Introduction
An ancient disease with a myriad of symptoms and equally disparate modes of transmission, tularemia has been documented across the Northern Hemisphere [Reference Sjostedt1], and more recently, in Australia [Reference Whipp2], Middle East [Reference Esmaeili3] and Africa [Reference Njeru4, Reference Ghoneim, Abdel-Moein and Zaher5]. The discovery of Francisella tularensis about a century ago allowed for serologic confirmation of sporadic and outbreak-related cases, providing the initial data to estimate tularemia incidence, characterise its multiple clinical presentations and investigate risk factors [Reference Sjostedt1, Reference Hestvik6, Reference Rossow7]. These investigations revealed that two subspecies cause disease in humans; F. tularensis subspecies tularensis (biovar A) found primarily in North America is the most virulent, and F. tularensis subspecies holarctica (biovar B) found across Europe and Asia which typically causes less severe disease [Reference Sjostedt1, Reference Ellis8]. Tularemia generally presents with non-specific infectious symptoms (e.g. fever, fatigue) followed by a wide range of clinical presentations that vary by site of infection and may not be easily identifiable as tularemia infection [Reference Maurin and Gyuranecz9]. Risk factors identified in outbreak investigations include environmental exposures (e.g. contaminated water), contact with infected animals (e.g. rodents, hares) and arthropods (e.g. ticks, mosquitos), occupational exposures (e.g. landscaper, veterinarian) and recreational activities (e.g. hunting) [Reference Hestvik6]. Case reports and outbreak investigations identified the presence of endemic areas serving as disease reservoirs, and prompted efforts to measure the background incidence of tularemia.
Human surveillance programmes were initiated about 90 years ago in the former Soviet Union to monitor the incidence of tularemia [Reference N10]. However, like many national surveillance programmes, these efforts have been marginally successful for monitoring endemic disease, finding relatively few cases between outbreaks. Improved surveillance in Georgia, like most countries in Eurasia, has occurred in the past two decades when international concerns about the use of tularemia as a biologic weapon increased [Reference Hestvik6]. Surveillance data have been used to identify cyclical incidence patterns in regions of Finland where mosquito transmission results in a predictable peak tularemia incidence in late summer with larger outbreaks occurring the year after the vole population peaks [Reference Rossow7, Reference Desvars11]. Continued surveillance over time and resulting investigations also identified recurrent risk factors for sporadic outbreaks, including hunting [Reference Hauri12] and landscaping [Reference Feldman13].
More recently, seroprevalence studies in endemic regions have revealed unreported tularemia infections and additional risk factors for infection. National and regional seroprevalence studies have also uncovered infections in new geographic areas without previously documented transmission [Reference Rossow7, Reference Desvars11, Reference Splettstoesser14, Reference Schmitt15]. These studies have shown consistency between seroprevalence and reported incidence: low seroprevalences in regions with small and infrequent outbreaks and higher seroprevalences in areas, such as parts of Scandinavia, where outbreak investigators and surveillance systems have identified higher incidence [Reference Rossow7, Reference Desvars11]. Neighbours of Georgia have high seroprevalence regions including tularemia foci areas of Azerbaijan [Reference Clark16] (15.5%) and farmers in Kars, Turkey (13.6%) [Reference Buyuk17]. Thus, there is potential for a relatively high seroprevalence in Georgia, prompting this multipronged study to provide a national assessment of tularemia.
The purpose of this report is to characterise the current epidemiology and knowledge of tularemia in Georgia by summarizing: (1) national surveillance data, (2) seroprevalence of antibodies to F. tularensis among military soldiers, and (3) seroprevalence of antibodies to F. tularensis and risk factors in regions with known tularemia activity. While we hypothesised that undetected prior infection would be identified among residents in endemic regions, no specific seroprevalence estimate was made a priori. Further, we hypothesised that key risk factors for seropositivity in endemic areas of Georgia would include farming activity and contact with rodents and ticks.
Methods
Three surveillance activities were conducted to estimate the incidence and seroprevalence of tularemia in Georgia. First, we used Georgia's National Centres for Disease Control and Public Health's (NCDC) Electronic Integrated Disease Surveillance System (EIDSS) to identify acute cases. The EIDSS is designed within the One Health concept to facilitate compliance with International Health Regulations. Second, blood testing of a sample of military soldiers was conducted to estimate the seroprevalence of F. tularensis nationally. Third, serology and structured interviews were conducted on a randomly selected sample of residents of endemic regions to describe the epidemiology and risk factors of tularemia in high-risk areas.
