Diabetes mellitus is an endocrine disorder that affects over 100 million people worldwide, and is becoming very common with changing lifestyles. Diabetes often leads to disability from the vascular complications of coronary artery disease, cerebrovascular disease, renal failure, limb amputation and blindness, in addition to neurological complications and premature death(Reference Goldstein and Massry1, Reference Weidmann, Boehlen and de Courten2). These diabetic complications rank high among the top ten causes of mortality throughout the world. The high fatty acid levels in plasma can lead to the development of atherosclerosis(Reference Steinberg, Paradisi and Hook3) in diabetic patients and a decrease in antioxidant molecules and enzymes, which at the later stages can amplify the diabetic complications(Reference West4).
Streptozotocin (STZ)-induced diabetes is a well-recognised diabetic experimental model that causes selective destruction of islet β-cells, associated with the generation of free radicals(Reference Thomas and Ramwell5). Hyperglycaemia is known to contribute to defective β-cell function in STZ-induced diabetes(Reference Briaud, Rouault and Bailbe6). Dietary intervention with non-digestible fibre is one of the main therapies proposed in the case of diabetic patients(Reference Luo, Yperselle and Rizkalla7). A few oligosaccharides and polysaccharides of natural or synthetic origin have gained importance for the treatment of chronic diseases such as IHD(Reference Trowell8) and cancer(Reference Bingham, Williams and Cummings9). Prebiotics are emerging functional products generally useful for the improvement of the nutritional quality of foods, and are defined as non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improve host health(Reference Gibson and Roberfroid10). Prebiotics possess interesting functional and physiological attributes such as low sweetness, non-cariogenicity, low energetic value, and hypolipidaemic and hypocholesterolaemic properties. They are indigestible by human gastrointestinal enzymes and are not degraded by low gastric pH; however, they are fermented in the large bowel and enhance the population of beneficial microbes such as lactobacilli and bifidobacteria(Reference Gibson and Roberfroid10).
Oligosaccharides such as fructo-oligosaccharides (FOS) are naturally present in wheat, asparagus, garlic, onion, artichoke, soya and milk, though in rather low amounts(Reference Van Loo, Cousement and De Leenheer11). Xylo-oligosaccharides (XOS) are naturally available in bamboo shoots, which are also produced from xylan, a major component of hemicelluloses(Reference Vazquez, Alonso and Dominguez12). FOS and XOS are found to increase the number of beneficial microbes such as bifidobacteria and lactobacilli in the colon and increase the body weight (BW), caecal weight and caecal SCFA(Reference Campbell, Fahey and Wolf13). FOS and XOS markedly decreased the caecal pH and serum TAG concentration, and increased total caecal weight and bifidobacteria population(Reference Hsu, Liao and Chung14). Daily intake of FOS (8 g/d) for a period of 14 d reduced blood glucose, cholesterol and LDL in diabetic patients(Reference Yamashita, Kawai and Itakura15). XOS improved growth retardation, hyperphagia, polydipsia and elevation of serum glucose, TAG and cholesterol in diabetic rats(Reference Imaizumi, Nakatsu and Sato16).
In the above context, the present investigation evaluated the beneficial effects of two oligosaccharides – XOS and FOS – in STZ-induced diabetic rats with respect to glycaemic status, BW, hypercholesterolaemia, activities of antioxidant enzymes and also pathophysiological conditions such as advanced glycation end (AGE) products in renal tissue and kidney pathology. In the absence of any detailed information on these aspects, the present study could provide for a better understanding of the antidiabetic profile of these oligosaccharides.
Experimental methods
Chemicals
STZ was procured from ICN Biomedicals Inc. (Asse-Relegem, Belgium). Heparin, glucose oxidase, peroxidase, o-dianisidine, bovine serum albumin, Coomassie Brilliant Blue G-250, cysteine hydrochloride, thiosemicarbazide and diacetyl monoxime were purchased from Sigma Chemical Co. (St Louis, MO, USA). Standards of FOS (1-kestose, 1-nystose and 1-fructofuranosyl nystose) were procured from Wako Pure Chemical Industries, Ltd (Osaka, Japan). Casein (refined grade) was procured from Nimesh Corporation (Mumbai, India). Salt mixture was procured from SISCO Research Laboratories Pvt. Ltd (Mumbai, India). All other chemicals and solvents used were of analytical grade.
