Persons with asymptomatic coronavirus disease 2019 (COVID-19) can transmit severe acute respiratory coronavirus virus 2 (SARS-CoV-2), including within healthcare systems. Early in the COVID-19 pandemic, the concern for asymptomatic SARS-CoV-2 transmission led to the practice of universal (symptomatic and asymptomatic) laboratory test-based screening programs for patients upon hospital admission. The main objective of this strategy is to prevent nosocomial spread of SARS-CoV-2 from asymptomatic patients through early identification and subsequent swift initiation of appropriate isolation precautions and other infection prevention measures. This intervention, however, may cause unnecessary delays in care and can strain available resources. Reference Sangal, Peaper and Rothenberg1,Reference Couture, Amjad, Lewis and Mycyk2 Whether the costs and logistic challenges surrounding admission screening for asymptomatic COVID-19 are outweighed by the benefits of such testing is unclear, particularly with the use of other hierarchies of control to prevent the spread of SARS-CoV-2, such as the universal use of face masks, visitor restrictions, environmental cleaning, and vaccination. Reference Guilabert, Aparicio and Medina-Prado3
Prior studies examining the impact of universal laboratory admission screening for SARS-CoV-2 infection are limited (1) by short study periods (ie, weeks to a few months) that occurred early in the COVID-19 pandemic, (2) by study in single hospital settings, and (3) by assessment during periods of low COVID-19 community incidence. Reference Krüger, Leskien and Schuller4–Reference Bai, Li and Alsalem10 As COVID-19 activity continues to wax and wane, it is important to evaluate the experience of admission screening programs. Defining any differences in program impact among various patient populations or during periods of differing community infection incidence can assist in decisions around the use of testing, laboratory, and personnel resources for future waves of COVID-19.
In the current study, we examined the trends and impact of a hospital admission testing program for asymptomatic SARS-CoV-2 infections across a large healthcare system that includes a tertiary-care adult hospital, a pediatric hospital, a psychiatric hospital, and a community-based hospital. Using expert opinion to determine a clinically meaningful number needed to test (NNT, ie, the number of asymptomatic patients tested necessary to identify 1 asymptomatic COVID-19 patient), the study defined a threshold of benefit for universal admission screening across a variety of patient populations and community COVID-19 incidence rates.
Methods
Setting and study period
This study was conducted at Vanderbilt University Medical Center (VUMC), a large academic healthcare system in central Tennessee. VUMC includes 4 distinct hospitals that serve different patient populations: a tertiary-care adult hospital, a free-standing pediatric hospital, a community-based hospital, and a behavioral health hospital. Beginning in April 2020, every patient admitted to VUMC inpatient facilities underwent SARS-CoV-2 nucleic acid amplification testing (NAAT) upon admission as part of an operational screening program. This screening continued once COVID-19 vaccines became widely available. Admissions testing was suspended in June 2021 for nonimmunocompromised asymptomatic patients who had been fully vaccinated with a COVID-19 vaccine. We conducted a retrospective descriptive analysis of this asymptomatic testing program among the patients admitted to any of the 4 VUMC inpatient facilities between April 20, 2020, and June 14, 2021, when the suspension of testing for fully vaccinated patients commenced. For a subgroup analysis by patient vaccination status, a truncated study period reflecting the time when COVID-19 vaccination was available (allowing for sufficient patient uptake) was used (February 1, 2021, through June 14, 2021). During this study period, the SARS-CoV-2 α (alpha) variant was the predominant circulating variant.
Testing program details
Patients admitted to any 1 of the 4 VUMC inpatient hospitals received a SARS-CoV-2 NAAT via nasopharyngeal or bilateral nares swab. Patients presenting with any symptoms concerning for SARS-CoV-2 infection and those who had had confirmed COVID-19 in the 90 days prior to admission were not included in the asymptomatic admission screening program. Patients with a SARS-CoV-2 NAAT collected within 72 hours prior to admission, patients tested at an outside facility prior to hospital transfer, and inborn infants admitted to the neonatal ICU or newborn nursery with maternal SARS-CoV-2 NAAT results were excluded from this analysis.