The seroprevalence study protocols were approved by research ethics committees in both Georgia and the USA. Informed consent was provided to those recruited for the seroprevalence studies and volunteers were enrolled only after reading and discussing the content of the informed consent form.
Study design
Incidence surveillance
All physicians, laboratories and hospitals are required to report suspected and confirmed acute cases of tularemia to NCDC's EIDSS. The surveillance system uses case definitions consistent with the World Health Organization guidelines. Confirmed cases must have a positive culture or a fourfold antibody titre difference in paired serum specimens taken at least 2 weeks apart. Presumptive cases have symptoms compatible with tularemia and one elevated titre (i.e. microagglutination test (MAT) of 1:128 or immunoglobulin enzyme-linked immunosorbent assay (>15 U/ml)). All other suspected cases were excluded from the official statistics and this report.
National incidence data from 1997 to August 2017 were used to assess trends in incidence over time, because prior to 1997 only outbreak-related cases were ascertained by the surveillance system. The surveillance data also allowed for examination of demographic characteristics (i.e. age, gender), potential exposure, form of tularemia and geospatial distribution of cases and outbreaks.
Seroprevalence study of military
Between October 2014 and February 2016, a convenience sample of soldiers visiting the military clinic responsible for conducting physical examinations were invited to participate in the study. Those providing informed consent were enrolled in the seroprevalence study of infections that included testing for F. tularensis antibodies. The limited data collected included gender, age and current residence.
Seroprevalence study in endemic regions
A cross-sectional study of residents in two rural areas was conducted between October 2013 and September 2016. Villages were selected from the two areas with naturally occurring foci of tularemia: the mountainous Meskhet-Javakheti region which includes Ninotsminda, Akhalkalaki, Akhaltsikhe and Aspindza regions; and the Kartl-Kakheti focus which covers the central and eastern areas of Georgia.
After the seroprevalence study was initiated, it was determined that population registries of some villages were out-of-date, and included far more people and households than actually live in the villages due to substantial migration to large urban areas. Population-based registries were therefore not an option for study subject selection within some selected villages. Thus, a hybrid sampling method was utilised that included direct random sampling of households from the registries when available, or using geographic location to sample households. Once a household was selected, all adults in the household who were in the village the day of sampling were enumerated, and one adult from each household was randomly selected. This convenience sample provides a reasonable approximation of adults living in the villages who are at risk for tularemia infection.
Village residents were eligible for the study if they were randomly selected, ages 18–65 years, spoke Georgian or Russian, lived in the village for at least the past three consecutive years, were competent to answer questions and volunteered to participate after both oral and written informed consent was provided.
A 20 min, standardised, interviewer-administered questionnaire was used to collect socio-demographic information; exposures associated with tularemia; diagnostic history of tularemia and vaccination; and awareness about tularemia. Potential risk factors were identified through a literature review and findings from past outbreaks. Five millilitres of venous blood was drawn from consenting volunteers for testing of tularemia antibodies.
Diagnostic methods for seroprevalence studies
All samples were tested for antibodies against tularemia using a MAT with an in-house antigen prepared according to a protocol provided by the US Centres for Disease Control and Prevention (CDC), Fort-Collins. A MAT of 1:128 or higher was defined as seropositive.
Data management and statistical analysis
The association between seropositive status for tularemia and potential risk factors was examined in bivariate analyses with likelihood ratio χ 2 tests. When frequencies for rare exposures were small, Fisher's exact test was utilised for tests of statistical significance. Logistic regression analysis was conducted with the intent to adjust for confounding. The strong associations between some exposures and seropositive status, however, resulted in complete separation and the models could not mathematically converge.
ArcGIS 10.4 software with the local region/settlement database was used for geocoding villages sampled in the seroprevalence study, and results were mapped based on the number of seropositive cases identified in the village. Outbreaks occurring during the last 70 years and sporadic cases from 2014 to 2017 were also mapped [Reference Klimukhina18, Reference Velijanashvili19].
Results
Incidence surveillance
Georgia experienced a tularemia outbreak in 2006–2007, with 35 reported cases (Fig. 1). The outbreak occurred in a small village with an unprotected water reservoir. Water from a spring inside the village that supplies water to the reservoir tested positive for F. tularensis. A rat was found in the spring reservoir.
