At birth the gastrointestinal tract is sterile, but it becomes rapidly colonized by microbes. The microflora changes in the first weeks after birth and its composition is influenced by, among other things, the type of feedingReference Fanaro, Chierici, Guerrini and Vigi1. Breast-fed infants are colonized predominantly with bifidobacteria and lactobacilli, whereas the microflora of formula-fed infants is composed of less bifidobacteria and lactobacilli and more anaerobic strains, such as bacteroides and clostridiaReference Harmsen, Wildeboer-Veloo, Raangs, Wagendorp, Klijn, Bindels and Welling2–Reference Penders, Thijs, Vink, Stelma, Snijders, Kummeling, van den Brandt and Stobberingh4. When solid foods are introduced into the diet, each individual develops a unique and complex microfloraReference Edwards and Parrett5. It has been shown in several studies that the intestinal bacterial flora plays a crucial role in the generation of an appropriate functioning immune systemReference Sudo, Sawamura, Tanaka, Aiba, Kubo and Koga6–Reference Macpherson and Harris8. Furthermore, an association between the composition of intestinal microflora and allergies has been observed in a study comparing microflora from allergic and non-allergic children. Allergic children were less often colonized with lactobacilli and bifidobacteria than non-allergic childrenReference Bjorksten, Naaber, Sepp and Mikelsaar9.
The association between composition of the microflora and allergies has brought up the idea that modulation of the microflora could beneficially influence the immune systemReference Perdigon, Locascio, Medici, Pesce de Ruiz Holgado and Oliver10–Reference Isolauri, Sutas, Kankaanpaa, Arvilommi and Salminen12. Supplementation of infant formulas with probiotics might alter the composition of the microflora of formula-fed infants in such a way that it resembles that of breast-fed infants. This may reduce the chance of developing an allergy. Evidence for beneficial effects of probiotics on allergy has been found in experimental animal modelsReference Murosaki, Yamamoto, Ito, Inokuchi, Kusaka, Ikeda and Yoshikai13–Reference Shida, Takahashi, Iwadate, Takamizawa, Yasui, Sato, Habu, Hachimura and Kaminogawa18. In human subjects, there are several studies that have shown improvement or prevention of atopic eczema in infants who received Lactobacillus rhamnosus strain GG (LGG)Reference Kalliomaki, Salminen, Poussa, Arvilommi and Isolauri19–Reference Rosenfeldt, Benfeldt, Nielsen, Michaelsen, Jeppesen, Valerius and Paerregaard21. However, another study showed that neither L. rhamnosus nor LGG could improve atopic eczemaReference Brouwer, Wolt-Plompen, Dubois, van der Heide, Jansen, Hoijer, Kauffman and Duiverman22. In addition, L. acidophilus did not prevent the development of atopic eczema in atopic infants. Notably, in infants who received this probiotic the rate of sensitization was higherReference Taylor, Dunstan and Prescott23. These studies show that the beneficial effects of probiotics on atopic manifestations are strain-dependent and that modulation of the immune system does not always lead to allergy prevention and can also increase allergic sensitization.
One of the mechanisms that could explain the beneficial effects of probiotics on allergies is the ability of some probiotic strains to stimulate T-helper (Th)-1 immunity, thereby reducing Th2 responses and thus (Th2-mediated) allergiesReference Matsuzaki24–Reference Pohjavuori, Viljanen, Korpela, Kuitunen, Tiittanen, Vaarala and Savilahti26. However, stimulation of Th1 immunity might aggravate Th1-mediated autoimmune diseases. We have previously demonstrated that L. casei Shirota (LcS), indeed, aggravated experimental autoimmune encephalomyelitis (EAE) in Lewis ratsReference Baken, Ezendam, Gremmer, de Klerk, Pennings, Matthee, Peijnenburg and van Loveren27. Similar results were found for L. reuteri in a mouse model for EAEReference Maassen, van Holten, Balk, Heijne den Bak-Glashouwer, Leer, Laman, Boersma and Claassen28. These experimental data suggest that certain probiotic strains can induce adverse effects.
Probiotics are considered to be safe due to their long-term use in the adult population without any adverse effects. The adverse effects on experimental autoimmunity, however, indicate that side effects could be possible. Infants might be more vulnerable to the adverse effects of probiotics, especially since the developing immune system is more susceptible to immunomodulation, as has been shown previously for dexamethasone. Neonatal treatment increased the severity and incidence of EAE in adult lifeReference Bakker, Kavelaars, Kamphuis, Cobelens, van Vugt, van Bel and Heijnen29. This could imply that infants are a group at risk. Although the short-term safety of probiotics in infants has been investigatedReference Kullen and Bettler30, long-term effects have not yet been investigated.