Community COVID-19 incidence rates
Weekly community incidence rates of COVID-19 (per 100,000 persons) were calculated using US 2019 Census and Tennessee Department of Health COVID-19 case data for the 8 surrounding counties within the Metropolitan Service Area for VUMC: Davidson, Williamson, Cheatham, Robertson, Sumner, Wilson, Rutherford, and Montgomery counties. For a subgroup analysis examining the program in the pediatric hospitalized population, the Tennessee Department of Health 2019 Census data for persons aged 0–18 years were utilized. For a subgroup analysis examining the program at the community-based hospital, the US 2019 Census data for the county where the hospital is located (Wilson County) were utilized to calculate the weekly community COVID-19 incidence rates. Weekly COVID-19 incidence rates were classified and grouped by the Centers for Disease Control and Prevention (CDC) level of community transmission 11 (ie, low, moderate, substantial, and high) based on the initial case rate definition in use when the study began.
Statistical analysis
To assess the potential clinical utility of admission SARS-CoV-2 testing, an established listserv comprised of 144 national experts in infection prevention and healthcare epidemiology was surveyed to ascertain a clinically meaningful NNT to identify a single asymptomatic infected patient (Supplemental Material online). For each week during the study, the test positivity rate and NNT were calculated for the overall study period, by each hospital type, by patient vaccination status (as ascertained through electronic health record linkages with the state immunization registry), and by CDC-defined levels of community transmission. The calculated NNTs were compared to the clinically meaningful NNT. Patients were classified as fully vaccinated if they had received 2 doses of an mRNA COVID-19 vaccine or 1 dose of an adenoviral vector vaccine 14 or more days prior to admission. Finally, to provide some financial context to the program analysis, operational laboratory data, which included testing supplies, reagents, and laboratory personnel costs, were used to determine the cost of testing (ie, $50 per test). The immediate form of 2-sample test of proportions was utilized to compare positivity rates. The analysis was performed using Stata version 17 software (StataCorp, College Station, TX). NNT values were compared using confidence interval (CI) analysis.
Results
Determination of a clinically meaningful NNT
In total, 46 (32%) of 144 hospital epidemiologists responded to the survey question. The most commonly selected threshold NNT was the identification of 1 positive patient per 100 patients tested (selected by 26% of respondents) (Table 1). In addition, 54% of the survey respondents selected a NNT 100 or lower, suggesting comfort with the comparatively more conservative threshold choice of 100 NNT to delineate clinical meaningfulness. A calculated NNT that does not meet the survey NNT (ie, is a higher number) suggests a transmission period in which asymptomatic testing may be of limited utility, whereas a calculated NNT that meets or is below the 100 NNT indicates a transmission period in which asymptomatic testing would have been clinically meaningful according to the survey group. A few respondents noted in optional free-text comments that some factors, such as the local proportion of semiprivate inpatient rooms and underlying patient comorbidities, would affect their selection of a clinically meaningful NNT, with some noting that a more conservative NNT (ie, >100) would be useful.
Table 1. Hospital Epidemiologist Survey Results to Define a Clinically Meaningful Number Needed to Test

Admission testing epidemiology
In total, 51,187 admission tests were collected during the study period. For the examination of the program by community transmission rates, no periods of low transmission were observed during the study period. Moreover, 40 weeks or two-thirds of the study were periods of high transmission; 13 weeks were periods of substantial substantial transmission; and 7 weeks were periods of moderate transmission. For the entire study period and study population, the asymptomatic admission positivity rate was 1.8% (Table 2). Asymptomatic admission positivity rates were significantly different during periods of high transmission compared to other transmission periods (2.3%; P < .05) (Table 2 and Supplementary Table 1 online). Positivity rates were the highest among community hospital patients (2.6%) and the lowest among behavioral health patients (0.9%). Within each hospital setting, positivity rates during high transmission levels were significantly higher than during other transmission levels (Supplementary Table 2 online).