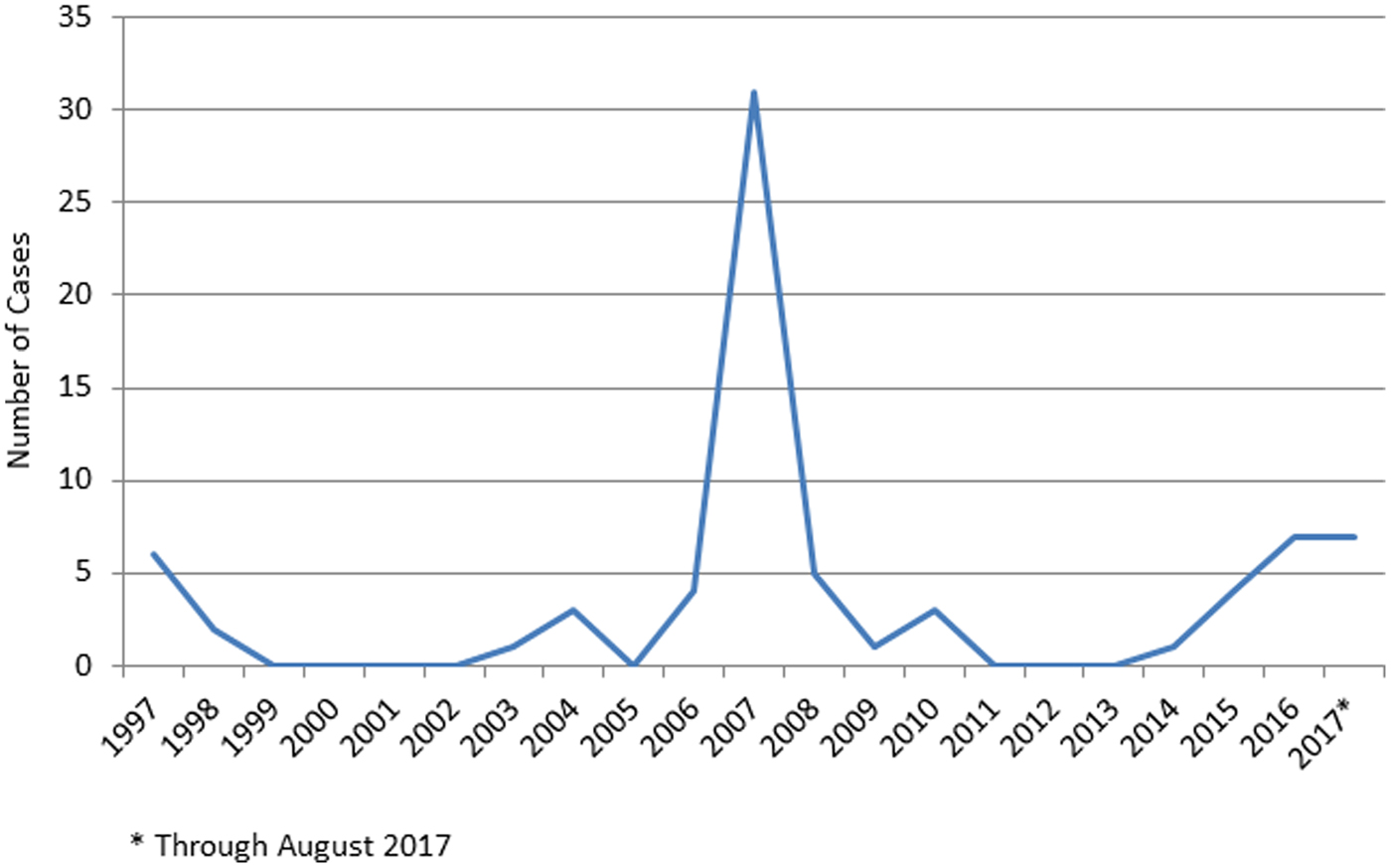
Fig. 1. Incident cases of confirmed and presumptive tularemia infections, Georgia, 1997 to August 2017.