Production and analysis of oligosaccharides
FOS (containing 90–93 % (w/w) FOS and designated as FOS-90) consisting of 57 % kestose, 30 % nystose and 5 % fructofuranosyl nystose were prepared by the selective removal of glucose and sucrose (our unpublished results) from FOS-56 (consisting of 56 % (w/w) FOS), which were derived previously from cane sugar using fungal fructosyl transferase(Reference Sangeetha, Ramesh and Prapulla17). XOS (consisting of 90–92 % (w/w) XOS) were prepared through a slight modification of the procedure used by Aachary & Prapulla(Reference Aachary and Prapulla18) under optimised conditions using commercial xylanase (Bioxyl P-40, Biocon Ltd, Bangalore, India). These two oligosaccharides were analysed by HPLC (LC-6A, Shimadzu, Kyoto, Japan) with a refractive index detector using a polar-bonded phase column (Exsil NH2, 4·6 mm × 25 cm, 5 μm) at an ambient temperature using acetonitrile–water (75:25) as a mobile phase at a flow rate of 1·0 ml/min. FOS were identified and quantified by comparing with standards of kestose, nystose and fructofuranosyl nystose(Reference Sangeetha, Ramesh and Prapulla17), while XOS were estimated according to Jeong et al. (Reference Jeong, Park and Kim19) and Aachary & Prapulla(Reference Aachary and Prapulla18). The refractive index detector used for the analysis of FOS and XOS has been standardised in our laboratory, and it was found to be satisfactory(Reference Sangeetha, Ramesh and Prapulla17, Reference Aachary and Prapulla18).
Animals and dietary treatment
The present animal study was carried out by taking all appropriate precautions and by strictly following the guidelines with regard to the use of animals for experimental purpose after due approval from the Institutional Animal Ethics Committee (CFTRI, Mysore, India). Influence of prebiotics on diabetic rats was examined using male Wistar rats (150–160 g) procured from the animal production facility of this institute. Rats were divided into the following groups: (1) control group fed with the basal diet; (2) control group fed with the basal diet containing XOS (10 %); (3) control group fed with the basal diet containing FOS (10 %); (4) control group fed with the basal diet containing XOS (5 %)+FOS (5 %); (5) diabetic control group fed with the basal diet; (6) diabetic group fed with basal diet containing XOS (10 %); (7) diabetic group fed with the basal diet containing FOS (10 %); (8) diabetic group fed with the basal diet containing XOS (5 %)+FOS (5 %). The basal diet consisted (%) of maize starch, 54; casein, 21; refined peanut oil, 10; powdered cane sugar, 10; Bernhardt-Tommarelli salt mixture, 4; and National Research Council (NRC) vitamin mixture, 1. The oligosaccharide diets contained either XOS (10 %) or FOS (10 %), or a combination of XOS (5 %) and FOS (5 %) at the 10 % level by replacing an equivalent amount of maize starch in the basal diet (w/w).
Induction of diabetes
Diabetes was induced by a single administration of STZ (intra- peritonially 40 mg/kg BW in 1 ml of 0·1 m-citrate buffer, pH 4·5) to overnight fasted rats. A parallel set of control rats (non-diabetic) were injected with citrate buffer only. Glucose (5 %) was given for 48 h following the intra-peritonial injection of STZ to prevent initial drug-induced hypoglycaemic mortality. Blood was drawn from the retro-orbital plexus 1 week after STZ administration, and it was used for the determination of fasting blood glucose. Animals having at least two and half times the normal fasting blood glucose were considered as hyperglycaemic.
The groups of rats were allowed to access the respective food and water ad libitum for 6 weeks. BW was recorded weekly. Twenty-four-hour urine samples were collected at weekly intervals from individual rats housed in metabolic cages, filtered and stored at − 20°C for further analysis of urinary protein and glucose excretion. Blood glucose was monitored biweekly, while urinary excretion of glucose and protein was monitored weekly.
At the end of the experiment, rats were killed, and blood was collected in heparinised tubes (20 U heparin/ml blood) and centrifuged at 4°C at 4000 rpm for 10 min (Remi centrifuge, Mumbai, India). The separated plasma was stored at − 20°C until processed. The caecum samples were collected and weighed wet. An aliquot from caecal content was used for microbial analysis, and the remainder was stored at − 20°C for further analysis. The kidneys were excised, weighed, homogenised in phosphate buffer using a Potter–Elvehjem homogeniser, and were then centrifuged at 8000 rpm at 4°C for 20 min. The supernatant was stored at − 20°C for further analysis.
