Genetic testing for high-penetrance mutations that follow Mendelian inheritance is increasing, generally in the context of pre- and post-test genetic counselling (e.g. using the Huntington's disease genetic testing protocols). By contrast, genotyping for low-risk susceptibility alleles is still in its infancy. Over the past decade, predictors of uptake and social impact of genetic testing for adult-onset disorders that follow Mendelian inheritance have been examined. Studies on uptake of genetic testing for hereditary cancers and Huntington's disease show that educational level, disease status and psychological factors (perceived risk, disease-related anxiety or distress) are consistently associated with interest in testing, more so than gender, age and marital status. Reference Meiser1,Reference Meiser and Dunn2 Studies of individuals receiving such genetic information suggest that those who do not carry ‘at risk’ genotypes derive psychological benefits, while those identified as ‘at risk’ show no adverse effects. Reference Meiser1,Reference Lerman and Shields3
In 2003, Caspi et al Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington4 demonstrated that multiple stressful life events were more likely to lead to depression in individuals with the s/s genotype of the promoter region of the serotonin transporter gene (5-HTTLPR) than those with the s/l and l/l genotypes, that is, there was a demonstrable gene–environment interaction in depression onset. This finding was replicated by seven other research groups including our own, Reference Eley, Sugden, Corsico, Gregory, Sham and McGuffin5–Reference Cervilla, Molina, Rivera, Torres-Gonzalez, Bellon and Moreno11 with two negative reports. Reference Gillespie, Whitfield, Williams, Heath and Martin12,Reference Surtees, Wainwright, Willis-Owen, Luben, Day and Flint13 Recently, the s/s genotype has also been associated with depression onset after hip fracture Reference Lenze, Munin, Skidmore, Dew, Rogers and Whyte14 and cardiac events. Reference Nakatani, Sato, Sakata, Shiotani, Kinjo and Mizuno15
There are no reports on predictors of uptake or impact of such genotype testing; data on its acceptance and impact are needed. We therefore decided to ‘test the water’ in our longitudinal cohort of individuals who had undergone genetic testing. We focused on this group as they had expressed interest, were articulate and were in a position to provide information about perceived benefits and concerns about testing for the 5-HTT genotype, which could then be examined in other groups. As they had also reviewed their personal history of depression, anxiety and adverse life events with the research team, and were past the peak age of depression onset, provision of the research results was thought less likely to lead to concerns about future onset of depression.
Method
Participants
Participants were from an initial group of 170 adults (114 women and 56 men) recruited in 1978 during a 1-year postgraduate teacher training programme. In 1983, 165 of the initial sample formed a cohort for a longitudinal study investigating risk factors of depression and were followed up at 5-year intervals. Reference Wilhelm, Parker and Ashari16 Cohort members were of a similar age (mean 23 years in 1978) with similar career and life opportunities and ethnic backgrounds; 160 were White from European backgrounds, 2 and 3 were of Chinese and Indian descent respectively. These shared demographic characteristics reduced the likelihood of psychosocial confounders.
By 2003, 149 of the original 165 individuals remained in the study (8 had died, 2 were unable to be located, 2 were too ill to continue and 4 refused further involvement). Criteria for the Composite International Diagnostic Interview-derived Reference Robins, Helzer and Burrows17 lifetime diagnosis of major depression had been met by 62 (42%) of the remaining participants, with mean age at onset of 30.7 years (s.d. = 8.2, range 15–50). Of the 149, 128 participants provided informed consent for collection of genetic material. On recruitment for the genetic study, they were given a page of general information about serotonin, the serotonin transporter gene and a summary of the study by Caspi et al. Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington4
After the genotype study Reference Wilhelm, Mitchell, Niven, Finch, Wedgwood and Scimone10 was completed, participants were invited to an information evening to discuss the results of the original genetics study, but not their own genotype. The issue of individual feedback was raised and interest level was high. Participants were also given information about the possible limitations, including the potential future obligation to provide results to insurance companies.