The years between outbreaks are marked by few, if any, reported cases. Between 2014 and August 2017, there were 10 confirmed and nine presumptive cases, with an estimated annual average incidence of 0.12/100 000. Of the 19 cases, 12 (63.2%) were men. The age range was 12–74 years, with just under half of the cases occurring between the years 20 and 29 (eight cases, 42%). There was no conclusive evidence of seasonal effect with six cases diagnosed from January to March, five from April to June, six from July to September and two from October to December. Most cases presented clinically as oropharyngeal (10 cases) or ulceroglandular (eight cases), with one case exhibiting gastrointestinal presentation. Volunteers with the ulceroglandular form of tularemia reported onset of symptoms between March and October.
Although most of the recently reported incidence cases have occurred in regions in known tularemia foci (Fig. 2a), there were two geographic areas without previously documented tularemia cases. One was an isolated incident of a resident of Tbilisi with no documented travel outside the city. The other region newly identified as a possible tularemia focus was Imereti in Western Georgia, with one case in 2015 and three in 2017. Two of the three cases in 2017 were neighbours in the village of Chkhari, with symptom onset approximately 2 weeks apart. Exposures in Chkhari included drinking water from an uncovered reservoir and working with oatmeal straw and shucking corn. Possible exposures reported by infected individuals elsewhere included agricultural work (e.g. hay straw preparation), drinking from wells and uncovered reservoir, hunting, contact with rodents, removing ticks from cattle and being bitten by ticks.
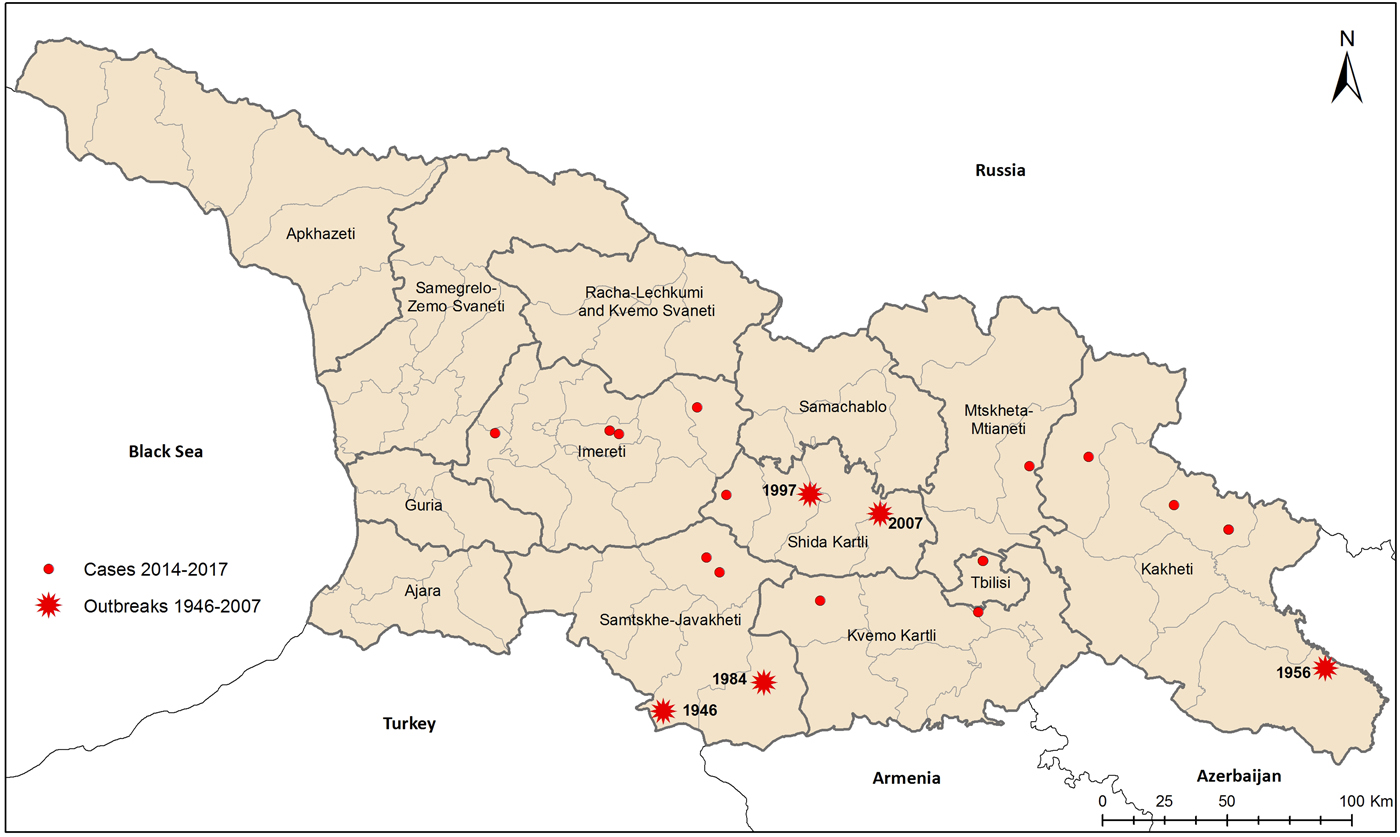
Fig. 2. Historic outbreaks of tularemia and incident cases, Georgia, 2014 to August 2017.
