (Table 1).
As HS inhibits DNA, RNA and protein synthesis, the downregulation of the RNA transport pathway (Fig. 2) could be related to the lower synthetic capacity observed under HS conditions (reviewed by Collier et al. Reference Collier, Collier, Rhoads and Baumgard2008). This could be due to protein aggregation, which is considered a mitigating effect of protein misfolding. In this sense, this response could explain the greater number of downregulated genes in various HS experiments in different tissues and organisms (current study; Kolli et al. Reference Kolli, Upadhyay and Singh2014; Stallings et al. Reference Stallings, Ippolito, Rakesh, Baer, Dennis, Helwig, Jackson, Leon, Lewis and Reifman2014). In accordance with this notion, mammary gland cells have lower protein synthetic capacity in heat-stressed goats (Hamzaoui et al. Reference Hamzaoui, Salama, Albanell, Such and Caja2013) and the EIF4EBP1 gene (inhibitor of protein synthesis) is upregulated in bovine mammary cells under heat stress (Salama et al. Reference Salama, Duque, Shahzad and Loor2015).
Proliferation and migration of immune cells
White blood cells or leukocytes are a diverse group of cell types that mediate the body's immune response. Leukocytes have a common origin in hematopoietic stem cells that differentiate into diverse functional cell types (Seita & Weissman, Reference Seita and Weissman2010). They circulate through the blood and lymphatic system and are recruited to sites of tissue damage and infection (Geissmann et al. Reference Geissmann, Auffray, Palframan, Wirrig, Ciocca, Campisi, Narni-Mancinelli and Lauvau2008). Immune cells have different lifespans and are continuously replaced. As the hematopoietic cell lineage (pathway 9) was downregulated by HS (Fig. 2), a reduction in the generation and differentiation of new leukocytes is expected, hence, negatively impacting the immune response. Results agree with those of Lacetera et al. (Reference Lacetera, Bernabucci, Scalia, Ronchi, Kuzminsky and Nardone2005) who observed a reduced proliferation of peripheral blood mononuclear cells collected from HS dairy cows in response to mitogenic stimulation. Additionally, heat stress reduced the proliferation of lymphocytes in sheep (Sevi & Caroprese, Reference Sevi and Caroprese2012).
The leukocyte transendothelial migration pathway (pathway 1), related to the movement from the blood to tissues, was also downregulated by HS (Fig. 2). In fact, both innate and adaptive immune responses are not acquired as long as leukocytes do not cross blood vessels (Muller, Reference Muller2011). This process occurs through diapedesis, in which the leukocytes have to go through 4 steps: rolling, activation, adhesion and finally, locomotion through the tight junctions or through the endothelial cell itself. This leukocyte movement is controlled by cell adhesion molecules (pathway 4), their ligands, and the interaction with extracellular matrix receptors (pathway 13) in endothelial cells (Etzioni, Reference Etzioni1996). As shown in Fig. 2, both pathways were downregulated by HS. In addition, for an efficient transmigration, the Ca2+ signaling transducer is needed for the loss of cellular junctions (Huang et al. Reference Huang, Manning, Bandak, Ratau, Hanser and Silverstein1993). The downregulation in cell adhesion molecules (pathway 4) and Ca2+ signaling (pathway 14; Fig. 2) together with the leukocyte transendothelial migration capacity (pathway 1; Fig. 2), clearly indicate a lower ability of leukocyte migration in HS animals that likely would increase the susceptibility to infectious diseases. These results could explain the slower somatic cell migration from blood to LPS-challenged mammary glands in HS compared with TN lactating dairy goats (Love, Reference Love2015).
Lipid metabolism of blood cells
A strong relationship exists between lipid composition of immune cells and their functions (Calder, Reference Calder2008). In the present study, we observed an altered lipid metabolism as HS downregulated the signaling pathways of peroxisome proliferator-activated receptor gamma (PPARγ) and adipocytokine signaling (pathways 10 and 11, respectively; Fig. 2).