In this paper we have investigated effects of LcS administered early in life (during lactation) on the development of respiratory allergy (in mice) or EAE (in rats), which was induced at the adult age. To compare the consequences of LcS administration early in life on allergy development at an adult age, similar experiments were performed in mice that received LcS as adults. Effects of LcS on EAE were compared with our previous study, in which rats were exposed to LcS as adultsReference Baken, Ezendam, Gremmer, de Klerk, Pennings, Matthee, Peijnenburg and van Loveren27.
Materials and methods
Bacteria
LcS, isolated from a commercially available drink (Yakult™, Yakult Nederland BV, Almere, The Netherlands), was cultured for 72 h at 30°C under anaerobic conditions in Man Rogosa Sharpe broth (CM359; Oxoid, Haarlem, The Netherlands). Thereafter, bacteria were washed twice with saline (0·9 % NaCl) containing 1 mg/ml peptone (saline/peptone) and resuspended in saline/peptone to a final concentration of 2 × 109 colony forming units (CFU)/ml. The number and viability of the lactobacilli were determined by aerobic culturing on Man Rogosa Sharpe plates (CM361; Oxoid) for 72 h.
Animals
Female and male BALB/c mice (6–8 weeks old) were obtained from our own breeding colony. For experiments where LcS administration started during lactation, 2-week old female and male BALB/c mice born to pregnant BALB/c mice obtained from our own breeding colony were used. Mice were bred specific pathogen free and kept under conventional conditions. The breeding colony of the animals was pre-screened/monitored for endogenous pathogenic viruses and bacteria and was found negative. For the autoimmunity experiments 2-week old female and male Lewis rats (LEW/HanHsD) born to pregnant Lewis rats obtained from Harlan (Horst, The Netherlands) were used. Mice and rats were fed Hope Farms chow pellets (Woerden, The Netherlands) and water ad libitum. The experimental set-up of all experiments was examined and agreed upon by the institute's Ethical Committee on Experimental Animals, according to national legislation.
Experimental design respiratory allergy
To study the effects of LcS exposure early after birth, young suckling pups were used. After birth, the pups were randomized and cross-suckled between the dams. Each nest contained the same number of pups with an equal male:female ratio. LcS was given via oral gavage and administration started when the mice were 2 weeks old until the end of the experiment. Mice received 2–4 × 108 CFU LcS or saline/peptone alone (controls) daily in a volume of 100 μl, except for the first week when 2–4 × 108 CFU was administered in 50 μl. At weaning (21 d after birth) mice were taken away from their mothers and housed in the experimental groups. Mice were sensitized and challenged with ovalbumin (eight females and eight males per experimental group) as described earlierReference Smit, van Loveren, Hoekstra, Nijkamp and Bloksma31 with some minor modifications. Mice were sensitized twice, first when they were 6 weeks old (day 0) and for a second time on day 14, by intraperitoneal injection with 10 mg ovalbumin (grade V; Sigma-Aldrich, Zwijndrecht, The Netherlands) adsorbed onto 2·25 mg aluminium hydroxide (AlumInject, Pierce, Rockford, IL, USA) in saline. Control mice (four females and four males per control group) were sensitized with saline. Mice were challenged on days 35, 38 and 41 by inhalation of ovalbumin or saline aerosols in a plexiglass exposure chamber for 20 min. Aerosols were generated by nebulizing a solution of 10 mg/ml ovalbumin in saline or saline alone using a nebulizer. At day 43, mice were killed and blood was collected, clotted and serum was collected for determination of ovalbumin specific Ig. Spleens were collected and cell suspensions were prepared for ex vivo stimulation with ovalbumin.
Adult (6–8 weeks old) female (eight for the experimental and four for the control group) and male (eight for the experimental and four for the control group) mice were used to compare effects of early administration of LcS on allergy. Oral gavage with LcS started 1 week before sensitization with ovalbumin. The sensitization and challenge protocol was the same as described earlier.