Caecal characteristics
After dissection, the rat caecum was immediately removed, and total net weight was recorded, and then it was stored at a refrigerated temperature for further analysis. Bifidobacteria and lactobacilli were enumerated from a known quantity of suitably diluted caecal matter using spread plate method in selective Bifidobacterium iodoacetate agar (anaerobically, 37°C for 48 h) and Lactobacillus MRS (de Mann Rogosa) agar (aerobically, 37°C for 24 h), respectively. Microbial counts were expressed as log colony-forming units/g wet sample. Aseptic conditions were maintained throughout the microbial enumeration. The pH of the caecal matter was also recorded (Analab Scientific Instrument Pvt. Ltd, Vadodara, India).
Analytical parameters
Fasting blood glucose and glucose excretion in urine were determined by the glucose oxidase and peroxidase method(Reference Huggett and Nixon20). Total plasma protein and protein excretion in urine were analysed by the Bradford method(Reference Bradford21). The method described by Folin & Wu(Reference Oser22) was used to estimate plasma and urinary creatinine. Plasma urea was estimated as described by Levine(Reference Oser23), and urinary urea was measured as described by Wyebenga et al. (Reference Wyebenga, Di Giorgio and Pileggi24). Formation of AGE products in renal tissue was determined as reported by Monnier & Cerami(Reference Monnier and Cerami25). Plasma total cholesterol was determined as described by Rudel & Morris(Reference Rudel and Morris26). Activity of catalase was assayed by the method of Takahara et al. (Reference Takahara, Hamilton and Nell27), and glutathione reductase (GR) activity was assayed using a standard procedure(Reference Racker28). All the experiments were carried out in triplicate.
Histological studies
Light microscopic observations were made with haematoxylin–eosin-stained thin sections of kidney previously fixed in 10 % formalin and embedded in paraffin.
Statistical analysis
Values were expressed as means and standard deviations of eight rats. Statistical analysis was carried out using Origin 6.1 statistical software (Originlab Corporation, Northampton, MA, USA). Results were analysed and the significance level was calculated using the Tukey–Kramer multiple comparison test, and results are considered significant at P < 0·05.
Results
Effect of xylo-oligosaccharides and fructo-oligosaccharides on body weight and mortality
The influence of prebiotic supplementation on the BW of diabetic rats and normal rats is presented in Table 1. Gain in BW was markedly suppressed in diabetic rats, whereas the diabetic rats fed with XOS and FOS diets showed a significant improvement in the BW during the feeding period in comparison with those fed with the basal diet. The BW of the control rats fed with oligosaccharide diets was not significantly increased compared with those fed with the basal diet. High mortality was generally observed in diabetic rats (as much as 50 %) fed with the basal diet, but oligosaccharide supplementation reduced the mortality by up to 8, 14 and 21 % in the case of FOS (10 %), XOS (10 %) and combination of XOS (5 %) and FOS (5 %) in diabetic rats, respectively (data not presented). Interestingly, the mortality was less in the case of FOS-fed diabetic rats compared with other diabetic groups.
Table 1 Effect of prebiotics on growth in diabetic rats
(Mean values and standard deviations of eight rats)
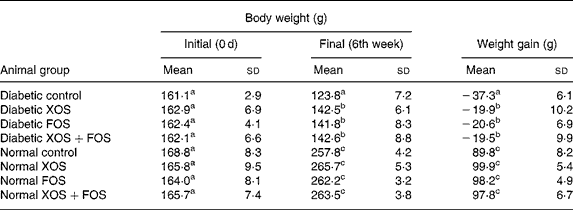
XOS, xylo-oligosaccharides; FOS, fructo-oligosaccharides.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P ≤ 0·05).