Following institutional ethics committee approval, cohort members were offered the opportunity to learn their own genotype and discuss any implications. Prior to divulgence of their genotype result, they were sent a ‘baseline’ self-rated questionnaire (see below), consent form and reply-paid envelope. After completion, an appointment was made, either in person or by telephone with the principal investigator (K.W.), a psychiatrist who had followed them throughout the study.
Participants were also offered the option of discussing their results with another clinician, and/or genetic counsellor, but none took up this offer.
At the interview, K.W. covered the following areas.
-
(a) Prior to disclosure, K.W. ascertained how much of the information provided had been accessed by the participant and each participant's knowledge of the relationship between the serotonin transporter genotype, stress and depression, with further details provided where necessary.
-
(b) The results were then given, together with further information about the implications for the participant or their family.
-
(c) After disclosure, K.W. raised the issue of participants' coping styles in times of stress, emphasising the need to review whether their coping styles served them well, with further time for questions.
-
(d) An offer of further discussion was made if indicated.
At the time of disclosure, those with the l/l allele were told that they were in the 30% of the population likely to have lower reactivity to a series of adverse life events; those with the s/l allele were told they were in the 50% with an intermediate level of reactivity; and those with the s/s allele were told they were in the 20% who were potentially more emotionally reactive when confronted with a series of life events, with an increased risk (∼twofold) for depression. Regardless of genotype, the importance of reflecting on how they dealt with stressful events was emphasised. Participants had already been told at the information night that the genetic effect seemed more relevant for the first onset of depression; that the peak age at onset of depression was in the 20–40 age band and those who were likely to develop depression had probably already done so. This was restated at the interview.
Participants choosing to learn their genotype (receivers) were mailed follow-up questionnaires 2 weeks and 3 months after learning their result. Participants electing not to learn their results (decliners) completed one follow-up questionnaire only, 3 months after the initial questionnaire.
Measures
Predictor variables
Data already collected from the 25-year follow-up included in this analysis were age, gender, number of children, personal and family history of depression, and 5-HTT genotype status. Reference Wilhelm, Mitchell, Niven, Finch, Wedgwood and Scimone10
The following measures were administered at baseline only.
Causes of depression. Measured on a five-point Likert scale, ranging from ‘totally owing to genetics’ to ‘totally owing to environment’, this single item assessed belief about the extent to which genetic v. environmental factors cause depression.
Short Positive and Negative Affect Scale (Short-PANAS). Reference Watson, Clark and Tellegen18 A 10-item measure of positive and negative affect was used to predict test uptake impact, with ten adjectives rated according to the extent participants described the way they felt ‘in general’.
Perceived benefits and limitations of testing. A 14-item measure that assessed perceived benefits and limitations of ‘testing for gene variations that influence the impact of stress on depression onset’ using a five-point Likert scale, ranging from ‘not at all important’ to ‘extremely important’. The items were developed on the basis of a qualitative study which explored the range of perceived benefits and limitations of genetic testing for bipolar disorder. Reference Meiser, Mitchell, McGirr, Van Herten and Schofield19 The two subscales comprising this measure demonstrated high internal consistency in the previous study on bipolar disorder Reference Meiser, Kasparian, Mitchell, Strong, Simpson and Tabassum20 and were adapted for this study by omitting items considered unsuitable for the current sample (e.g. items related to decision-making about marriage and childbearing). Using data from the current study, an exploratory factor analysis using maximum likelihood extraction followed by oblique (promax) rotation produced a two-factor solution with each item loading on a factor (>0.4) and the factors matching the a priori scales for the perceived benefits and limitations of serotonin transporter genotyping. The benefits factor (eight items) explained 31.4%, and the limitations factor explained 25.1% (six items) of the total variance and the correlation between factors was low (r=0.06), supporting their independence. They demonstrated good reliability, with Cronbach's alphas of 0.88 (benefits scale) and 0.85 (limitations scale).
