Introduction
Elective single embryo transfer is a new technology in assisted reproduction (AR) that has contributed to an increase in pregnancy rate (Bissonnette et al., Reference Bissonnette, Cohen, Collins, Cowan, Dale, Dill, Greene, Gysler, Hanck, Hughes, Leader, McDonald, Marrin, Martin, Min, Mortimer, Mortimer, Smith, Tsang, van Vugt and Yuzpe2007). Therefore, identification of embryo quality predictors for reasonable embryo selection is of current importance (Dale et al., Reference Dale, Menezo and Coppola2015; Albertini, Reference Albertini2016).
In the first 5–6 days of preimplantation development, the human embryo is located inside the zona pellucida (ZP), which plays a major role in fertilization and early embryo development (Wassarman, Reference Wassarman2008). Hatching is a process of blastocyst escape through the ZP that depends on embryonic and endometrium enzymes, and is characterized by repeated contraction and expansion of the blastocyst (Sathananthan et al., Reference Sathananthan, Menezes and Gunasheela2003; Quesada et al., Reference Quesada, Sánchez, Alvarez and López-Otín2004).
According to in vitro experiments, up to 75% of morphologically normal human blastocysts cannot spontaneously leave the ZP. The inability of the blastocyst to hatch can be one of the major causes of recurrent implantation failure in mammalians, including humans (Seshagiri et al., Reference Seshagiri, Sen Roy, Sireesha and Rao2009).
Assisted hatching is a technique that is widely used for in vitro fertilization treatment (IVF), although there is no evidence that assisted hatching improves the chances of clinical pregnancy (Carney et al., Reference Carney, Das, Blake, Farquhar, Seif and Nelson2012). Furthermore, non-viability of assisted hatching may be due to desynchronization between delayed endometrial development and blastocyst-stage embryo (especially in frozen–thawed embryo transfer cycles). The use of assisted hatching also may be associated with an increased risk for monozygotic twinning (Kanter et al., Reference Kanter, Boulet, Kawwass, Jamieson and Kissin2016). Hence, the investigation of hatching mechanisms is of particular interest. Despite the large number of studies conducted on animal models, biomechanical and molecular mechanisms of hatching have still not been completely studied.
The objective of this study was to identify cell and genetic predictors of human blastocyst hatching success in AR programs. The null hypothesis of the study included the statement regarding no influence of cell factors (stage of development of the blastocyst, oocytes and embryo quality, ZP thickening and oocyte dysmorphisms) and genetic factors [mRNA expression of cathepsin V (CTSV), GATA binding protein 3 (GATA3) and chorionic gonadotropin beta (CGB) in blastocysts] on hatching success.
Materials and Methods
Samples were recruited over the period 2014–2015. All blastocysts (n = 62), which were donated by 28 married couples, were included in a prospective case–control study. The couples had applied for AR programs at the Center for Obstetrics, Gynecology and Perinatology, and had given signed informed consent to donate blastocysts for research purposes. The mean age of the women was 31 (25–36) years; and the mean of their body mass indexes was 22.5 kg/m2 (19.5–26.7 kg/m2). All patients had been unable to conceive naturally for at least 1 year before entering the study, and had indications for IVF treatment (tubal or male factor). Standard regimens for controlled ovarian stimulation were recombinant follicle-stimulating hormone (FSH) or human menopausal gonadotropins with a gonadotropin-releasing hormone (GnRH) antagonist to prevent a premature luteinizing hormone (LH) surge. Monitoring of the IVF cycles was performed through routine practices. Oocyte maturation was induced with purified urinary human chorionic gonadotropin (hCG; 7500–10,000 IU) when the lead follicles reached 17 mm.
The blastocysts were divided into two groups. The hatching group (Group 1) included 15 blastocysts (24.2%) that underwent spontaneous hatching. The control group (Group 2) included 47 blastocysts (75.8%) that did not undergo spontaneous hatching.
Embryos that had developmental arrest and degeneration before the fifth day of culture or were earlier exposed to the assisted hatching procedure were excluded from the study.