Table 2. Admission Testing for SARS-CoV-2 Infection in Hospitalized Patients, Overall and by Specific Patient Population Tested
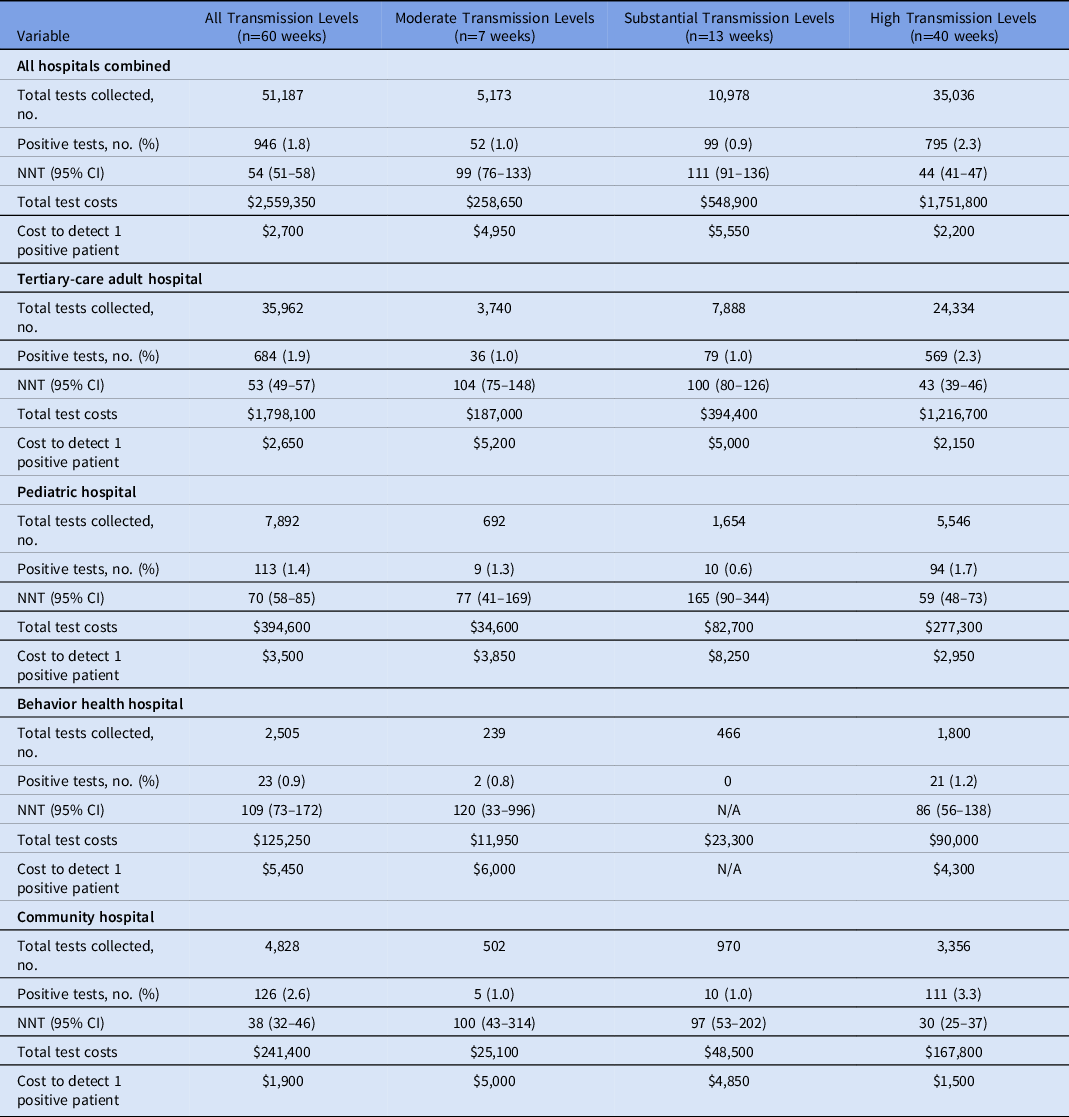
Note. NNT, number needed to test to identify 1 positive patient; CI, confidence interval. No periods of low transmission were observed during the study period.
NNT analysis
Differences in NNT by transmission level
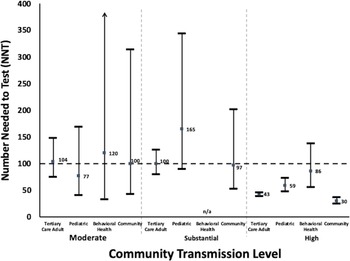
Taking all hospitals collectively, the NNT significantly met the clinically meaningful NNT threshold and was significantly different during high transmission periods compared to other transmission periods (NNT, 44; 95% CI, 41–47) (Fig. 1A, Table 2). For the moderate and substantial transmission levels, the point estimate of the NNT did meet the clinically meaningful threshold, but these values were not statistically significant by CI analysis. When examined by the individual patient populations and by the community transmission levels (Fig. 2), the NNT point estimates during high community levels met the clinically meaningful threshold (with only the behavioral health population not meeting statistical significance). During periods of other transmission levels, the NNT did not meet statistical significance even though many point estimates fell near or met the NNT threshold of 100.

