INTRODUCTION
During the 2009 influenza A(H1N1) pandemic (pH1N1), North American Indigenous populations suffered disproportionately compared to the general population, as shown by higher rates of influenza-like illness [Reference Dee1], hospitalizations [Reference Wenger2–Reference Thompson4], intensive care unit admissions [Reference Thompson4–Reference Zarychanksi6] and a fourfold increased rate of death [7]. We conducted a case-control investigation to determine risk factors for death due to pH1N1 in five US states with large American Indian/Alaska Native (AI/AN) populations. Our objectives were to determine: (1) whether AI/AN racial status was an independent risk factor for death and, (2) the risk factors for death within the AI/AN population.
METHODS
This investigation was conducted in Alaska, Arizona, New Mexico, Oklahoma and Wyoming. Case-patients (cases) were state residents who died related to infection with laboratory-confirmed influenza A from 15 April 2009 to 31 January 2010. Influenza infection was defined by a positive polymerase chain reaction (PCR) test, viral culture confirming pH1N1, a rapid influenza A test, or a direct fluorescent antibody test on a specimen collected from 15 April to 31 December 2009. We excluded cases who had no contact with a healthcare provider in the 14 days before death, or whose death and illness onset occurred when the person was located outside their state.
We attempted to match cases with two control-patients based on state of residence and influenza specimen date (within 14 days). Controls were state residents who had laboratory-confirmed pH1N1 infection (confirmed by PCR or culture) from 15 April to 31 December 2009 and who were not hospitalized for influenza within 30 days after their specimen collection date. Cases and controls were identified from death certificates and notifiable disease reports. Death certificates and medical records were abstracted using a standard form. Additionally, we interviewed a case proxy, defined as an adult who lived with or cared for the case prior to their illness, or a relative or close friend who lived nearby, or a relative knowledgeable of the case. If no case proxy was interviewed, we abstracted seven of the questions from the medical record. Interviews began in October 2010 and ended March 2012. Data on fatalities from influenza for the US population was obtained from national surveillance.
Four of the states required reporting of positive laboratory tests for pH1N1 (PCR and culture). Oklahoma obtained laboratory data from tests performed by its Public Health Laboratory. Outpatients with pH1N1 were randomly selected and contacted for an interview. Participants' individual medical records were obtained and we abstracted demographic information, height, weight, health insurance status, medical and vaccination history, and influenza illness treatments. From interviews we obtained self-identified race, household characteristics, access to healthcare, past medical history, tobacco and alcohol habits, income and educational attainment.
Data were double-entered into Paradox v. 9·0 (Corel, Canada). Univariable tests were run in a conditional logistic regression model using the Wald χ 2 statistic. Households with ⩾1·5 persons per room were considered crowded and poverty was defined as having an annual household income <US$ 25 000 [Reference Blake, Kellerson and Simic8]. Obesity was defined as a body mass index (BMI) ⩾30 (adults), a BMI ⩾95th percentile for age (2–17 years), or ⩾95th percentile of weight for age (<2 years). Influenza-like illness was defined by a reported fever, and either a cough or a sore throat. Age was modelled using three age groups (<18, 18–44, ⩾45 years). Receipt of antiviral medications was categorized into three levels: none, received ⩽2 days after symptom onset, received ⩾3 days after symptom onset.