PPARγ belongs to the nuclear receptor superfamily and acts as a lipid sensor in various tissues and cell types to modulate gene expression by binding DNA. PPARγ controls many lipid metabolism-related genes (Chawla et al. Reference Chawla, Barak, Nagy, Liao, Tontonoz and Evans2001). Therefore, its downregulation by HS in the present study could be associated with a dysregulation in lipid formation and metabolism, leading to significant defects in immune cells function (Calder, Reference Calder2008).
Adipocytokines (leptin and adiponectin) derive from the adipose tissue or the immune cells that infiltrate fat depots. In the current study, the downregulation of the adipocytokine signaling pathway (presumably leptin signaling) by HS could have a negative effect on immune cell function. Leptin has a diversity of physiologic roles associated with metabolism and energy homeostasis, and transmits information on energy availability and immune capability (Matarese et al. Reference Matarese, Moschos and Mantzoros2005). Leptin regulates adaptive and innate responses both in normal and pathological conditions (Fernández-Riejos et al. Reference Fernández-Riejos, Najib, Santos-Alvarez, Martín-Romero, Pérez-Pérez, González-Yanes and Sánchez-Margaret2010). Furthermore, altered levels of leptin are related to diverse inflammatory conditions (Fantuzzi, Reference Fantuzzi2005), affecting cell–cell signaling, thymic homeostasis, hematopoietic cell lineage, and cytokine production (Matarese et al. Reference Matarese, Moschos and Mantzoros2005).
Inflammatory response and tissue repairing
Heat stress induces an inflammatory state as observed by the increase of TNF-α and IL-6 in long-term heat-stressed dairy cows (Min et al. Reference Min, Zheng, Zhao, Cheng, Yang, Zhang, Yang and Wang2016). The mononuclear phagocytic system is part of innate immunity and, in the current study, the phagosome pathway was downregulated by HS (pathway 17; Fig. 2). According to Murray & Wynn (Reference Murray and Wynn2011), macrophages mediate defense of the host from a variety of pathogens, have anti-inflammatory function, regulate wound healing by engulfing pathogens and apoptotic cells, and produce immune effector molecules. Through their ability to clear pathogens and instruct other immune cells, macrophages are essential for protecting the organism, but also contribute to the origin and development of inflammatory diseases.
Because both are essential for platelet activation and aggregation, the downregulation of both PPARγ and calcium signaling compromises the repair of damaged tissues (Razzell et al. Reference Razzell, Evans, Martin and Wood2013). Furthermore, it is well known that PPARγ inhibits the expression of proinflammatory genes and also activates arginases (Munder, Reference Munder2009) that have been shown to decrease the magnitude of inflammatory responses and promote adequate wound healing (Murray & Wynn, Reference Murray and Wynn2011). Accordingly, we observed a downregulation of arginine and proline metabolism pathway (pathway 15; Fig. 2) by HS.
Conclusion
Heat stress negatively affected milk yield and milk components in dairy goats, and resulted in a dramatic increase in rectal temperature and respiratory rate, especially during the first few days of heat stress. The impaired lactational performance was accompanied by immune-dysfunction. The decrease in the hematopoiesis and leukocyte diapedesis might compromise the innate and the adaptive immune response. In addition, the disruption of lipid metabolism would significantly affect immune cell functionality due to altered PPARγ activation and, thus, an inadequate modulation of gene expression. Overall, a lower capacity of phagocytosis not only would compromise the defense of the organism against an eventual infection, but could also lead to a pathological inflammatory state with a decrease in the capacity of platelet activation and aggregation for tissue repair.
This work is part of a research project funded by the Spanish Ministry of Economy and Finance (Program I + D + I oriented to Society challenges; Project AGL2013-44061-R) and was also supported by a research scholarship to Alexandra Contreras Jodar from the Spanish Ministry of Economy and Competitiveness (Reference BES-2012-052602). The authors are also grateful to the team of SGCE (Servei de Granges i Camps Experimentals) of the UAB for the care of the animals.