Fig. 1. Number needed to test (NNT) with 95% confidence intervals (A) by CDC-defined community transmission level and (B) by hospital population (A) NNT of all hospitals combined by community transmission level. (B) NNT of all transmission levels combined by hospital population. A square data point indicates a calculated NNT point value from admissions data. Horizontal line indicates survey values NNT = 100. NNT values above the horizontal line (NNT=100) indicate the uncertain utility of asymptomatic admission testing. NNT values that met or are below the horizontal line (NNT=100) indicate a likely benefit for asymptomatic admission testing. No periods of low transmission were observed during the study.

Fig. 2. Number needed to test (NNT) with 95% confidence intervals by CDC-defined community transmission levels and by hospital population. A square data point indicates a calculated NNT point value from admissions data. A horizontal line indicates survey values NNT = 100. NNT values above the horizontal line (NNT = 100) indicate uncertain utility of asymptomatic admission testing. NNT values that met or are below the horizontal line (NNT = 100) indicate a likely benefit for asymptomatic admission testing. No periods of low transmission were observed during the study.
Differences in NNT by hospital populations
The NNT point estimates for the entire study period for all hospital sites combined (NNT, 54; 95% CI, 51–58) significantly met the clinically meaningful NNT threshold (Table 2), as did the full study period individual NNT for the tertiary-care adult hospital, the pediatric hospital, and the community hospital populations (Fig. 1B). The subgroup analyses examining pediatric admissions testing using pediatric rates of community COVID-19 incidence and community hospital admissions testing using community-specific COVID-19 rates for that hospital’s specific county data did not result in any significant changes to the findings from the overall analyses above (Supplementary Tables 5 and 6 online).
Impact of patient vaccination on admission testing utility
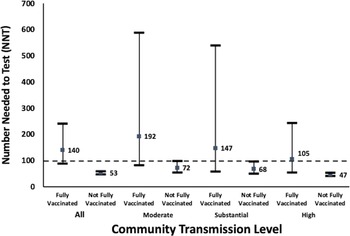
For all transmission periods, fully vaccinated patients had a significantly lower positivity rate (0.7%) compared to those who were not fully vaccinated (1.9%; P < .05) (Table 3 and Supplementary Table 3 online). Positivity rates were higher in non-fully vaccinated patients for all levels of transmission, but this difference was not significant for the substantial transmission period (Table 3). During the overall study period as well as by specific COVID-19 transmission levels, all NNT point values for non-fully vaccinated patients significantly met the clinically meaningful threshold (Fig. 3). This contrasts with the fully vaccinated patient NNTs that did not meet the clinically meaningful threshold and were much higher than the non-fully vaccination population point estimates. The NNT values between the vaccinated and non-fully vaccinated were significantly different during times of high transmission as well as all transmission periods collectively.
Table 3. Admission Testing for SARS-CoV-2 Infection in Hospitalized Patients, by Patient Vaccination Status
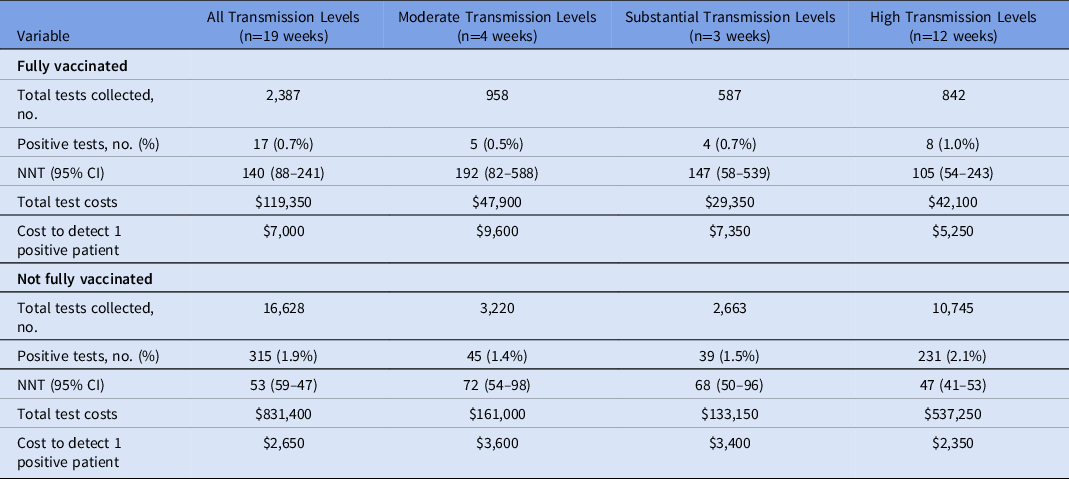
Note. NNT, number needed to test to identify 1 positive patient. No periods of low transmission were observed during the study period. Data from study period following vaccine availability and subsequent time to develop immunity (February 1, 2021, through June 14, 2021). Fully vaccinated = receipt of 2 doses of mRNA COVID-19 vaccine or 1 dose of adenoviral vector vaccine.