Effect of prebiotics on caecal characteristics
Data on caecal wet weight, pH, bifidobacteria and lactobacilli count are presented in Table 2. Total wet weight of the caecum was significantly (P ≤ 0·05) increased in diabetic and non-diabetic rats consuming XOS and FOS than in the rats fed with the basal diet. In addition, rats fed with oligosaccharides showed a significant decrease in pH from 6·73 (sd 1·6) up to 6·35 (sd 0·08) and an increase in the bifidobacteria and lactobacilli count in the caecum compared with rats fed with the basal diet. Rats fed with the XOS-containing diet, either 10 % or 5 %, showed a significant increase in bifidobacteria compared with rats fed with FOS (10 %). The rats fed with the FOS-containing diet, either 10 or 5 %, showed a significant concentration of lactobacilli compared to rats fed with XOS. Thus, the result explains the prebiotic efficacy of XOS and FOS.
Table 2 Effect of prebiotics on total caecum weight, pH, bifidobacteria and lactobacilli population of the caecum contents
(Mean values and standard deviations of eight rats)
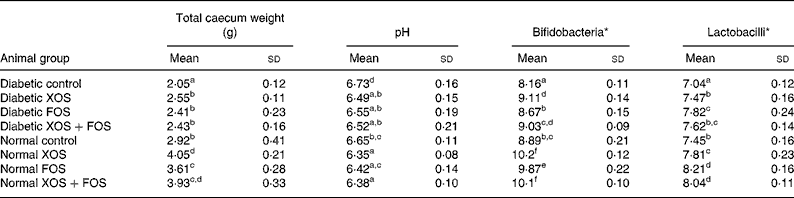
XOS, xylo-oligosaccharides; FOS, fructo-oligosaccharides.
a,b,c,d,e,f Mean values within a column with unlike superscript letters were significantly different (P ≤ 0·05).
* Log10 colony-forming units/g caecal wet content.
Effect of xylo-oligosaccharides and fructo-oligosaccharides on fasting glucose, cholesterol, creatinine and urea in plasma
Hyperglycaemia was ameliorated throughout the experimental period in the case of diabetic rats fed with the XOS or FOS diet. Treatment of diabetic rats with prebiotics at the 10 % dietary level for a period of 6 weeks brought down hyperglycaemia significantly (P ≤ 0·05; Fig. 1), and a similar trend was observed in the urinary glucose excretion pattern in oligosaccharide-fed diabetic rats (Fig. 2). In addition, oligosaccharide diets significantly (P ≤ 0·05) reduced the plasma cholesterol, creatinine and urea concentration in diabetic rats in comparison with diabetic rats fed with basal diet. Effects on plasma cholesterol, creatinine and urea are presented in Table 3.

Fig. 1 Fasting blood glucose in diabetic rats fed with prebiotics. Diabetic control (–♦–); diabetic xylo-oligosaccharides (XOS, –□–); diabetic fructo-oligosaccharides (FOS, –▲–) and diabetic XOS+FOS (– × –). * Mean values are significantly different from those of diabetic rats fed with prebiotic groups (P ≤ 0·05).

Fig. 2 Urinary glucose excretion in diabetic rats fed with prebiotics. Diabetic control (–♦–); diabetic xylo-oligosaccharides (XOS, –□–); diabetic fructo-oligosaccharides (FOS, –▲–) and diabetic XOS+FOS (– × –). * Mean values are significantly different from those of diabetic rats fed with prebiotic groups (P ≤ 0·05).
Table 3 Effect of prebiotics on protein, creatinine, urea, cholesterol in plasma and kidney weight
(Mean values and standard deviations of eight rats)

BW, body weight; XOS, xylo-oligosaccharides; FOS, fructo-oligosaccharides.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P ≤ 0·05).
Effect of xylo-oligosaccharides and fructo-oligosaccharides on plasma protein, kidney weight and advanced glycation end products
Table 3 shows the effect of XOS and FOS on plasma protein and kidney weight in rats. Diabetic rats fed with the basal diet showed a high degree of plasma protein depletion (59·0 (sd 2·9) mg/l) compared with control rats (65·6 (sd 1·1) mg/l). In diabetic rats fed with oligosaccharides, the plasma protein destruction was reduced up to 3–4 % compared with diabetic control rats. The urinary protein excretion pattern substantiates the above finding (Fig. 3). Countering of proteinuria by dietary oligosaccharides in diabetic rats was progressive with the duration of the diet regimen, and it was maximum in the FOS group. As is typical in diabetes, the increase in the kidney weight in diabetic rats was markedly high compared with the control rats. A reduction in kidney weight was observed in the case of diabetic rats fed with oligosaccharides compared with diabetic control rats. The increased kidney weight corresponds to the presence of diabetic nephropathy such as heavy AGE product formation and subsequent damage in renal tissues. XOS and FOS diets also decreased the level of AGE products in renal tissue of diabetic rats compared with those fed with the basal diet. The relative intensity of 15·3 for diabetic controls corresponds to an increased number of AGE products, which was found to be significantly reduced to a relative intensity of 12·83, 13·20 and 13·14 at 440 nm in the diabetic rats fed with XOS, FOS and a combination of XOS+FOS, respectively. The control rats fed with prebiotics showed a relative intensity in the range of 10·8–10·9, which was comparable to that shown by normal control rats fed with the basal diet.