Bronchoalveolar lavage
Bronchoalveolar lavage was performed by flushing the lungs with 1 ml sterile PBS. Bronchoalveolar lavage fluid was centrifuged at 1200 rpm for 10 min. Cell pellets were used for determination of total cell number and for cytospin preparations. Cytospins were stained with May-Grünwald (Fluka, Seelze, Germany) and Giemsa (Merck, Darmstadt, Germany) and on each preparation 400 cells were counted.
Culture of spleen cells
Spleens were collected and single-cell suspensions were prepared under aseptic conditions by pressing the spleen through a sterile 70 μm nylon cell strainer. Cells were washed in RPMI 1640 (Gibco, Life Technologies, Breda, The Netherlands) with 5 % heat inactivated fetal calf serum (PAA, Linz, Austria), 100 U/ml penicillin and 100 μg/ml streptomycin (standard medium) (10 min, 4°C, 300 g) and resuspended in 1 ml standard medium with 10 % fetal calf serum. Cell suspensions (2 × 106 cells/ml, 75 μl per well) were cultured for 96 h with 100 μg/ml ovalbumin (75 μl per well). Supernatants were collected for cytokine measurements.
ELISA specific for ovalbumin IgE and IgG1
Specific ovalbumin IgE and IgG1 titres in sera were determined by ELISA. Incubations were followed by extensive washing on an automatic plate washer with PBS containing 0·1 % Tween-20. To measure ovalbumin-specific IgE, 96-well plates (Nunc-Immuno Plate, Nunc A/S, Roskilde, Denmark) were coated overnight at 4°C with 2 μg/ml rat-anti-mouse IgE (rαm IgE; Zymed Laboratories, San Francisco, CA, USA) diluted in sodium carbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were blocked by adding 0·05m-Tris buffered saline with 1 % bovine serum albumin, pH 8 (Sigma) for 1 h at 37°C. Thereafter, serial dilutions of mouse serum samples and a pooled positive reference serum were incubated for 1 h at 37°C. All dilutions were done in blocking buffer plus 0·05 % Tween-20. Then, wells were incubated for 1 h at 37°C with digoxigenin-3-O-succinyl-ɛ-aminocaproic acid (DIG)-conjugated ovalbumin. The coupling of ovalbumin to DIG (molar mixture 1:10) was performed according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). Then, wells were incubated with anti-DIG Fab fragments labelled with peroxidase (Roche Diagnostics) for 2 h at 37°C. Plates were incubated with tetramethyl benzidine (TMB) substrate and the enzyme reaction was stopped with 2 m-H2SO4 and absorbance was read at 450 nm.
To measure ovalbumin-specific IgG1, wells were coated overnight at 4°C with 10 μg/ml ovalbumin/ml PBS (grade V; Sigma). Blocking buffer was added and wells were incubated for 1 h at 37°C. Thereafter, serial dilutions of mouse serum samples and a pooled positive reference serum were added to the wells and incubated for 2 h at room temperature (RT). Biotinylated rat-anti-mouse IgG1 (Zymed Laboratories) was added and wells were incubated for 1·5 h at RT, followed by incubation with poly-horseradish peroxidase labelled streptavidin for 45 min at RT. Then plates were incubated with TMB substrate and the enzyme reaction was stopped with 2 m-H2SO4 and absorbance was read at 450 nm. Extinction values of the positive reference serum were used to calculate the amount of IgG1 and IgE in the samples and extinction values were expressed as arbitrary units.
Bioplex for cytokines
Th1 and Th2 cytokines were measured in supernatants of spleen cells that were cultured with ovalbumin. Cytokine levels were detected with a Bioplex 5-plex cytokine assay kit that contained antibodies specific for IL-4, IL-5, IL-10, IL-13 and interferon (IFN)-γ (Biorad Life Science, Hercules, CA, USA) according to the manufacturer's instructions. Cytokine measurements were performed on a Luminex® (Biorad Life Science) and Luminex software was used to calculate the amount of cytokines (pg/ml supernatant). The range of detection for each cytokine was: for IL-4 0·18–424 pg/ml; for IL-5 1·3–1051 pg/ml; for IL-10 14·6–3556 pg/ml; for IFN-γ: 0·52–3566 pg/ml; for IL-13: 4·3–30 782 pg/ml.
Experimental design for experimental autoimmune encephalomyelitis
After birth, the pups were randomized and cross-suckled between the dams. Each nest contained the same amount of pups with an equal male:female ratio. Oral administration of LcS started when the rats were 2 weeks old. Rats received 1–2 × 109 CFU daily in a volume of 500 μl. Control rats received 500 μl saline/peptone daily. At weaning (21 d old), rats were taken away from their mothers and housed in the experimental groups.