Missing data ranged from 0% (age) to 25% (income). Missing data imputation procedures were employed, assuming data were missing at random. Data were imputed using Markov chain Monte Carlo iterations assuming a multivariable normal distribution [Reference Allison9–Reference Lee and Carlin11]. The imputation model included variables for state of residence and specimen collection date, and all variables in the univariable analyses and case-control membership. Dichotomous variables derived from continuous variables were imputed in their continuous form; other dichotomous variables were imputed as dichotomous indicators [Reference Allison9, Reference Bernaards, Belin and Schafer10]. We created 20 imputed datasets for the multivariable models [Reference Graham, Alchowski and Gilreath12]. A single chain was used with 200 burn-in iterations and 100 iterations between datasets. Each imputed dataset was analysed using conditional logistic regression and then estimates were combined accounting for the parameter variability estimates and the variability associated with the imputation process. Analyses were conducted in SAS using logistic and imputation procedures [Reference Yuan13]. Multivariable models used purposeful forward selection and included variables with a univariate P value <0·25 [Reference Hosmer and Lemeshow14]. After determining main effects, all two-way interactions with appropriate sample sizes were evaluated for statistical significance. Variable selection was repeated on the imputed datasets using the combined Wald χ 2 statistic that incorporated the between- and within-imputation variation components [Reference Wood, White and Royston15]. Multivariable models included: the imputation model and a complete case analysis, where matched pairs or observations were entered or missing based on whether they had missing data for any given risk factor in the model.
A multivariable model restricted to AI/AN persons evaluated a subset of factors, due to sample size limitations, including demographics, healthcare access, one socioeconomic variable, and all other variables that had adequate sample sizes. The variables that were used to match cases and controls were considered independent predictors in this model. Model diagnostics were run to identify influential observations or matched pairs in terms of model fit and parameter point and variance estimates. All P values were two-sided and a value <0·05 was considered statistically significant.
Human participant protection and tribal review
The investigation was determined to be a non-research public health practice investigation by CDC and the Indian Health Service. Additional human subjects review and approval was obtained: Alaska Area IRB; Arizona: Navajo Nation Health Research Review Board, Arizona Department of Health Services – Human Subjects Review Board, Gila River Indian Community – Research Review Committee; New Mexico: New Mexico State University IRB, Southwest Tribal IRB, Navajo Nation Human Research Review Board; Oklahoma State Department of Health IRB; Wyoming Department of Health IRB. The protocol was approved by the following Tribal entities: Arizona: San Carlos Apache Tribe – Health Committee, San Carlos Apache Tribe Tribal Council, Tohono O'odham Nation – Health and Human Services Committee, Tohono O'odham Legislative Council, Alaska Native Tribal Health Consortium; Oklahoma City Area Intertribal Health Board; Wyoming: Eastern Shoshoni and Northern Arapahoe Tribes Montana/Wyoming Tribal Leaders Council/Rocky Mountain Tribal Epidemiology Center.
RESULTS
A total of 257 fatalities associated with influenza A infection were reported in these states (annualized mortality: 6·6/100 000 persons per year). Of these, 145 met the case definition (Table 1). The epidemic peak occurred during calendar weeks 40–44, similar to the overall US epidemic (Fig. 1). Thirty-two fatalities were excluded, principally because they did not see a healthcare provider prior to death (90%); 69% of excluded cases were from Arizona. Eighty fatalities from Arizona were not included in analysis because of incomplete data or because Tribal approvals were not obtained. These persons were similar to the cases included from Arizona with regard to sex, age, diagnosis date, and underlying medical conditions; however, those not included were more likely to be of AI/AN racial status than the included fatal cases (26% vs. 11%, P = 0·01).

Fig. 1. Fatal influenza cases by week of death, 2009, for five states (Alaska, Arizona, New Mexico, Oklahoma, Wyoming) and overall United States.
Table 1. Characteristics of participants, influenza mortality investigation for five states (Alaska, Arizona, New Mexico, Oklahoma, Wyoming), 2009

* Twelve outpatient controls did not have a chart review completed and sex was not determined.
† Residence type only available for 88 (61%) of the cases and 210 (89%) of the controls.
‡ Twenty-two persons listed ‘Hispanic’ as their race and one person listed ‘Filipino’.
§ Results reflect medical chart review results. All controls were reported to the state health department as having a positive culture or PCR for pH1N1.
We recruited 236 controls; 91 (63%) cases were matched to two controls and 54 were matched to one control. Proxy interviews could not be obtained for 43 (30%) fatal cases. Univariate risk factors for death are shown in Table 2. Multivariable analysis identified four independent risk factors for a fatal infection (Table 3): older age, having a pre-existing medical condition, being a smoker and receiving antivirals ⩾3 days after illness onset. Using the imputed dataset, a fifth independent variable was identified: a financial or transportation-related barrier to healthcare access. AI/AN race was not significantly associated with death after adding age and pre-existing conditions to the models. There were no significant two-way interactions between the risk factors in the final models.