Fig. 3. Number needed to test with 95% confidence intervals by patient vaccination status. A square data point indicates a calculated NNT point value from admissions data. Horizontal line indicates survey values NNT = 100. NNT values above the horizontal line (NNT = 100) indicate uncertain utility of asymptomatic admission testing. NNT values that met or are below the horizontal line (NNT = 100) indicate a likely benefit for asymptomatic admission testing. No periods of low transmission were observed during the study.
Assessment of laboratory costs of admission testing
Using the NNT and cost per test ($50), the laboratory cost to detect 1 positive patient over the study period was highest at the behavioral health hospital ($5,450) and lowest at the community hospital ($1,900). The laboratory cost to detect 1 positive patient was the lowest during periods of high transmission across all patient populations compared to moderate and substantial transmission periods (Table 2).
Discussion
The use of a laboratory screening program to identify asymptomatic COVID-19 upon admission was frequently implemented during the pandemic to reduce the risk of healthcare-associated spread of the SARS-CoV-2. Published experiences on the use of such programs are limited. This study, through the analysis of a large asymptomatic SARS-CoV-2 NAAT hospital admission testing program, provides insight on the use of the program across 4 distinct patient populations and during varying levels of community transmission for a prolonged period of implementation. Among the study population, there was a potential benefit for asymptomatic admission testing across each of the 4 different hospital settings even as the community COVID-19 rates decreased. The greatest yield to admission testing occurred in periods with the highest community transmission levels as well as among patients who were not fully vaccinated. Notably, although the behavioral health hospital setting had a NNT that met our clinically meaningful threshold during high transmission, it was the only setting during this transmission period to not meet statistical significance. The lower denominator of admission tests obtained at this hospital setting, likely due to the relative lower patient census in comparison to other settings, could have contributed to its relatively wider NNT confidence intervals. Overall, such findings may provide important insight for healthcare facilities as they assess testing and associated resources.
Published analyses of other institutional experiences with admission testing for asymptomatic SARS-CoV-2 infection are limited. Many of these earlier studies were conducted during the initial months of the COVID-19 pandemic, often during periods of low COVID-19 incidence, Reference Uchida, Uwamino and Uno12 and usually captured a short period of time. Reference Sutton, Fuchs, D’Alton and Goffman13 Similar to the current study, prior studies also noted a relationship between the asymptomatic screening results and community COVID-19 rates. Reference Gilner, Kansal and Biggio14 Although these studies looked at the overall study period case incidence, Reference Jung, Kim, Lim, Kim, Kim and Kim5,Reference Nakamura and Itoi6 the current study was able to provide a more granular analysis on a weekly basis. Reference Abbas and Yusuf15 Other studies looking at universal admission testing may be limited in how they are screening patients, as if symptomatic patients are defined as just having fever, cough, or shortness of breath, patients could potentially have been mislabeled as asymptomatic if they had mild or atypical COVID-19 symptoms not fitting these strict descriptors. This approach could have swayed results toward a seemingly larger number of asymptomatic cases. Reference Saidel-Odes, Shafat, Nativ, Borer and Nesher16 In our study, even though symptom determination was based solely on provider input, providers ordering admission COVID-19 tests had been educated to label and categorize patients with any potential COVID symptoms as symptomatic. However, even with adequate education, the use of provider determination of symptom determination is imperfect.