Acute EAE was induced at the age of 7 weeks as described previouslyReference Hendriks, Alblas, van der Pol, van Tol, Dijkstra and de Vries32. Rats (eight females and eight males per experimental group) were injected subcutaneously in the left ankle. Males were dosed with an emulsion (100 μl) containing 20 μg guinea pig myelin basic protein (MBP; Sigma), 500 μg Mycobacterium tuberculosis type H37RA (Difco, Detroit, MI, USA), 50 μl complete Freund's adjuvant (Difco) supplemented with saline (0·9 % NaCl) to reach a volume of 100 μl. Females were dosed with the same solution (80 μl). The dose for immunization with MBP is dependent on body weight and females were lighter than males at the age of 7 weeks. Control rats (four females and four females per control group) were not immunized and received either LcS or saline/peptone. After induction of EAE, body weight was recorded daily. Also, neurological signs were scored daily and graded from 1 to 5: 0, no clinical signs; 0·5, loss of tonicity in distal half of tail; 1, flaccid tail; 1·5, unsteady gait; 2, partial hind limb paralysis; 2·5, complete hind limb paralysis; 3, paralysis of the complete lower part of the body up to the diaphragm; 4, paraplegia; 5, death due to EAE. Rats were killed 27 d after induction of EAE. The clinical score is expressed as the cumulative clinical score per rat and was calculated by cumulating the daily clinical scores. In addition, duration of EAE was defined by the number of days that rats display clinical signs of EAE per animal. The cumulative disease index was defined as the sum of the cumulative daily scores per group divided by the amount of days that clinical disease signs were observed in the group.
Power calculations
The number of animals per experimental group was calculated with a power analysis using data from previous experiments. ExpDesign software, a freely available tutorial program, was used for power analysisReference van Wilgenburg, van Schaick Zillesen and Krulichova33. The P value was set at < 0·05 and the number of animals per group was considered to be sufficient when the power was >0·8.
Statistical analysis
In both the allergy and the EAE experiments, both sexes were included in the experiments, because at initiation LcS administration was performed in litter. Previously, it has been demonstrated that females are more susceptible for the sensitization with ovalbuminReference Melgert, Postma, Kuipers, Geerlings, Luinge, van der Strate, Kerstjens, Timens and Hylkema34 and immunization with MBPReference Bebo, Schuster, Vandenbark and Offner35, Reference Bebo, Schuster, Vandenbark and Offner36. In addition, the immunization with MBP was based on body weight and since females were lighter than males they were immunized with less MBP, because overdosing can be lethal. The statistical analysis was therefore done in males and females separately.
Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, IL, USA). Data are presented as means with their standard errors. The P value for statistical significant differences was set at < 0·05. When data were not normally distributed they were 10log transformed prior to analysis.
To determine statistically significant differences in cell number in lung lavage fluid, a one-way ANOVA was used. The Levene test was used for homogeneity of variance. When the variance was not equal, the Games–Howell test was used as a post hoc test, otherwise Bonferroni's post hoc test was used. Statistical significant differences in ovalbumin-specific cytokine production and ovalbumin-specific IgE and IgG1 levels were determined with a two-tailed Student's t test comparing the two experimental groups that were sensitized and challenged with ovalbumin. Statistical difference of clinical symptoms and duration of symptoms in the EAE experiment between the experimental groups that were immunized with EAE were determined with a one-tailed Mann–Whitney test.
Results
Respiratory allergy
Bronchoalveolar lavage: cell counts and differentiation. Table 1 shows the cell counts in lung lavage fluid when LcS administration started during lactation. Ovalbumin sensitized and challenged female and male mice showed significantly increased numbers of total cells compared with controls. This increase was predominantly due to an increase in eosinophils and lymphocytes. Influx of eosinophils and lymphocytes after challenge with ovalbumin was more pronounced in female mice than in male mice. In sensitized female mice that received LcS, total cell number and eosinophils were higher, but these differences were not significantly different from sensitized mice that received the vehicle. The observed increase in lymphocytes, however, was significantly different from the sensizited group (P = 0·026). In males, total cell number was slightly higher in sensitized mice that received LcS, but this was not significant. The number of lymphocytes was not different between both sensitized groups. A significant increase in eosinophils was observed in sensitized mice that received LcS (P = 0·018).