Fig. 3 Urinary protein excretion in diabetic rats fed with prebiotics. Diabetic control (–♦–); diabetic xylo-oligosaccharides (XOS, –□–); diabetic fructo-oligosaccharides (FOS, –▲–) and diabetic-XOS+FOS (– × –). * Mean values are significantly different from those of diabetic rats fed with prebiotic groups (P ≤ 0·05).
Histopathology of kidney sections
Histological examination of the kidney sections revealed pronounced glomerulosclerosis and tubular lesions and cellular infiltration in all diabetic rats maintained on the basal diet. The diabetic rats fed with XOS and FOS showed a decreased degree of renal pathology compared with diabetic rats fed with the basal diet (Fig. 4).

Fig. 4 Kidney sections of diabetic rats maintained on prebiotics (stained with haematoxylin–eosin; × 100 magnification). (a) Normal control (normal renal morphology); (b) diabetic control (characterised by increased glomerulosclerosis and tubular lesions); (c) normal xylo-oligosaccharides (XOS)+fructo-oligosaccharides (FOS; normal renal morphology); (d) diabetic XOS+FOS (showing reduced renal pathology).
Effect of xylo-oligosaccharides and fructo-oligosaccharides on the activities of plasma catalase and glutathione reductase
Results showed that the activity of antioxidant enzymes, with catalase and GR, was lowered in diabetic rats compared with normal control rats, but diabetic rats fed with oligosaccharides showed improved (P ≤ 0·05) activities of catalase and GR compared with diabetic control rats fed with the basal diet (Table 4). Such an increase, however, was not observed in normal rats fed with XOS or FOS.
Table 4 Effect of prebiotics on plasma catalase and glutathione reductase activities in diabetic rats*
(Mean values and standard deviations of eight rats)
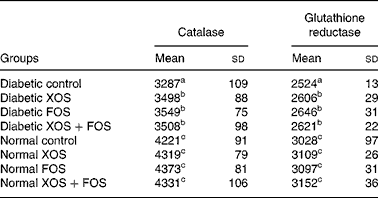
XOS, xylo-oligosaccharides; FOS, fructo-oligosaccharides.
a,b,c Mean values within a column with unlike superscript letters were significantly different (P ≤ 0·05).
* All values are expressed as units/l.
Discussion
In the present study, consumption of oligosaccharides FOS and XOS showed desirable effects in STZ-induced diabetic rats. Oligosaccharide diets markedly improved the BW in diabetic rats, reduced the mortality and significantly increased the bifidobacteria and lactobacilli population in the caecum. The tested oligosaccharides at 10 % dietary concentration beneficially countered fasting hyperglycaemia associated with diabetes, improved plasma albumin and significantly attenuated the rise in plasma concentrations of cholesterol, creatinine and urea. In addition, diabetes-induced reduction in the activity of antioxidant enzymes catalase and GR in plasma was ameliorated. Attenuation of diabetic nephromegaly by dietary oligosaccharides was accompanied by a reduction in AGE products in the renal tissue. Dietary levels of XOS and FOS (10 %) used in the present study conform to the levels used in our previous report on the beneficial effect of FOS(Reference Mabel, Sangeetha and Platel29). Health beneficial hypocholesterolaemic and antidiabetic influences of various dietary fibres included up to 10 % in the diet in animal studies have been reported by various authors without any adverse effects. Thus, we used the 10 % level of oligosaccharides either individually or in combination.