Outcome variables
Perceived future risk of developing depression. A one-item measure, administered at baseline and the 2-week and 3-month follow-up to both receivers and decliners, assessed perceived future risk of depression on a numerical differential scale (ranging from 0 to 100). In addition, receivers completed the following measures at both follow-up periods.
-
(a) Test-related distress and positive experiences. This questionnaire comprises ten items from a validated instrument, the Multidimensional Impact of Risk Assessment Scale Reference Cella, Chang, Peterman, Wenzel, Marcus and Hughes21 assessing distress (six items, e.g. ‘feeling upset about my genetic risk factor result’) and positive experiences (four items, e.g. ‘feeling relieved about my genetic risk factor result’). Response options range from ‘never’ (0) to ‘often’ (5), and scores range from 0 to 30 and 0 to 20 for the distress and positive experiences scales respectively.
-
(b) Recall and interpretation of testing result. This scale asked receivers whether their genotype effected low, normal or high risk (l/l, s/l or s/s respectively) or was not recalled.
-
(c) Satisfaction with the decision to undergo genotyping. This questionnaire asked receivers whether they felt pleased about, unsure or regretted having learned their result.
Statistical analysis
Mann–Whitney U-tests were carried out using the ‘coin’ software Reference Hothorn, Hornik, van de Wiel and Zeileis22 and other analyses were conducted using SPSS (version 14) for Windows. Receivers and decliners were compared across a number of likely predictor variables using logistic regressions for categorical variables and Mann–Whitney U-tests for continuous variables as these variables were non-normal and could not be transformed into a normal distribution.
Controlling for the presence of lifetime major depression, we ran a repeated measures linear regression using mixed-effects modelling to assess whether the perceived risk of developing future depression differed between study groups (s/s, s/l, l/l genotypes and decliners) or across time (baseline, 2-week and 3-month follow-up) and also whether there was an interaction between these variables.
Results
Response rate and analysis of participation bias
As shown in Fig. 1, 102 (80%) participants returned their baseline questionnaire prior to receiving their genotype result. Individuals who completed baseline questionnaires were significantly more likely to subsequently choose to learn their genotype results (χ2=35.5, d.f.=1, P<0.001).

Fig. 1 Flow chart of the group and assessment structure.
Uptake of genotyping results
Of the 128 individuals (Fig. 1) offered the opportunity to learn their results, 84 (66%) chose to receive their results (receivers). When only those 102 participants returning baseline questionnaires are included in the denominator, the percentage of receivers is higher, with 79 (78%) receivers and 23 (23%) decliners. Of the 84 receivers, 80 elected to learn their results by telephone and four face to face. Receivers learned their results between 0 and 181 days (mean=62, s.d.=52) after completion of baseline assessment.
Perceived benefits and limitations of testing
Figure 2 shows the rates of endorsement for each item pertaining to the perceived benefits and limitations of genetic testing by receiver and decliner status. Overall, the items most frequently endorsed by the total group (n=102) as ‘quite/extremely’ important benefits of receiving such genotyping information were that it: (a) allowed for earlier intervention (84%); (b) provided the potential to prevent the onset of depression (83%); and (c) helped people proven to have a gene variation to avoid stressors or triggers that may lead to the onset of depression (77%). The most frequently endorsed items seen as ‘quite/extremely important’ limitations of receiving genotype information for the total group were that it could: (a) lead to insurance discrimination (73%); (b) lead to discrimination by employers (72%); and (c) make people who have a gene variation more likely to feel stressed, depressed or vulnerable (62%).