The number needed to test to identify 1 asymptomatic case helps to describe trends of collective findings of an admissions screening program. The current analysis does not definitively answer the question of utility of asymptomatic admission laboratory screening regarding the number needed to prevent a case of nosocomial transmission, but these findings can be used to guide discussions at other institutions regarding such a program. Asymptomatic testing can slow down operations of patient care, can delay pertinent imaging or procedures, can increase length of stay, and can contribute to inappropriate use of isolation or PPE. Implementing an asymptomatic patient admission testing program can provide clinically relevant value, even during lower periods of transmission and in different patient populations. Limiting admission testing to non-fully vaccinated patients during periods of lower transmission may be a strategy to address cost and resource concerns around this practice. Although these findings can be used as a reference for how asymptomatic testing may apply to other hospital systems, some unique infection prevention considerations may also impact these decisions. For example, hospitals with a higher proportion of semiprivate rooms or congregate settings (eg, in behavioral health facilities) may have a more conservative threshold to relax admission screening programs, given the challenges of compliance with other infection prevention practices such as patient masking. Reference Brody, Shi and Shaffer17,Reference Füszl, Bouvier-Azula and Van Den Nest18 Individual patient immune status or admission to a unit typically houses other severely immune compromised patients may also consider a more conservative threshold as well, given the more detrimental ramifications of missing an asymptomatic case.
Although these findings provide important insight on the impact of an admission screening program, this analysis has some limitations. The utility of testing during periods of low community transmission could not be assessed because no periods of low transmission occurred during the study. In addition, the study period occurred prior to the emergence of the SARS-CoV-2 δ (delta) and (omicron) variants, which have had their own distinct effects on transmissibility and vaccination protection. Importantly, however, even though vaccinated individuals have lower likelihood of acquiring infection, the immune evasive nature of some of the SARS-CoV-2 variants, such as the δ (delta) variant, may still contribute to comparable transmission risk in vaccinated compared to unvaccinated individuals. Reference Riemersma, Haddock and Wilson19 Patients with screening testing performed outside of VUMC were not included, but these were anticipated to be a small proportion of the total number of admitted patients. To identify a clinically meaningful anchor for examining admission testing, a cohort of infection prevention experts was utilized; however, this group was small and may not be fully representative of other perspectives. Also, we used a conservative estimate of the cost of resources with regard to asymptomatic testing. The estimated cost per test did not fully reflect factors related to testing, such as impacts upon personnel time, and logistic impacts around an increased number of specimens (eg, improperly collected specimens and resultant personnel time devoted to address these issues). The impact and costs of widespread universal testing, especially during community surges of COVID-19, can acutely strain the existing laboratory workforce, particularly when compounded by staffing shortages noted with personnel exposures and infections. The laboratory used in this study did not have the capacity to run cycle thresholds on each asymptomatic positive test. Notably, however, several other studies have reported that most asymptomatic COVID-19 cases were noninfectious. Reference Uchida, Uwamino and Uno12,Reference Alsuhaibani, Kobayashi and Trannel20,Reference Aslam, Singh and Robilotti21 Finally, in this analysis, we could not definitively determine whether asymptomatic testing led to a reduction in nosocomial transmission, given the numerous factors that may influence such spread (eg, community adherence to mitigation measures by healthcare personnel). The NNT cannot be compared to the number needed to prevent 1 transmission because this number is unknown and not possible to calculate based on current data. No evidence has demonstrated that the added value of asymptomatic testing is superior to other infection prevention controls. The detection of a case in the setting of other infection prevention controls does not definitively mean that detection alone would have prevented transmission.
Even with these limitations, the current analysis provides further insight into SARS-CoV-2 admission screening programs. As the pandemic progresses and COVID-19 incidence becomes similar to that of other respiratory viruses, and COVID-19 vaccination coverage increases, healthcare systems will need to assess the need for these screening programs and their triggers. The concept of NNT, which has been used to help analyze asymptomatic testing in other settings, Reference Requena-Méndez, Mougkou and Hedberg22 as well as patient vaccination status, Reference Tande, Pollock and Shah23,Reference Singh, Walker and Paul24 may be helpful indicators for these assessments. More studies such as this are needed to support and inform future infection prevention measures especially with the emergence of SARS-CoV-2 variants.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.301
Acknowledgments
Financial support
The study was supported by institutional funds.
Conflicts of interest
T.R.T. reports serving on the Board of Directors for OmniSolve. R.M.H. reports serving as a consultant for Roche, bioMerieux, Qiagen and ThermoFisher. All other authors report no conflicts of interest relevant to this article.