Table 2. Univariate risk factors for death due to H1N1 in a matched case-control investigation, five states (Alaska, Arizona, New Mexico, Oklahoma, Wyoming), 2009

mOR, Matched odds ratio; CI, confidence interval; BMI, body mass index; AI/AN, American Indian/Alaska Native. * Odds provided for a 10-year increase in age; odds for a single year increase was 1·04.
† Odds, case numbers, percentages and P values for those aged ⩾7 years.
‡ Odds and P value for persons aged ⩾18 years.
§ Alcohol abuse noted in chart or ⩾15 drinks/month.
|| Includes asthma, chronic lung disease, cardiovascular disease, diabetes, other chronic metabolic disease, cancer (last 12 months), renal disease, liver disease, neuromuscular disease, blood disorder, aged <19 years with aspirin therapy, immunosuppressive condition, and alcohol abuse.
Table 3. Risks factors for influenza mortality, multivariable results for matched case-control investigation, for five states (Alaska, Arizona, New Mexico, Oklahoma, Wyoming), 2009

mOR, Matched odds ratio; CI, confidence interval; AI/AN, American Indian/Alaska Native; n.s., not significant, removed from model.
* n = 258 observations in the final model for all races (from 106 case-control matches), and n = 63 observations in the final model for AI/AN race.
† n = 381 observations the final model for all races (from 145 case-control matches), and n = 70 observations in the final model for AI/AN race.
‡ For AI/AN race model, OR are not matched.
Mortality risk factors for the 70 AI/AN participants are given in Table 4. Multivariable analysis identified two independent risk factors for death: pre-existing medical conditions and obesity (Table 3). Using the imputed data, a third independent risk factor was identified: having been a smoker.
Table 4. Univariate risk factors for influenza mortality in American Indian/Alaska Native persons (alone or in combination with other races), for five states (Alaska, Arizona, New Mexico, Oklahoma, Wyoming), 2009
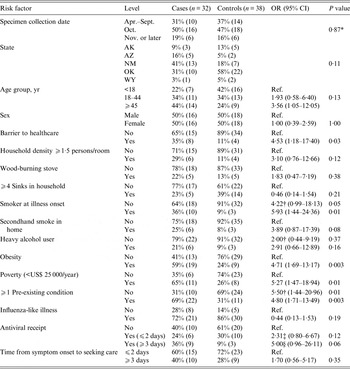
OR, Odds ratio; CI, confidence interval.
* Test if overall mean specimen date differs between American Indian/Alaska Native cases and controls.
† In those aged ⩾18 years.
‡ Comparing those who received antivirals (⩽2 days and ⩾3 days combined) to those who did not.
§ Comparing those who received antivirals ⩾3 days after symptom onset to those receiving antivirals ⩽2 days after symptom onset.
DISCUSSION
In this case-control investigation from US states with substantial AI/AN populations, we used medical records and interviews to assess potential risk factors for pH1N1 influenza A mortality. Three of the risk factors are modifiable (smoking, delayed receipt of antiviral medications, and a barrier to accessing healthcare). The association of death with smoking and a barrier to healthcare access have not been previously described for influenza [Reference Epstein, Reynaldo and Nelson El-Amin16, Reference Regan17]. Two other risk factors (older age and underlying medical conditions) are well recognized. AI/AN persons were overrepresented in the fatal cases (22% vs. 16% of controls, P = 0·05); however, AI/AN racial status was not an independent risk for death. The risk factors for death in the AI/AN population (pre-existing medical conditions, smoking, obesity) are similar to other North American populations [Reference Regan17–Reference Morgan19]. This is the first population-based investigation to evaluate AI/AN race as a potential risk factor for influenza mortality and the first to evaluate influenza mortality risk factors in the AI/AN population.