The blastocysts were evaluated over 6 days from oocyte fertilization until outcome detection (hatching or no hatching). Evaluation of the blastocyst stage of development, oocytes and embryo quality (Gardner & Schoolcraft, Reference Gardner and Schoolcraft1999), ZP thickening, oocyte dysmorphisms detection and hatching detection was performed by light microscopy (Nikon TE 300, total increase ×400 magnification).
Fertilization of mature oocytes was implemented by intracytoplasmic sperm injection (ICSI). Morphology of the oocytes was evaluated at the time of ICSI. Morphological characteristics of the oocytes were classified as exhibiting cytoplasmic dysmorphisms or extracytoplasmic dysmorphisms (Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Minasi, Romano, Ferrero, Sapienza, Baroni, Litwicka and Greco2008).
Hatching success assessment was performed after 144–146 h post-fertilization (Menezes et al., Reference Menezes, Gunasheela and Sathananthan2003). An analysis of the ejaculate was performed according to WHO recommendations (2010) (Tocci & Lucchini, Reference Tocci and Lucchini2010).
The expression of CTSV, GATA3 and CGB gene subunits 3, 5, 7 and 8 was detected by quantitative real-time polymerase chain reaction (qRT-PCR; DNA Technology, Russia). The RNeasy Mini Kit 250 (Qiagen) was used for DNA extraction. Primers were designed using Oligo6 software. B2M was considered as the reference gene.
Statistical analysis was performed using Statistica V10 software (USA). A two-sided P < 0.05 value was considered to indicate statistical significance. Categorical data were presented as rates and assessed using the χ2 test. Continuous data were presented as medians with lower and upper quartiles [Me (Q25–Q75)] and assessed by Mann–Whitney or Kruskal–Wallis tests. The measurement of association for comparing binary data was odds ratio (OR) ± 95% confidence interval (CI). The study was approved by the local Institutional Review Board (IRB).
Results
In total, 62 blastocysts were divided into a hatching group (n = 15, 24.2%) and a control group (n = 47, 75.8%). ZP quality did not affect the success of hatching. The rate of blastocysts with thickening or other defects of the ZP was 16.1% (n = 10) and was similar in both groups [two in the hatching group (13.3%) and eight in the control group (17.0%), P = 0.7350].
Most oocytes, from where the embryos were obtained, did not have dysmorphisms. The rate of cytoplasmic dysmorphisms was 14.5% (n = 9); the rate of extracytoplasmic dysmorphisms was 9.7% (n = 6); and the rate of combined dysmorphisms was 3.2% (n = 2). Single types of oocyte dysmorphisms or combination of cytoplasmic and extracytoplasmic dysmorphisms had no effect on the hatching success (P = 0.4170).
Assessment of sperm quality effect on blastocyst hatching ability did not reveal any influence of pathology of the male gametes on hatching efficacy. For asthenozoospermia, hatching failure OR was 1.37 (95% CI = 0.96, 1.96), and for teratozoospermia it was 1.32 (95% CI = 0.90, 1.93).
The quality of blastocysts on the fifth day of culture by Gardner classification affected their ability to hatch from the ZP. Most blastocysts were at the fourth stage of development (n = 45, 72.5%). The rates of blastocysts at the fifth and sixth stages of development were significantly higher in the hatching group, and blastocysts in the second and third stages of development were detected only in the control group (P = 0.0001) (Fig. 1 A).

Figure 1 Embryo assessment by Gardner scale. (A) Blastocyst maturity for spontaneous hatching success (Group 1) and hatching failure (Group 2). (B) Inner cell mass (ICM) quality. (C) Trophectoderm (TE) quality.
In 79% of cases, inner cell mass (ICM) was evaluated as grades A (n = 24, 38.7%) and B (n = 25, 40.3%). Grade A ICM was detected in 66.7% of blastocysts in the hatching group and in only 29.8% of blastocysts in the control group (P = 0.0226) (Fig. 1 B).