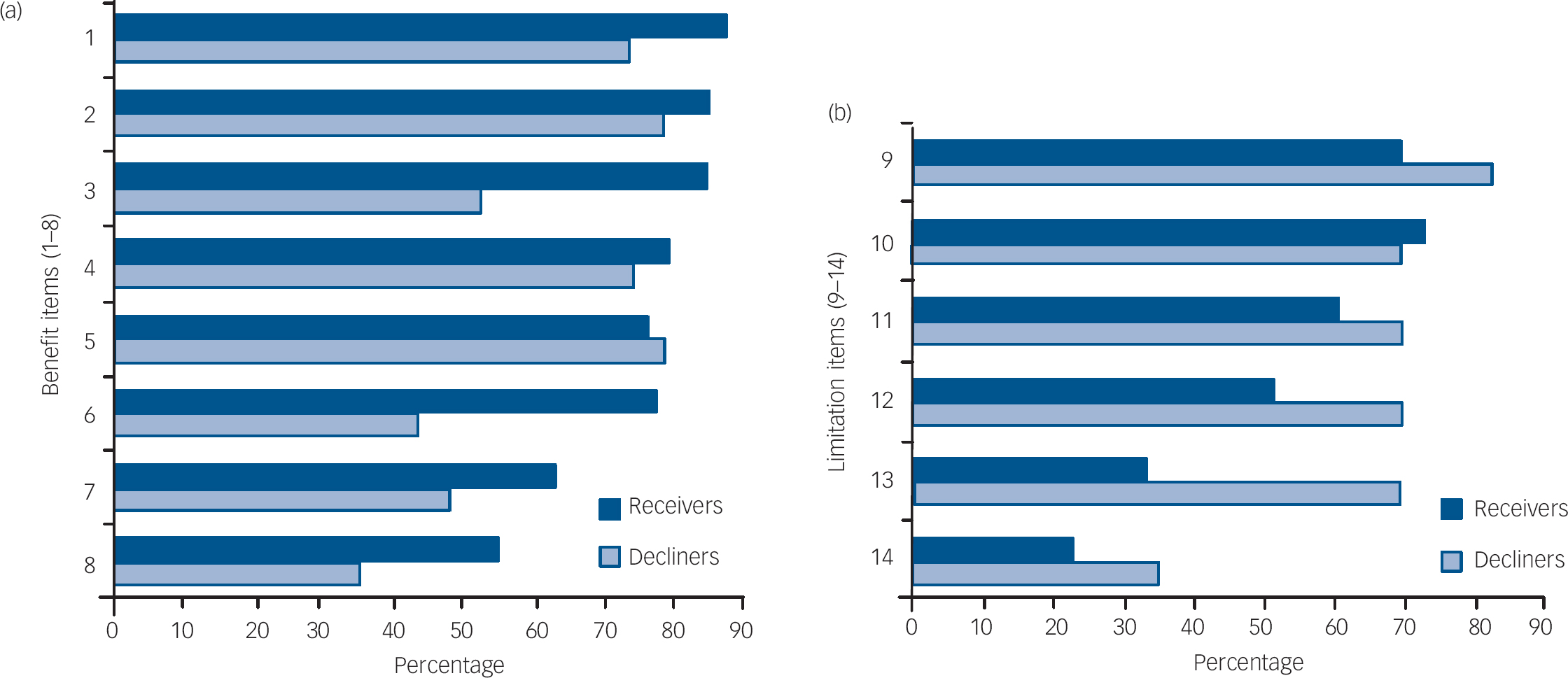
Fig. 2 Percentage of receivers and decliners endorsing perceived (a) benefits and (b) limitations of serotonin transporter genotyping as quite/extremely important factors (n = 102) Receivers who completed baseline questionnaire after learning their results (n = 5) were excluded from the analysis. 1 Allows for earlier intervention. 2 Provides the potential to prevent the onset of depression. 3 Helps people proven to have a gene variation to avoid stressors or triggers that may lead to the onset of depression. 4 Helps research into this illness. 5 Provides a basis for tailoring medications to specific gene variations to improve treatment outcomes. 6 Potentially allows for early diagnosis. 7 Allows improved basis for planning the future. 8 Allows increased certainty about my risk. 9 Could lead to insurance discrimination 10 Could lead to discrimination by employers. 11 Could mean that people who have a gene variation may be more likely to feel stressed, depressed, or vulnerable. 12 Could increase worry in people who have a gene variation where depression has not yet developed or may never develop. 13 Could mean living with uncertainty if genetic risk factor testing indicated probability of disease onset only. 14 Could increase stigma because of labelling.