While dietary XOS and FOS resulted in a similar influence on various parameters studied, a striking difference between them was their effect on microbial flora. While FOS stimulated the growth of lactobacilli, XOS stimulated the growth of bifidobacteria. Several in vitro and in vivo studies have demonstrated that diets containing oligosaccharides such as FOS and XOS selectively increase the bifidobacteria and lactobacilli population, and decrease the pH of caecal content(Reference Gibson and Roberfroid10, Reference Campbell, Fahey and Wolf13, Reference Hsu, Liao and Chung14). In the present study, their dietary supplementation at the 10 % level significantly increased the caecum total weight, bifidobacteria and lactobacilli population, and decreased the pH of the caecal content. The reduction in the pH of caecal content after the supplementation of prebiotics could be attributed to an increase in the SCFA concentration by fermentation of bifidobacteria and lactobacilli. Among the two oligosaccharides, XOS was found to be more bifidogenic, which is in agreement with earlier reports of XOS (1 g/d) producing a selective increase in bifidobacteria(Reference Okazaki, Fujikawa and Matsumoto30–Reference Kleessen, Hartmann and Blaut32). The growth of lactobacilli was enhanced by FOS as observed previously by Campbell et al. (Reference Campbell, Fahey and Wolf13). These beneficial microflora which can utilise XOS and FOS produce SCFA such as lactate, acetate, propionate and butyrate, which in turn influence the glucose and lipid metabolism(Reference Venters, Vorster and Cummings33, Reference Laurent, Simoneau and Marks34).
The present study has shown that with the administration of XOS or FOS for a period of 6 weeks, the plasma glucose level was significantly decreased in the diabetic rats. The reduction in high glucose level was observed in diabetic rats from the second week of prebiotic supplementation, and the reduction was found to be maximum in FOS-fed diabetic rats at 6 weeks. It has been reported earlier that the intake of FOS (syrup containing 56 % FOS) at 10 and 5 % in the diet for a period of 6 weeks did not affect the blood glucose level in diabetic rats(Reference Kleessen, Hartmann and Blaut32). The positive effect of FOS on the severity of hyperglycaemia in diabetic rats observed in the present study is in contrast to an earlier report(Reference Mabel, Sangeetha and Platel29), possibly because of the higher concentration of FOS (syrup containing 90 % FOS) in the present study compared with FOS (syrup containing 56 %) used earlier. Intake of FOS (8 g/d) for a period of 14 d has been reported to decrease fasting blood glucose level in diabetic subjects(Reference Yamashita, Kawai and Itakura15). Dietary supplementation with XOS (4 g/d) improved blood sugar and lipids in type 2 diabetes(Reference Sheu, Lee and Chen35). These two reports and the present study thus suggest that FOS- or XOS-containing diets are beneficial to diabetic subjects in alleviating the severity of hyperglycaemia.
In the present study, the dietary oligosaccharides significantly reduced the plasma cholesterol level in diabetic rats. It is suggested that the oligosaccharides could reduce blood cholesterol by reducing the cholesterol absorption and increasing the excretion of bile acid and cholesterol in faeces(Reference van Bennekum, Nruyen and Schulthess36) or by decreasing the enzymes involved in fatty acid synthesis(Reference Williams37). Several studies have shown that intake of FOS reduces TAG and cholesterol associated with LDL and VLDL(Reference Kok, Roberfroid and Robert38). Supplementation of XOS (10 %) in diet for a period of 5 weeks significantly reduced cholesterol and TAG in diabetic rats(Reference Imaizumi, Nakatsu and Sato16), and also XOS (4 g/d) reduced the total cholesterol and LDL-cholesterol values in type 2 diabetes(Reference Sheu, Lee and Chen35). It has been reported that plasma cholesterol and TAG might be reduced by an increase in the amounts of SCFA (propionic acid) in the large intestine during fermentation of non-digestible carbohydrates(Reference Beylot39), and it has been demonstrated that propionic acid supplementation decreased the fasting serum glucose and insulin sensitivity and influenced lipid metabolism in healthy volunteers(Reference Venters, Vorster and Cummings33). The decrease in plasma cholesterol in diabetic rats fed with XOS or FOS could be due to the inhibition of cholesterol synthesis by an increase in propionate or modifications in the bile acid metabolism during oligosaccharide supplementation, and hence it can be used as an adjunct against hypercholesterolaemia.