Seroprevalence study of military
Of the 500 military solders tested, 10 (2%) had antibodies for F. tularensis (MAT of 1:128 or higher) (Table 1). The seropositive soldiers were men, the majority of whom were between 30 and 39 years of age. Seven cases had current residences in known endemic areas (i.e. Kakheti, Samtskhe-Javakheti, Kvemo Kartli, Shida Kartli, and Tbilisi). Three were from areas without previously known F. tularensis transmission (i.e. Imereti).
Table 1. Socio-demographic characteristics for participants in two tularemia seroprevalence studies, Georgia
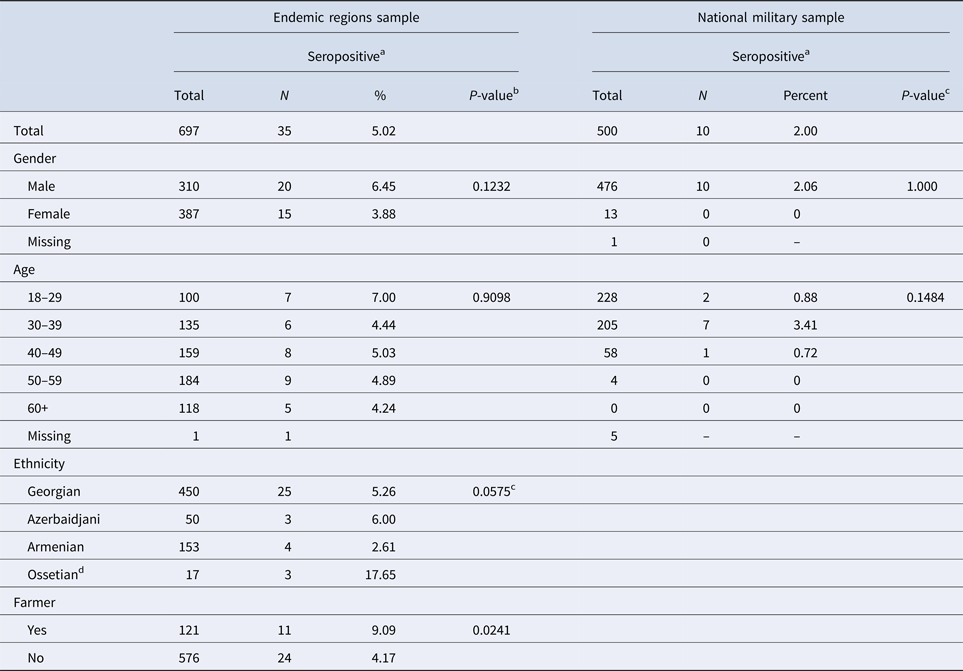
a Seropositive if MAT ⩾ 1:128.
b Likelihood ratio χ 2 test unless otherwise specified.
c Fisher's exact test utilised due to small sample sizes.
d The small number of Ossetians enrolled resulted in an unstable estimate of seroprevalence.
Seroprevalence study in endemic regions
Of the 783 residents approached to participate in this study, 697 (89.0%) volunteered and provided sufficient information to be included in these analyses. Overall, 35 (5.0%) volunteers met the study definition for a seropositive antibody response (i.e. MAT of 1:128 or higher) to F. tularensis. Seroprevalence was not statistically significantly associated with age or gender, although men had seroprevalence more than two-thirds that of women (6.45% vs. 3.88%, respectively, P = 0.123) (Table 2).