Predictors of decision to learn genotyping results
Tables 1 and 2 shows the variables assessed as predictors of the decision to learn results (for all individuals invited into the study). The only factor significantly associated with the decision to learn genotyping results was higher ‘perceived benefits of testing’ scale scores (P=0.001) (Table 2).
Table 1 Demographics of study participantsa

| Variable | Decliners, n (%) | Receivers, n (%) | OR (95% CI) | P |
|---|---|---|---|---|
| Gender | ||||
| Male | 19 (44) | 24 (56) | ||
| Female | 25 (29) | 60 (71) | 1.90 (0.9-4.1) | 0.10 |
| Childrenb | ||||
| No | 10 (40) | 15 (60) | ||
| Yes | 34 (33) | 69 (67) | 1.35 (0.6-3.3) | 0.51 |
| Genotype result | ||||
| s/s | 12 (44) | 15 (56) | 1, Reference | |
| s/l | 23 (37) | 40 (64) | 1.39 (0.6-3.5) | 0.48 |
| l/l | 9 (24) | 29 (76) | 1.61 (0.9-2.7) | 0.08 |
| Prior episodes of major depressionb | ||||
| None | 25 (34) | 49 (66) | 1, Reference | |
| One | 6 (29) | 15 (71) | 1.28 (0.4-3.7) | 0.65 |
| Two or more | 13 (39) | 20 (61) | 0.89 (0.6-1.4) | 0.58 |
| Family history of depressionb | ||||
| No | 19 (31) | 43 (69) | ||
| Yes | 19 (33) | 38 (67) | 0.88 (0.4-1.9) | 0.75 |
Table 2 Factors explored for association with decision to learn serotonin transporter genotype result (n= 128)a

| Decliners | Receivers | Difference in means (95% CI) | ||||
|---|---|---|---|---|---|---|
| Variable | n | Mean (s.d.) | n | Mean (s.d.) | P | |
| Age | 84 | 50.4 (2.1) | 44 | 50.8 (3.1) | -0.44 (-1.46 to 0.59) | 0.91 |
| Perceived lifetime riskb,c | 77 | 44.0 (28.8) | 22 | 39.2 (28.4) | -5.24 (-18.81 to 8.34) | 0.45 |
| PANAS-Shortc,d | ||||||
| Positive Affect | 76 | 17.5 (3.4) | 22 | 17.5 (3.3) | 0.035 (-1.54 to 1.61) | 0.89 |
| Negative Affect | 76 | 9.2 (4.0) | 23 | 8.4 (2.6) | -0.78 (-2.52 to 0.99) | 0.88 |
| Perceived benefits of testingc | 78 | 4.1 (0.7) | 23 | 3.5 (0.8) | -0.57 (-0.91 to -0.24) | 0.001 |
| Perceived limitations of testingc | 79 | 3.4 (0.9) | 23 | 3.7 (0.9) | 0.27 (-0.15 to 0.70) | 0.18 |
| Causes of depressionc | 76 | 3.0 (0.6) | 23 | 3.3 (0.8) | 0.13 (-0.08 to 0.56) | 0.20 |
Causation of depression
The majority of receivers (59%) considered that genetics and environment were equally causative of depression, and 18% judged genetic factors and 22% judged environmental factors as more important in causing depression. No one indicated that depression was caused exclusively by either genetic or environmental factors. There were no differences by genotype (χ2=1.2, d.f.=2, P=0.54).