In an equal number of cases, trophectoderm (TE) was evaluated as grades A (n = 23, 37.1%), B (n = 18, 29.0%) and C (n = 21, 33.9%). Grade A TE was detected in 80% of blastocysts in the hatching group but only in 23.4% of blastocysts in the control group. Grade C TE was detected in 44.7% of control blastocysts and was not detected in the hatching blastocysts (P = 0.0001) (Fig. 1C).
The analysis demonstrated that the blastocysts in the control group had lower mRNA expression for the CTSV, GATA3 and CGB genes (Table 1). At the embryo stage of development, ICM and TE quality were associated with mRNA expression of CTSV and GATA3 genes, which was higher in the embryos of the fifth to the sixth stages of development degree with grade A ICM and grade A TE (Tables 2–4).
Table 1 Association between embryonic gene expression and spontaneous hatching
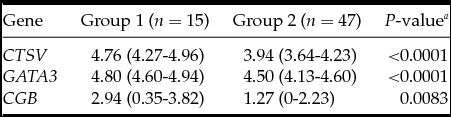
Medians with interquartile range; a Mann–Whitney test.
Table 2 Association between embryonic gene expression and blastocyst stage of development
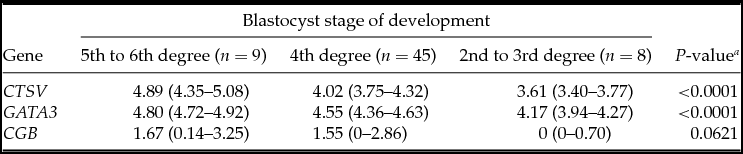
Medians with interquartile range; a Kruskal–Wallis test.
Table 3 Association between embryonic gene expression and the inner cell mass (ICM) quality
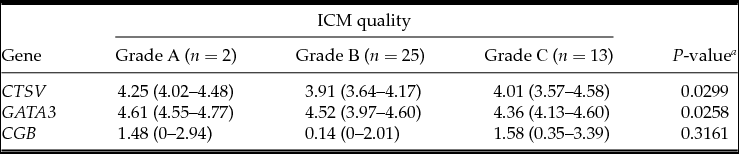
Medians with interquartile range; a Kruskal–Wallis test.
Table 4 Association between embryonic gene expression and trophectoderm (TE) quality
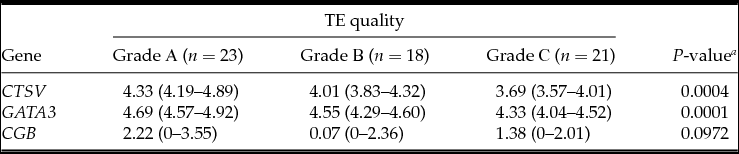
Medians with interquartile ranges; a Kruskal–Wallis test.
Discussion
In our study, the rate of spontaneous hatching (24.2%) was consistent with the available scientific data, according to which 75% of blastocysts cannot leave the ZP spontaneously (Practice Committee et al., 2014).
ZP thickening should adversely affect the hatching process, because a greater amount of lytic enzymes is required for ZP dissolution (Sathananthan et al., Reference Sathananthan, Menezes and Gunasheela2003), however we did not find an association between the rate of hatching failure and ZP thickness. This result suggests that high-quality embryos have enough adaptation possibilities for the timely release from both the normal and the thickened ZP.
The quality of gametes has a direct effect on the quality of the embryos and their capacity for implantation (Rienzi et al., Reference Rienzi, Ubaldi, Iacobelli, Romano, Minasi, Ferrero, Sapienza, Baroni and Greco2005; Sirard et al., Reference Sirard, Francois, Patrick and Claude2006; Cohen & Alikani Reference Cohen and Alikani2013). However, there are no scientific data confirming the influence of oocyte dysmorphisms and sperm quality on the blastocyst's ability to hatch. In our study, the quality of female and male gametes did not influence the hatching process.