Table 2. Association between risk exposures and tularemia seroprevalence, Endemic Regions, Georgia, 2014–2016

a Seropositive if MAT ⩾ 1:128.
b Likelihood ratio χ 2 test unless noted.
c Includes domestic animals (i.e. dogs, cats, bunnies, cows, goats, sheep and pigs) and wild animals (i.e. birds, rodents, hares, wolves, squirrels, bats, jackals and deer).
d Fisher's exact test utilised due to small sample sizes.
Fewer than half of the villages studied had any seropositive residents (44.4%, 28/63, Fig. 3), and no village had more than two residents, of the average of 10 sampled, testing positive for F. tularensis antibodies. The small number of seropositive residents in any given village precluded identification of specific water sources, such as spring, river, covered or uncovered well, covered or uncovered reservoir, or centralised pipeline, as being associated with seropositivity.
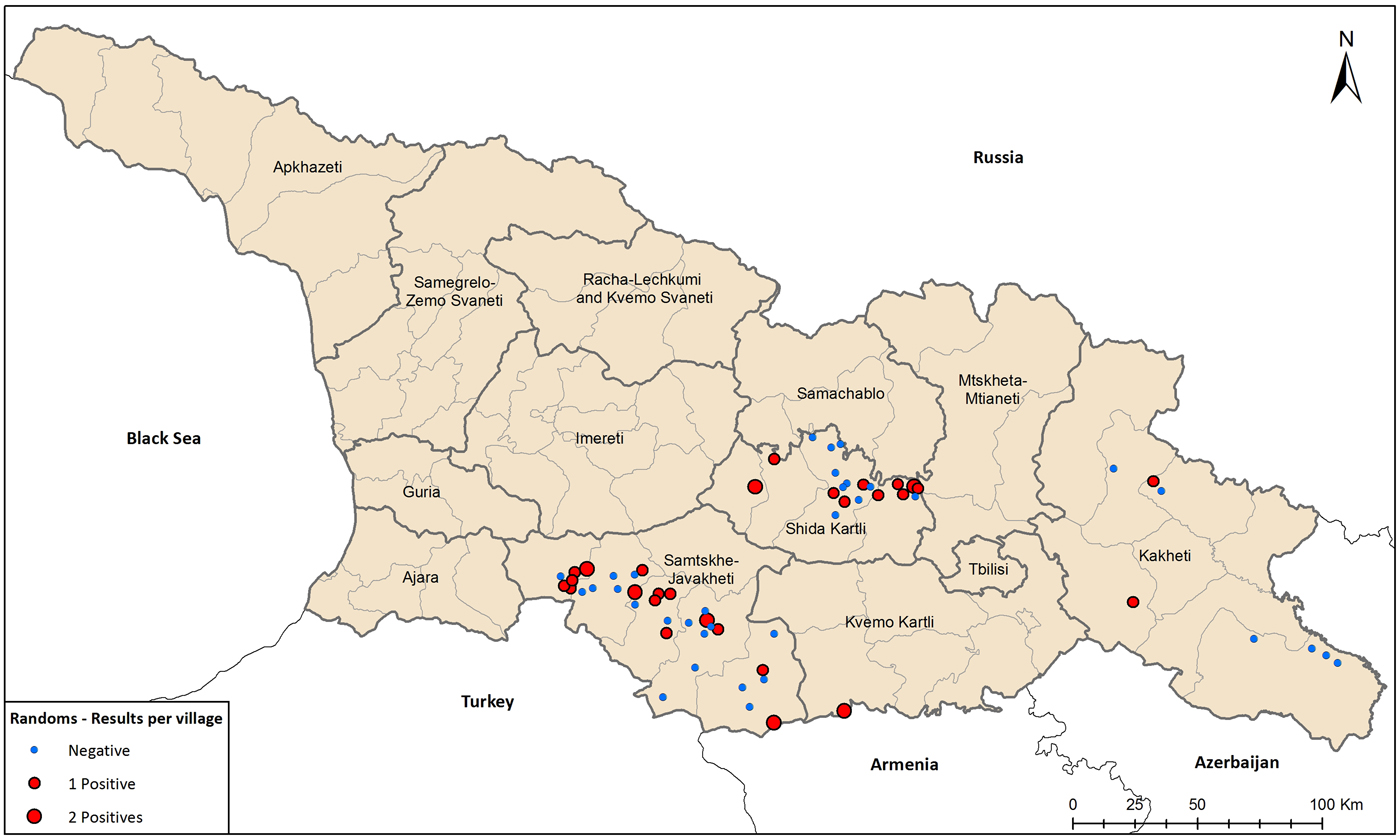
Fig. 3. Seroprevalence study of tularemia in foci regions, Georgia, 2014 to August 2017.