Impact of learning test result on perceived risk of depression
Figure 3 shows changes in perceived lifetime depression risk across time points for decliners and receivers, grouped by testing result. A general reduction in perceived depression risk from baseline to each follow-up time point is reflected by the mixed-effects analysis. Although controlling for history of major depression, there was a significant effect for time (F(2,163) = 8.09, P<0.001) and for the study group (F(3,98) = 3.02, P=0.033 but the interaction between group and time was not significant (F(5,163) = 0.50, P = 0.773). The covariate, history of major depression, was also found to exert a significant effect on one's perceived risk of depression (F(1,97) = 15.15, P<0.001). Controlling for history of major depression, contrast tests showed significantly higher estimates of risk of future episodes of depression among those with s/s genotypes than each of the other study groups (s/l and l/l genotypes, and decliners) prior to disclosure of genotype results. These estimates remained significantly higher at 2 weeks post-disclosure among receivers with the s/s genotype.
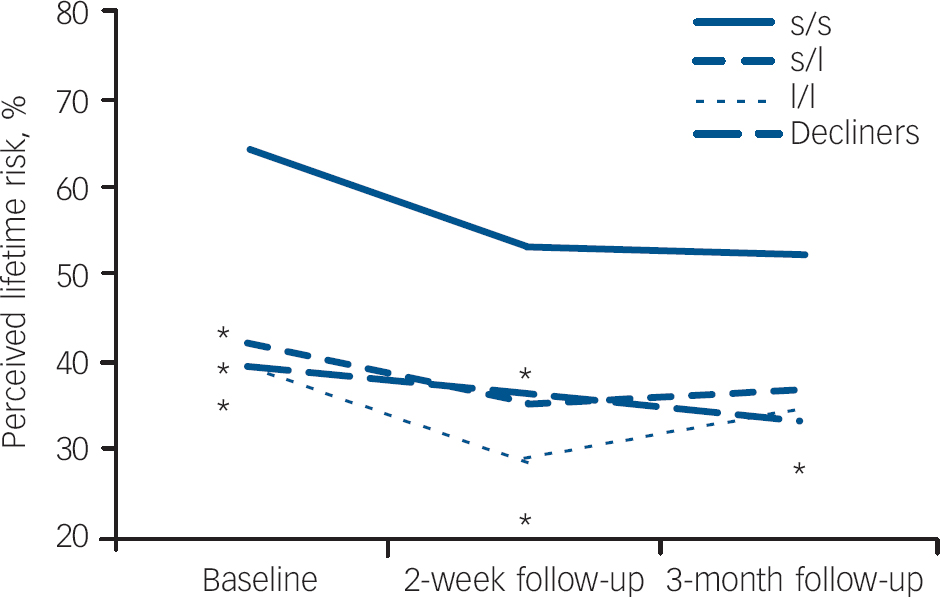
Fig. 3 Perceived risk of future episodes of depression across time for decliners and receivers (n=102)a a. Receivers who completed baseline questionnaire after learning their results (n=5) were excluded from the analysis. Note: data is based on estimated marginal means from the mixed-model analysis. *Differs significantly from s/s scores (P<0.05).
Emotional response and recall after disclosure of genotyping result
Participants with the s/s genotype demonstrated significantly higher distress levels after learning their result at the 2-week (χ2=11.5, d.f.=2, P=0.003) and 3-month follow-up (χ2=13.0, d.f.=2, P=0.001) compared with the other genotypes (Fig. 4). There were no differences between groups in terms of test-related positive experiences at either follow-up.

Fig. 4 Test-related (a) distress and (b) positive experiences among receivers over time by genotype result Receivers who completed baseline questionnaire after learning their results (n = 5) were excluded from the analysis. *Differs significantly from s/s scores (P<0.01).
At both 2-week and 3-month follow-up after result disclosure, 92% of receivers reported feeling pleased that they had learnt their result, 8% were not sure and none regretted learning their result. At the 2-week and 3-month follow-ups 92% and 87% of receivers respectively correctly stated their genotyping result.