Plasma creatinine level was higher in diabetic rats compared with normal animals, which is an indication of increased muscle wasting during this metabolic disorder. Dietary oligosaccharides lowered the blood creatinine concentration in diabetic rats. This indicates that these oligosaccharide diets counter muscle wasting normally associated with diabetes mellitus, and this is in concurrence with the improvement in BW observed in all the experimental diet-fed diabetic rats. Urea is another end product of the metabolic process and high blood urea levels indicate the impaired renal function, and oligosaccharide supplementation lowered the plasma urea concentration compared with that of diabetic control rats. A study demonstrated that the addition of oligosaccharides and gum arabic to the diet decreased blood urea by 20–30 % and renal nitrogen excretion relative to the control(Reference Younes, Garleb and Behr40).
In the present study, oligosaccharide supplementations were found to considerably protect this loss of protein in blood as indicated by decreased proteinuria in diabetic rats towards the end of the experimental period. These results are in agreement with the earlier study, wherein an intake of FOS (56 %) at 10 and 5 % dietary levels for a period of 6 weeks was found to protect diabetic rats from the urinary loss of albumin compared with diabetic rats fed with the basal diet(Reference Mabel, Sangeetha and Platel29).
An abnormally elevated blood glucose level causes oxidative stress and leads to the formation of AGE products(Reference Henri, Mule and Svend-Aage41). Dietary oligosaccharides reduced the formation of AGE products in the renal tissue of diabetic rats compared with those fed with the basal diet, suggesting that it would inhibit oxidative damage caused by the protein glycation reaction under diabetic conditions. These results showed that the administration of prebiotics at the 10 % dietary level might effectively alleviate the pathogenesis of diabetic complications caused by impaired glucose metabolism and the glycosylation of tissue proteins, eventually resulting in an alleviation of the diabetic pathological conditions. The increased weight of kidney in diabetic rats was also countered in oligosaccharide-fed diabetic animals. Administration of these test materials for 6 weeks reduced protein depletion, nephromegaly and glycation of renal tissue proteins. Light microscopy of kidney sections revealed nephromegaly and damaged glomeruli and basement membrane in diabetic rats. The magnitude of these changes was less in FOS- or XOS-fed diabetic rats. Presumably, this beneficial ameliorating influence of dietary oligosaccharides on diabetic nephropathy is attributable to their lowering effects on blood cholesterol levels(Reference Henri, Mule and Svend-Aage41).
In the present study, the activity of catalase in the blood of diabetic rats fed with FOS or XOS was significantly (P ≤ 0·05) higher compared with diabetic rats fed with the basal diet. In contrast, supplementation of XOS (4 g/d) has been reported to reduce the activity of catalase in erythrocytes but not the activity of superoxide dismutase and glutathione peroxidase in type 2 diabetes(Reference Baynes42). GR is another important oxidative defence enzyme, which converts the GSSG to GSH, an antioxidant molecule. Reduction of GSSG to GSH was found to be decreased in diabetic rats compared with diabetic rats fed with prebiotic diets. Prebiotic-mediated protection of plasma protein could be implied in improved activity of catalase and GR. The increase in GR activity in blood in turn neutralises superoxide anions and counteracts oxidative stress in diabetes.
Conclusions
The present investigation indicates that the supplementation of oligosaccharides (XOS and FOS) at the 10 % dietary level confers beneficial effects in STZ-induced diabetic rats with respect to plasma glucose, cholesterol and several other metabolic parameters. The reduced hyperglycaemia and hypercholesterolaemia by oligosaccharides may have in turn contributed to a decrease in the AGE products in the renal tissue and lowered nephromegaly in diabetic rats. The present study indicates that XOS and FOS at the 10 % dietary level can be used as an adjunct to dietary therapy to derive antidiabetic benefits, and to delay secondary complications. A detailed study on the mechanisms of these beneficial effects exerted by these oligosaccharides would be challenging and merits further investigation.
Acknowledgements
The authors thank Life-science Research Board, DRDO, New Delhi, for funding the project. The animal study was designed by S. G. P. and K. S. D. G., A. N. M. and G. P. performed the animal study, analytical work and statistical analysis of data. D. G. drafted the manuscript; K. S. and S. G. P. reviewed and interpreted the experimental data and refined the write-up of the present manuscript. The authors thank the Director of CFTRI for supporting the work. There are no conflicts of interest whatsoever among the authors.