Table 1 Number of cells in lung lavage fluid in mice that received Lactobacillus casei Shirota (LcS) from lactation onward§
(Mean values with their standard errors)
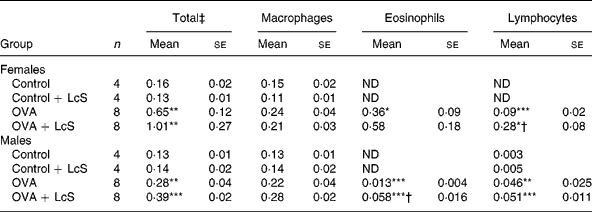
Mean values were significantly different from those of the control group: *P < 0·05; **P < 0·01; ***P < 0·001. Data were log transformed before statistical analysis. To determine statistical significance a one-way ANOVA with Bonferroni's post hoc test or Games-Howell (when variance was not equal in the Levene's test) post hoc test was used.
Mean values were significantly different from those of the ovalbumin (OVA) group: †P < 0·05.
‡ Cell numbers are expressed in 106 cells.
§ For details of animals and procedures, see Materials and methods.
ND, not detected.
Table 2 shows the cell counts in lung lavage fluid when LcS was administered to adult mice. In sensitized females, total cell number, macrophages, eosinophils and lymphocytes were significantly higher than the control group. Administration of LcS did not affect cell number in sensitized mice. The total cell number in control males was higher than male controls in the other experiment (Table 1) and in the controls that received LcS. A significant increase in eosinophils was found in both sensitized groups compared with the control group. Total cell number was significantly higher when compared with the controls that received LcS (P = 0·020 and P = 0·018 for sensitized males that received vehicle or LcS, respectively). Also in males, LcS had no effects on the inflammatory lung response.
Table 2 Number of cells in lung lavage fluid in mice that received Lactobacillus casei Shirota (LcS) at adult age‡
(Mean values with their standard errors)
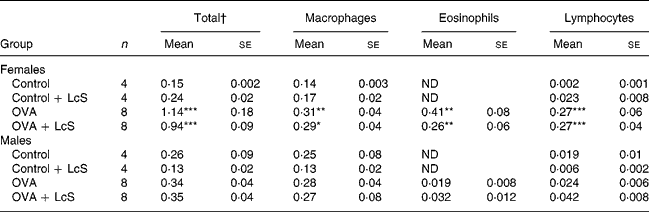
Mean values are significantly different from the control group: *P < 0·05; **P < 0·01; ***P < 0·001. Data were log transformed before statistical analysis. To determine statistical significance a one-way ANOVA with Bonferroni's post hoc test or Games-Howell (when variance was not equal in the Levene's test) post hoc test was used.
† Cell numbers are expressed in 106 cells
‡ For details of animals and procedures, see Materials and methods.
OVA, ovalbumin; ND, not detected.
Ovalbumin-specific cytokine production
To assess if cytokine profiles were affected by LcS administration, spleen cells were cultured with ovalbumin to detect specific cytokine production. In mice that received LcS from lactation onward, no differences in specific cytokine production could be detected (data not shown). In adult mice that received LcS, however, cytokine production was enhanced (Table 3). In females, all Th2 cytokines were increased in the LcS group. For IL-5 and IL-13 this was significant, for IL-4 (P = 0·089) and IL10 (P = 0·063) this was not. IFN-γ levels were comparable in both groups. In males, all Th2 cytokines were significantly higher in the LcS group. IFN-γ was also higher in this group but this was not significant (P = 0·09).
Table 3 Ovalbumin-specific cytokine production by spleen cells from mice that received Lactobacillus casei Shirota (LcS) at adult age‡
(Mean values with their standard errors)
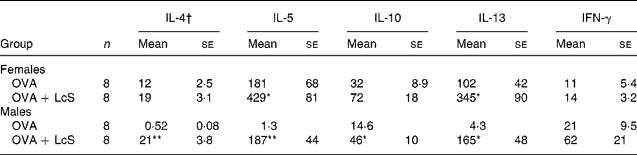
Mean values are significantly different from the ovalbumin (OVA) group: *P < 0·05; **P < 0·01 (to determine statistical significance a two-tailed Student's t test was used).
† Cytokines are expressed as pg/ml supernatant.
‡ For details of animals and procedures, see Materials and methods.
IFN, interferon.