Discussion
In this study, 66% of the 128 participants offered the opportunity to learn their genotype elected to do so, suggesting high acceptance of genotyping for risk of depression under stress. These results are consistent with findings from previous surveys of attitudes about (rather than uptake of) genetic testing for psychiatric disorders, which found that between 69% Reference Jones, Scourfield, McCandless and Craddock23 and 97% Reference Smith, Sapers, Reus and Freimer24 of respondents expressed interest in genetic testing for psychiatric disorders, including bipolar disorder, Reference Meiser, Kasparian, Mitchell, Strong, Simpson and Tabassum20,Reference Jones, Scourfield, McCandless and Craddock23–Reference Trippitelli, Jamison, Folstein, Bartko and DePaulo25 schizophrenia Reference Austin, Smith and Honer26,Reference DeLisi and Bertisch27 and psychiatric disorders in general. Reference Laegsgaard and Mors28,Reference Illes, Rietz, Fuchs, Ohiraun, Prell and Rudinger29 These studies included psychiatrists, Reference Jones, Scourfield, McCandless and Craddock23,Reference Smith, Sapers, Reus and Freimer24,Reference DeLisi and Bertisch27 people with a diagnosis of a psychiatric disorder, Reference Smith, Sapers, Reus and Freimer24,Reference Austin, Smith and Honer26,Reference Laegsgaard and Mors28,Reference Illes, Rietz, Fuchs, Ohiraun, Prell and Rudinger29 families with multiple members with a psychiatric disorder Reference Meiser, Kasparian, Mitchell, Strong, Simpson and Tabassum20,Reference Jones, Scourfield, McCandless and Craddock23,Reference Trippitelli, Jamison, Folstein, Bartko and DePaulo25,Reference DeLisi and Bertisch27 as well as the general population. Reference Illes, Rietz, Fuchs, Ohiraun, Prell and Rudinger29
Attitudinal surveys of interest in genetic testing for other adult-onset disorders report that actual uptake of testing is lower than expressed intentions and that the decision to forego testing is associated with a greater perceived likelihood of adverse emotional effects. Reference Lerman, Croyle, Tercyak and Hamann30 Our uptake rate exceeds the 10–20% rates among those at risk for Huntington's disease approached by registries Reference Harper, Lim and David31 and the 40–60% rates of test uptake in families with identified mutations predisposing to hereditary cancer. Reference Meiser1 The high uptake is likely to reflect a number of factors. Namely, participants were tertiary educated, well informed about previous study findings and had a trusting relationship with the research team. Further, participants had taken part in a genetic study of vulnerability to depression and may have been more disposed to accept a genetic explanation for mood disorders than the general population. Testing was free of charge and participants had the option of receiving their results by telephone. Finally, their mean age exceeded 50 years, well past the mean age at onset of depressive disorders. Reference Weissman, Bland, Canino, Faravelli, Greenwald and Hwu32 Additionally, interest among participants may have reflected the high lifetime incidence of major depression (42%) recorded in this cohort. Reference Wilhelm, Parker, Dewhurst-Savellis and Asghari33,Reference Wilhelm, Parker and Hadzi-Pavlovic34
To know or not to know
The most significant difference between those electing and not electing to receive information was the relative weight placed on personal benefits of testing. Those who wished to know their results emphasised the benefits of genetic testing to themselves and society. The most important perceived benefits were that genetic testing potentially allows for prevention and earlier intervention of depression, particularly for those with the s/s genotype. These findings contrast with attitudes to genetic testing for Huntington's disease and hereditary cancer, Reference Mastromauro, Myers and Berkman35 where the most important reasons for genetic testing are ‘to be certain’ and ‘to learn one's children's risk’ respectively. This highlights the participants' appreciation of the preventive potential for the current genetic testing knowledge combined with effective environmental (stress) management.