According to our data, the high blastocysts quality of all three Gardner scale parameters had a positive effect on hatching success. This result indicates that hatching is a special stage of blastocyst development, characterized by a chronological and, largely, chronogenetic determinism. Blastocysts that had not developed enough by the sixth day of culture were not capable of hatching. From an evolutionary point of view, this factor may be a mechanism that prevents the implantation of a defective embryo with retarded development or other development disorders.
The role of cathepsins in hatching has currently been investigated only in animal models (Sireesha et al., Reference Sireesha, Mason, Hassanein, Tonack, Navarrete and Fischer2008). Expression of cathepsin genes has been shown in TE and ICM cells (Adjaye, Reference Adjaye2005), however the role of cathepsins in human blastocyst hatching has not been ultimately determined. Our data demonstrated a significant increase in CTSV mRNA expression in the hatching blastocysts, which suggested the direct involvement of cathepsin V in human blastocyst hatching mechanisms. Moreover, we showed the association between CTSV expression and blastocyst quality by Gardner classification (Gardner & Schoolcraft, Reference Gardner and Schoolcraft1999). Thus, CTSV gene expression (the launch of one of the direct hatching mechanisms) was determined by blastocyst quality and a sufficient degree of its stage of development.
According to the published literature, GATA3 shows constitutive expression over the different stages of preimplantation embryo development, and its expression is different between TE and ICM cells (Ozawa et al., Reference Ozawa, Sakatani, Yao, Shanker, Yu, Yamashita, Wakabayashi, Nakai, Dobbs, Sudano, Farmerie and Hansen2012; Sozen et al., Reference Sozen, Can and Demir2014). It has been suggested that GATA3 plays a crucial role in early embryo development. GATA3 expresses in embryonic TE and is responsible for the regulation of Cdx2 gene expression. GATA3 gene knockdown leads to a significant reduction in Cdx2 expression, which causes an embryo blastulation disorder and renders blastocyst formation impossible. There is only indirect evidence of the participation of GATA3 in human blastocyst hatching regulation in the scientific data. The results obtained in our study suggested an influence of the GATA3 gene on hatching; the level of GATA3 mRNA expression was significantly higher in blastocysts capable of spontaneous hatching and in high-quality blastocysts.
Expression of CGB gene mRNA was also higher in the group of blastocysts capable of spontaneous hatching. In the scientific literature, there are no data on the role of hCG in the regulation of human blastocyst hatching. β-hCG secretion by human blastocysts in vitro has been reported previously (Atwood & Vadakkadath Meethal, Reference Atwood and Vadakkadath Meethal2016). Given the higher CGB gene expression level in the hatching group, we can assume that hCG is involved in the regulation of the hatching process. With regard to GATA3, the relationship between high levels of CGB gene expression and good blastocyst quality is evident.
It should be noted that the correlation between gene expression and protein abundance depends on various biological and technical factors that we could not be recognised due to the small number of cells in the embryo. Identification and quantification of cellular proteins in the embryo according to its ability to hatch would be the objective of future studies.
As a result, the efficacy of spontaneous hatching of human blastocysts is not determined by the quality of the ZP and gametes, but by the quality of the blastocysts themselves. Probably, the blastocyst can model its further development through its own genetic factors. Expression of CTSV, GATA3 and CGB genes is lower in low-quality blastocysts and does not allow them to commit spontaneous hatching and to implant into the endometrium.
The limitation of this study was its small sample size, particularly in the hatching group. The study had a selection bias, as the controls did not match with the cases because of the sample collection challenge. Impact of collapse and expansion on ZP thinning using morphokinetic analysis is of particular interest, but absence of time-lapse microscopy is an another limitation of this study.
However, the unique sampling of human blastocysts was the main strength of our study. Moreover, we did not use multivariate regression analysis to control confounding variables, as we did not find such variables in the univariate analysis. The single cell predictor that affected hatching success was blastocyst quality.
Financial support
Funding for this study was provided by the Ministry of Health of the Russian Federation (grant no. 4A-A15 reg. #115123110127-7).
Declaration of interest statement
The authors report no financial or commercial conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