Ovalbumin-specific IgG1 and IgE levels
Both ovalbumin-specific IgG1 and IgE titres were detectable after sensitization and challenge with ovalbumin. IgE titres were significantly higher in females than in males, IgG1 titres were similar. Administration of LcS either during lactation or when mice were adults did not affect IgG1 or IgE titres significantly (data not shown).
Experimental autoimmune encephalomyelitis
After immunization with MBP, the first clinical symptom that was observed was loss of tonicity in the tail. In males, there was no difference on the day of onset of EAE for vehicle-treated rats (between days 14 and 18) and LcS-treated rats (between days 15 and 21) after immunization. In Table 4 several clinical parameters are summarized. In vehicle-treated rats, six out of eight animals developed clinical symptoms, whereas all LcS-treated rats developed EAE. Other parameters, such as clinical score per rat, duration of symptoms and cumulative disease index, were not affected by LcS. In females, day of onset was similar in vehicle-treated rats (between days 12 and 18) and LcS-treated rats (between days 11 and 18). Table 4 shows that all females that received LcS developed clinical symptoms, while six of the eight rats that received vehicle developed EAE. Furthermore, after LcS administration, clinical score per animal and cumulative disease index were higher. The effect on clinical score was not significant however. In addition, LcS significantly increased the duration of symptoms, the disease symptoms lasted almost 3 d longer (P = 0·026).
Table 4 Summary of clinical parameters of experimental autoimmune encephalomyelitis (EAE)**
(Mean values with their standard errors)
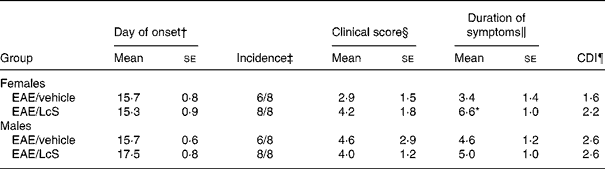
Mean values were significantly different from those of the EAE/vehicle group: *P < 0·05.
† The first day a rat displayed clinical symptoms for all rats with symptoms.
‡ The number of rats with clinical symptoms.
§ The cumulative clinical scores per rat at the end of the experiment.
‖ The number of days an animal displayed symptoms.
¶ The sum of the daily clinical scores for a group over a given number of days divided by the number of days, given as a cumulative disease index (CDI).
** For details of animals and procedures, see Materials and methods.
LcS, Lactobacillus casei Shirota.
Discussion
We have investigated the effects of administration of the probiotic LcS, starting during lactation, on the development of allergy and autoimmunity at an adult age. Early administration of LcS stimulated both allergic and autoimmune responses. Although effects were moderate, these data confirm that LcS has immunostimulatory properties.
When LcS was given during lactation, the inflammatory lung response was enhanced in males and females. Remarkably, when LcS administration started when mice were adults, there was no effect on the influx of inflammatory cells. The stimulatory effects of LcS were confined to the lungs, because no effect of ovalbumin-specific IgE was observed. This is in contrast with previous studies that demonstrated a reduction of serum IgE by LcSReference Shida, Takahashi, Iwadate, Takamizawa, Yasui, Sato, Habu, Hachimura and Kaminogawa18, Reference Yasui, Shida, Matsuzaki and Yokokura37. This discrepancy might be explained by the different models that were used and the different route of exposure. In a model for food allergy in transgenic (OVA-TCR) mice, LcS was injected intraperitoneallyReference Shida, Takahashi, Iwadate, Takamizawa, Yasui, Sato, Habu, Hachimura and Kaminogawa18, while in the present study LcS was given via the oral route. However, in mice that were sensitized to ovalbumin by intraperitoneal injection oral administration of LcS decreased ovalbumin-specific IgE levelsReference Yasui, Shida, Matsuzaki and Yokokura37. The observed effects on IgE were attributed to a shift in Th1/Th2 balance of cytokine production towards Th1. We could only detect effects on cytokine production in adult mice that received LcS and LcS did not induce a shift of the immune balance towards Th1, but stimulated predominantly Th2 cytokines and, to a lesser extent, Th1. The ability of probiotics to stimulate both Th1 and Th2 cytokines was previously shown for L. rhamnosus HN001 in a model for respiratory allergyReference Cross, Mortensen, Kudsk and Gill38.