The most important perceived limitations of testing were that the genotype result could lead to discrimination by insurance companies or employers, and that those with the s/s genotype may become more stressed, depressed or vulnerable. These findings contrast with results from surveys in the hereditary cancer setting, where only a minority were concerned about discrimination. Reference Lerman, Narod, Schulman, Hughes, Gomez-Caminero and Bonney36–Reference Harper and Clarke38 However, they are consistent with findings from a study of ethical issues related to the genetics of smoking, Reference Shields, Lerman and Sullivan39 where nearly two-thirds of Americans stated that they would refuse a genetic test if employers or health insurers were able to access the results. Heightened concern about discrimination in our participants relate to greater perceived stigma for depression and psychiatric illness overall. Reference Westbrook, Legge and Pennay40
Participants' responses indicated that they appreciated that depression is caused by both genetic and environmental factors. Although we ascertained the participants' knowledge of the interrelationship between genotype, stress and depression and their perception of causation of depression, we did not assess their knowledge of genetics and depression more broadly. Future research could consider whether the extent of participants' knowledge relates to testing uptake.
Cohort-specific issues
Given the age of the sample, a first depression onset subsequent to genetic testing was thought unlikely. The s/s genotype is considered to affect risk of first onset (or early episodes) of depression Reference Caspi, Sugden, Moffitt, Taylor, Craig and Harrington4–Reference Cervilla, Molina, Rivera, Torres-Gonzalez, Bellon and Moreno11 and studies investigating the interaction between environmental stress and 5-HTT genotype on the onset of depressive episodes in older-aged samples have found no such effect. Reference Gillespie, Whitfield, Williams, Heath and Martin12,Reference Surtees, Wainwright, Willis-Owen, Luben, Day and Flint13 However, prior to genetic testing, individuals with the s/s genotype perceived a higher risk of future depression (which would include further episodes as well as new episodes) than other genotype groups and this may account for their lower uptake of test disclosure.
We have previously found that the s/s genotype is associated with a lower use of problem-solving coping strategies Reference Wilhelm, Siegel, Finch, Hadzi-Pavlovic, Mitchell and Parker41 within the cohort from which the current study sample was drawn. Furthermore, brain imaging studies Reference Hariri, Drabant, Munoz, Kolachana, Mattay and Egan42 have demonstrated greater stress-induced amygdala activation in s/s carriers. We have hypothesised that deficient problem-solving coping and/or a hyper-reactivity to stressors may convey an increased risk of future depressive episodes among the s/s genotype group which could explain their perceptions of heightened depression risk. Alternatively, participants may have assessed their own future likelihood of depression based on their previous reactions following stressful events and past depression history.
Each group across the genetic risk spectrum (s/s, s/l and l/l genotypes) reported some reduction in the perceived chance of a future depressive episode following disclosure of their genotype results. We speculate that the information was provided in a manner that empowered the participants to actively address their coping styles rather than view themselves as passive recipients. However, those declining to learn their results also demonstrated a reduction in their perceived risk of depression from baseline to 3-month follow-up.
There was little indication of marked distress due to learning one's genotype result as each genotype group reported more positive feelings than distress. However, the s/s genotype group experienced more distress associated with receipt of the test results compared with those with the s/l and l/l genotypes (who reported almost no negative emotional impact).
Study limitations
The limitations of this study should be mentioned. The sample was a highly select and homogeneous group with regard to educational levels, professional and ethnic backgrounds and age range, which considerably limits the generalisability of the findings. Also, participants were from a cohort of an existing longitudinal study on risk factors of depression and their participation may have altered interest in genetic testing for depression risk. Furthermore, they were a select group of this cohort who had consented to genetic testing. Finally, we observed participation bias in that participants who had declined information about their genotype were also less likely to participate in this study. However, the very same features of this cohort were what influenced our ethics committee to approve the study. We see this study as a first step and, clearly, further studies involving samples that are more heterogeneous with regard to age, educational level and cultural background are required to ensure the best means of providing information to participants and to assess the acceptability and psychosocial impact of genotyping for depression risk in more representative population samples.
Acknowledgements
This study was granted approval by the University of New South Wales Human Research Ethics Committee. The authors thank Dusan Hadzi-Pavlovic for statistical advice, and Ian Blair and Anna Scimone for genetic analyses. We also give special thanks to the participants for their continuing interest and generous donation of their time and samples.









eLetters
No eLetters have been published for this article.