Smoking has not been previously identified as a risk factor for influenza mortality [Reference Epstein, Reynaldo and Nelson El-Amin16]. Other studies of mortality risk during this pandemic either did not evaluate smoking [Reference Regan17, Reference Fowlkes18] or did not show an association when they compared fatal and hospitalized patients [Reference Zarychanski20, Reference Kumar, Zarychanski and Pinto21]. The prevalence of smoking in controls (11% for those aged ⩾7 years) was much lower than the adult smoking prevalence in these states, which ranges from 19·2% to 26·1% [22]. This could have led to an overestimation of the magnitude of this risk factor. Despite this potential limitation, smoking is a biologically plausible risk factor and should be investigated further.
The financial or transportation-related barriers to healthcare access may have caused patients to delay seeking care after illness onset, resulting in more severe illness that was less amenable to treatment. Although 93% of the fatal cases had health insurance, insurance coverage differs with regard to costs paid by the patient and healthcare-seeking behaviour is complicated. Further investigation is needed to identify ways to reduce barriers to healthcare in high-risk individuals, with and without health insurance.
Whether AI/AN race is, by itself, a risk factor for death or is a marker for other factors has substantial implications. Finding that AI/AN racial status was a risk factor might imply an undiscovered genetic susceptibility to severe influenza. A genetic explanation offers limited prevention options and could have a chilling effect on efforts to reduce influenza mortality in AI/AN populations [Reference Jones23]. Because AI/AN race is a marker for other risk factors, we can now focus on those modifiable risks in AI/AN persons. Three risk factors from the AI/AN-specific model were common among fatal cases (obesity 61%, smoking 35%, pre-existing conditions 69%). Similar to the overall population, we observed trends in AI/AN persons for higher mortality for older age, delay in antivirals (cases 36%, controls 9%), and healthcare access barriers (cases 35%, controls 11%). Accessing healthcare is a problem for AI/AN persons with influenza [Reference Dee1]. Of adults in 2009, AI/AN persons had the highest frequency of influenza-like illness of any racial group (16·2% vs. 8·2% overall), yet were the least likely to seek healthcare (37·4% vs. 42·1% overall). Further efforts are indicated to improve access and healthcare-seeking behaviour in AI/AN persons. Environmental determinants for lower respiratory tract infections common in AI/AN populations deserve further attention. These include household crowding [Reference Bulkow24, Reference Singleton25], limited access to in-home water and sanitation services [Reference Hennessy26–Reference Wenger28], and household air pollution from wood-burning stoves or second-hand tobacco smoke [Reference Robin29].
The influenza mortality disparity between AI/AN persons and the general population is a challenge for influenza preparedness [Reference Groom30]. Prior to 2009, AI/AN individuals were not prioritized to receive vaccine or antiviral medications on the basis of racial status in the United States. However, recommendations now include AI/AN persons as a high-risk group [31, 32]. Some might consider that a risk factor-based strategy would be sufficient to identify AI/AN persons at risk for influenza complications. However, using the criteria for receipt of antiviral medications, 30% of fatal AI/AN cases would not have been considered high risk. By contrast, only 16% of influenza fatalities in whites would not have been considered high risk. AI/AN persons during 2009 also suffered disproportionately from influenza illness, hospitalizations and intensive care unit admissions. This is similar to the experience of AI/AN persons throughout the past two decades [Reference Groom33]. Thus, a risk factor-based strategy may not be comprehensive enough to address these long-standing influenza disparities. Designating AI/AN persons as high-risk can allow a more rapid delivery of vaccine, antiviral medications and education through the US Indian Health Service, tribal and urban Indian clinics. Thus, maintaining the high-risk designation may be more likely to reduce the health disparity than a risk factor-based approach.
Influenza immunization uptake in AI/AN persons is similar to the general United States population, but should be improved [34, Reference Lindley35]. Efforts to reduce smoking prevalence and obesity in AI/AN persons may be beneficial in reducing influenza mortality. Smoking prevalence in AI/AN adults (31·4%) far exceeds the overall US population (19·0%) [36]. Likewise, obesity in AI/AN adults (39·6% prevalence) is more common that in non-Hispanic whites (26%) [37].