Early administration of LcS, however, did not elicit different or more pronounced effects in the EAE model compared with adult administration. Previously, we have shown that LcS aggravated EAEReference Baken, Ezendam, Gremmer, de Klerk, Pennings, Matthee, Peijnenburg and van Loveren27. When LcS was given during lactation, an increase of incidence and duration of clinical symptoms in females was observed. In contrast, in males LcS increased the incidence of EAE, but did not affect clinical symptoms. It is important to note that the increase in incidence is an observational one, which is not tested statistically because the study was not powered to perform statistical analysis. These observations are in line with our previous studyReference Baken, Ezendam, Gremmer, de Klerk, Pennings, Matthee, Peijnenburg and van Loveren27. Our data suggest that LcS in general adjuvates both Th1 and Th2 responses, rather than skewing the immune system towards Th1. Stimulation of the immune response by LcS has been shown previously in a host resistance model for Listeria monocytogenesis Reference de Waard, Claassen, Bokken, Buiting, Garssen and Vos39, Reference de Waard, Garssen, Bokken and Vos40.
The effects of probiotics on the immune system are clearly strain-dependentReference Maassen, van Holten, Balk, Heijne den Bak-Glashouwer, Leer, Laman, Boersma and Claassen28, Reference Forsythe, Inman and Bienenstock41, Reference Maassen, van Holten-Neelen, Balk, den Bak-Glashouwer, Leer, Laman, Boersma and Claassen42, but the mechanisms behind this are not completely understood. One way in which the immune system could recognize probiotics is via toll-like receptors (TLR). These receptors are present on immune cells and play an important role in the recognition and initiation of immune responses against pathogenic micro-organismsReference Takeda and Akira43. There is some evidence from studies in experimental animals that TLR are involved in the immune effects elicited by probiotics. In an animal model for colitis, TLR9 mediated the anti-inflammatory effects induced by a mix of eight probiotic bacteria (VSL#3)Reference Rachmilewitz, Katakura and Karmeli44. TLR9 was also involved in the suppression of allergic airway inflammation in a mouse model by L. reuteri Reference Forsythe, Inman and Bienenstock41. Amelioration of experimental colitis by E. coli Nissle 1917 was dependent on TLR-2- and TLR-4-signallingReference Grabig, Paclik and Guzy45. TLR are present on dendritic cells, which are the principal stimulators of naïve T cells. As such, they play a crucial role in polarization of the immune response to Th1, Th2 or regulatory T cell responsesReference Kapsenberg46. In vitro studies have shown that probiotics can activate dendritic cells and influence T cell polarization towards Th1 or regulatory T cellsReference Christensen, Frokiaer and Pestka47–Reference Smits, Engering and van der Kleij49. Hence, dendritic cells seem to be involved in orchestrating the immune response induced by probiotics.
In our opinion, the proper probiotic strain should be selected in order to reach the goal for which the specific application is meant. This is illustrated by a recent study, in which it was shown that probiotics increase allergic sensitization in atopic infants who received L. acidophilus in the first 6 months of their lifeReference Taylor, Dunstan and Prescott23. Probiotics are considered to be safe because of long-term use in the adult population, but information of safety in infants is scarce. The follow-up of most intervention studies in infants is relatively short to get insight into long-term beneficial and adverse effects. In atopic infants who received LGG in the first 6 months after birth, a follow-up until the age of 4 years was reported and, although the incidence of atopic dermatitis was significantly lower in the LGG group, the incidence of rhinitis was diagnosed twice as often. This finding was not significant due to the small number of infants with rhinitisReference Kalliomaki, Salminen, Poussa, Arvilommi and Isolauri19.
In conclusion, modulation of the immune system does not necessarily lead to beneficial effects. Therefore, more research is needed to elucidate the complexity of the mechanisms underlying these immune effects. For a better understanding of effects of intervention with probiotics early in life, well-controlled studies in infants are needed that focus on both short-term and long-term beneficial and adverse effects.
Acknowledgements
We acknowledge the Food and Consumer Product Safety Authority (VWA) for financial support. For excellent technical assistance we thank Arja de Klerk, Eric Gremmer, Bert Verlaan, Bianca Matthee, Liset de la Fonteyne and Yvonne Wallbrink from the Laboratory for Toxicology, Pathology and Genetics of the RIVM. We thank Hans Strootman, Dirk Elberts, Piet van Schaaik, Christine Soputan and Jouke van de Siepkamp from PMP department of the experimental animal laboratory of the Dutch Vaccine Institute (NVI) for the biotechnical support.






