Bones generate electricity and it has been known for a while that this plays a critical role in their regeneration. However, it was not clear where the electricity came from or how it choreographed the healing of a broken bone. An international group of researchers from Catalonia, Costa Rica, Iran, and Switzerland have identified a phenomenon called flexoelectricity, most commonly found in ceramics, to be an important actor in this process.
Bones are composed of living cells, biopolymers, and minerals. The most common bone mineral is hydroxyapatite, a phosphate of calcium, which provides most of the load-bearing strength. These minerals are bound together by a tough protein called collagen that allows a bone to be more flexible. Collagen, it turns out, is piezoelectric; it is able to convert an applied mechanical stress to electric signals, for example when the bone is bent. Besides collagen’s piezoelectricity, streaming ions also produce movement of charges, leading researchers to believe that this is the source of bone electricity. However, it was discovered that new bone-forming cells or osteoblasts accumulate on the surface of pure hydroxyapatite, which has neither collagen nor streaming ions. The researchers wondered what was attracting these osteoblasts to a pure mineral.
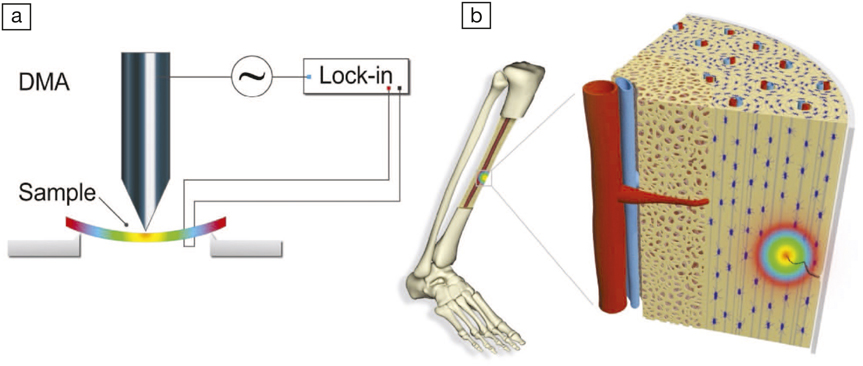
(a) Schematic of the experimental setup for measuring flexoelectricity; (b) strain gradients, and thus flexoelectricity, apparent at crack tips in bone mineral. DMA is dynamic mechanical analyzer. Credit: Advanced Materials.
As reported in a recent issue of Advanced Materials (doi:10.1002/adma.201705316), G. Catalan of the Barcelona Institute of Science and Technology and colleagues first looked at whether hydroxyapatite itself was piezoelectric. This is still a contentious issue; piezoelectricity originates from a fundamental asymmetry in the distribution of charges in a mineral, and structural studies disagree about whether the symmetry of hydroxyapatite is consistent with piezoelectricity. Laboratory measurements of the piezoelectric properties of hydroxyapatite by the team, however, revealed that the mineral is not piezoelectric.
However, even if a material is microscopically symmetric, a differential strain acting on the body can cause an asymmetry. This is called flexoelectricity, which is a coupling between a strain gradient in a body (flexure) and the amount of charge generated on the surface. As the name suggests, “flexure” leads to electricity. Tiny hairs in the inner ear, for example, have evolved to use this phenomenon for converting air waves to neural signals, thereby making flexoelectricity fundamentally important in auditory functioning. The researchers then set out to carefully flex natural bone and synthetic hydroxyapatite in the laboratory, measuring the bending-induced current using a lock-in amplifier. The flexure-induced current in synthetic hydroxyapatite and natural bone were remarkably similar, suggesting that flexoelectricity does indeed play a major role in generating electricity in bones. Moreover, because synthetic hydroxyapatite has no collagen, the quantitative similarity between the results rules out collagen piezoelectricity as a relevant contributor to the flexoelectricity of bone.
To fully understand the extent that flexoelectricity plays in bone regeneration, the researchers calculated the flexoelectric potential at a micro-crack in a bone. They found that even a small stress can be magnified at the apex of a crack tip, generating very high flexoelectric potentials. These flexoelectric potentials were compared with the electric fields that biologists had previously established to be sufficient to “electrostimulate” the beginning of the process of healing. As the crack starts healing, the apex shifts thereby serving as a beacon in directing the flow of healing cells. Thus flexoelectricity allows a micro-crack to call for help without the need for collagen piezoelectricity or streaming ions.
Brian Rodriguez at the University College Dublin, who is not connected to this research, says, “This work underscores the significance and ubiquity of flexoelectricity and clarifies several nagging and, until now, unresolved issues related to our understanding of bone remodeling.”