All of the respondents who were seropositive had contact with animals, while those reporting no contact or contact with one type of animal were all seronegative (Table 2). Only contact with dogs was statistically associated with seropositivity in bivariate analysis. However, all respondents who were seropositive who reported contact with dogs also reported contact with at least one other animal. Contact with other pets (i.e. cats, bunnies), livestock (i.e. cows, goats, sheep, pigs) and wild animals (i.e. birds, rodents, hares, wolves, squirrels, bats, jackals and deer), were not individually associated with seropositivity. Exposures correlated with animal exposures were similarly found to be statistically associated with seropositivity: buying and selling animals, finding ticks on their pets and livestock, and working with hay or wheat (Table 2). Exposure to woodlands and hunting were not associated with previous tularemia infection. Counterintuitively, consuming raw or undercooked meat was more common in seronegative volunteers (P = 0.0237).
The seroprevalence study also assessed knowledge of tularemia. Only 11% of participants reported awareness of tularemia, including only one person who was seropositive. Only one person reported having been vaccinated.
Discussion
Disease surveillance, coupled with seroprevalence studies, provide valuable insight into the extent of F. tularensis subspecies holarctica transmissions to humans in Georgia. In endemic regions, 5% of volunteers had antibodies for F. tularensis. The national study of military volunteers found a 2% seroprevalence. These findings suggest that cases identified during outbreaks and through routine disease surveillance grossly underestimate tularemia incidence. While clinical disease is underestimated by routine surveillance, it is unlikely severe cases of the ulceroglandular form, or tularemia outbreaks, go unrecognised. The substantial discordance between incidence and prevalence data is not unique to Georgia, it has been seen in Germany [Reference Splettstoesser14], Findland [Reference Rossow7] and Turkey [Reference Buyuk17] and believed to be the norm worldwide [Reference Hestvik6]. In low- and middle-income countries with limited laboratory resources, the discordance between incidence and seroprevalence estimates may be greater.
Historically, human, environmental, small mammal and ectoparasite surveillance in Western Georgia, which is geographically separated from east part by Lesser Caucasus mountains system, had not found F. tularensis. Human surveillance provided the first evidence that F. tularensis is circulating in Imereti. Because the majority of Georgians living in cities visit ancestral villages often, it can be difficult to determine the location of exposure reliably. The two neighbours in the Imereti village of Chkhari who developed symptoms within 2 weeks of each other provide strong evidence that the infections were locally acquired. Similarly, the lack of any travel prior to an acute infection in a resident of Tbilisi, the capital and most populous city, also established the first local transmission in the city. While the epizooty in rodents in Tbilisi is consistent with F. tularensis spillover to humans [Reference Elashvili20] and prior case reports suggested transmission in Tbilisi occurred, travel outside the city obfuscated the evaluation of prior reports.
While clinical disease is underestimated by routine surveillance, it is unlikely severe cases of the ulceroglandular form, or tularemia outbreaks, go unrecognised. The substantial discordance between incidence and prevalence data is not unique to Georgia, it has been seen in Germany [Reference Splettstoesser14], Findland [Reference Rossow7] and Turkey [Reference Buyuk17]. In low- and middle-income countries with limited laboratory resources, the discordance between incidence and seroprevalence estimates may be greater.
Sporadic acute cases of tularemia throughout the known endemic regions suggest the potential for ongoing transmission to humans via multiple reservoirs and modes of transmission. In Georgia, a half century of extensive environmental, small mammal and ectoparasite surveillance has identified F. tularensis foci thought to be maintained by the common vole-tick cycle; however, population dynamics of the common vole have not been associated with human tularemia incidence [Reference Elashvili20]. This assessment may be confounded by multiple transmission mechanisms. The hypothesis in Georgia, based on historical data, is that outbreaks mostly occurred in winter when rodents move closer to human settlements to find food, and in the process, contaminate food and water. In summer and autumn, people spend more time in nature and agricultural work in the fields providing opportunities for transmission via direct contact with rodents and ticks. All individuals with the ulceroglandular form had symptom onset between March and October, consistent with this hypothesis. The three individuals with symptom onset in the winter had the oropharyngeal form, consistent with the consumption of contaminated water or food. The oropharyngeal form occurred throughout the calendar year and may be related to water sources as ongoing reservoirs for transmission. Two biggest water-borne outbreaks in 1984 and 2007 had a place in winter [Reference Velijanashvili19, Reference Chitadze21]. Given that the infectious dose can be as few as 100 organisms [Reference Ellis8], drinking water from a local source after an infected carcass has contaminated the water is plausible.