These findings may not be generalizable to the entire United States or to all AI/AN persons. Because controls had access to healthcare and telephone service, comparisons for related socioeconomic factors may have been limited by design. By modelling only three age groups our ability to detect gradations in risk within age groups is limited. Missing data was addressed through multiple imputation which improved the power but would not solve potential bias related to representativeness of the population. We may have underestimated the number of AI/AN persons in the fatalities, since misclassification of AI/AN decedents has been documented [Reference Arias38]. Since approval was not obtained from all Arizona tribes, there was systematic under-recruitment of AI/AN persons and reduced representativeness of the included cases. Glucocorticoids used as a fever-reducing agent was identified as a risk factor for influenza death in China [Reference Han39]. This is not a recommended practice in the United States [40]. However, because we did not obtain the timing or dosage of corticosteroid administration and nearly 50% of the fatal cases had received steroids, this remains a potential, uncontrolled confounder.
During the 2009 pandemic, AI/AN racial status was not independently associated with death, but was a marker for other modifiable factors. Keeping AI/AN race in the high-risk conditions for influenza complications should be considered as an appropriate response to the elevated risk of morbidity and mortality in this population. Increased efforts to reduce influenza mortality are needed to address this longstanding health disparity.
APPENDIX. Investigative Team
Alaska: Steve Bentley, James Keck, Sassa Kitka, Gail Thompson, Donna Fearey, Diana Redwood, Jessica Craig, Ellen Provost. Arizona: Keisha Robinson. New Mexico: Rhonda Noble. Oklahoma: Laurence Burnsed, Kendra Dougherty, Anthony Lee, Christie McDonald-Hamm, Laura Smithee, Jean Williams. Wyoming: Reginald McClinton. Council of State and Territorial Epidemiologists: Annie Tran. Indian Health Service: John Redd, James Cheek. CDC: Ralph Bryan, Michael Jhung. Other US States: Nato Tarkhashvili (South Dakota), Richard Danila (Minnesota), Richard Leman (Oregon), Eden Wells (Michigan), Kathy Lofy (Washington), Tracy Miller (North Dakota).
ACKNOWLEDGEMENTS
Alaska: Jeremy Wood, Greg Raczniak, Michele Toomey. Arizona: Sara Imholte, John Meyer, Steve Baty, Anita Thorne, Zeenat Mahal, Sibeso Joyner, Eva Torres, Erica Weis, Justin Weddle, Tina Szopinski-Rubin, Reva Litt, Amritha Panachanathan. Oklahoma: Alfred Aiyanyor, Rachel Clinton, Margaret Selby. Wyoming: Tracy Murphy, Clayton Van Houten. Council of State and Territorial Epidemiologists: Edward Chao, Jennifer Lemmings, Monica Huang. Other states: Melissa Powell (OR), Lon Kightlinger (SD), Rachelle Boulton (UT), Anthony Marfin (WA), Megan Hoopes (NPAIHB), Thomas Kim (CTEC), John Mosely Hayes (USETI), Thomas Weiser (NPAIHB), Lindsey VanderBusch (ND), Steven Helgersen (MT), Folorunso Akintan (RMTEC), Rachel Herlihy (UT), Ruta Sharangpani (MI), Jennie Finks (MI), Catherine Lexau (MN), Craig Morin (MN), Aaron DeVries (MN), Melissa Morrison (AL/CDC). CDC: Alicia Fry, Scott Santibanez, Jay Wenger.
Funding support was contributed by the Council of State and Territorial Epidemiologists (Cooperative Agreement Number 1U38HM000414 from CDC), plus in-kind contributions by CDC, state health departments and tribal epidemiology centers.
The findings and conclusions in this publication are those of the authors and do not necessarily represent the official views of the U.S. Centers for Disease Control and Prevention (CDC) or the Indian Health Service.
DECLARATION OF INTEREST
None.