There have been multi-year periods of quiescence, suggesting that Georgia may have inter-epizootic periods where the reservoir is maintained by other small animal-tick cycles. In Hungary a hare (Lepus europaeus)-tick cycle explains their inter-epizootic period [Reference Gyuranecz22] and should be studied in Georgia. Contaminated water also is likely to serve as a reservoir [Reference Janse23–Reference Hightower26].
Investigation of risk factors in endemic regions, not surprisingly, identified exposures related to farming and contact with domestic animals [Reference Leblebicioglu27, Reference Rossow28]. In fact, the vast majority of seropositive individuals reported taking ticks off animals and working with hay, both well-known exposures associated with tularemia [Reference Hestvik6, Reference Chitadze21, Reference Inci29]. While men had a higher seroprevalence than women, it was not significantly different. Given that women help in the fields when needed, and take care of domestic animals, the relative opportunity for exposure among women may be higher in Georgia than in other countries.
Surprisingly, the seroprevalence was remarkably scattered across the region. Because local water contamination has caused outbreaks in the past [Reference Elashvili20, Reference Schulze25, Reference Leblebicioglu27], we expected to find villages with undetected outbreaks due to subclinical or mild infections. The data reflect the endemic regions have widespread, sporadic infections.
One must be careful when attributing disease to specific exposures in endemic regions. We could not narrow down exposures using regression analysis because seropositive volunteers almost uniformly had multiple exposures, in particular, contact with several animals and hay. The amount of exposure, or time-at-risk, appears to increase opportunity for infection. Thus, with more opportunity for infection, there may be a greater likelihood for infection. We constructed a summary measure that summed the number of animals with reported exposure as a crude estimate of exposure opportunity. The animal summary measure had a strong association with seropositivity, with all seropositive individuals having been exposed to at least two animals, typically one of which was a dog. Most of the participants remember taking a tick off an animal, providing a mechanism for infection. Some of these individuals remember being bitten by tick themselves, others did not. In the future, time at risk and extent of exposure should be measured in endemic regions, as was constructed for a study of risk among landscapers in Martha's Vineyard [Reference Feldman13].
Surveillance, both incident and seroprevalence, has inherit limitations. As previously noted, substantial underdiagnosis is likely, thus the national communicable (incident) surveillance system underestimates the number and rate of tularemia. Suspected exposures for acute cases are based on self-report of exposures prior to onset of symptoms; no biological link was attempted or noted. Typically only outbreaks result in environmental, animal and ectoparasite sampling to investigate causes. The seroprevalence study conducted in endemic regions consisted of a random sample; however, the probability weights of being selected could not be validated and thus were not used in the analysis. If official population estimates are used as weights, the seroprevalence estimate also would be 5.0%. The sample of military volunteers was designed to gauge, not estimate, seroprevalence nationally. Lastly, we used a strict definition for seropositive which likely underestimates seroprevalence slightly, particularly among older residents whose antibody titres may have waned. These imperfections are not unique to Georgia when monitoring rare infectious diseases and, even with these limitations, the data paint a more detailed picture of tularemia than previously available.
Conclusions
This multipronged surveillance study serves as a reminder that ancient diseases remain viable and dynamic, requiring nimble surveillance systems and ongoing clinician training. In this evaluation, a summary of the data available on human infections with F. tularensis subspecies holarctica in Georgia, documenting the first human cases in Imereti, and confirmed transmission in Tbilisi. Surveillance systems in low- and middle-income countries have finite resources, thus laboratory testing of rare diseases is typically limited to severe disease, if available at all. Seroprevalence studies conducted periodically may enhance our understanding of tularemia infections in countries with dramatically underestimated incidence rates due to lack of clinical recognition and diagnostic testing.








