Introduction
Feather fingergrass (Chloris virgata Sw.) is a warm-season annual C4 nonnative weed in Australian cropping systems (Ngo et al. Reference Ngo, Krishnan, Boutsalis, Gill and Preston2018). It can grow up to 1-m tall, can produce more than 600 g m−2 of dry matter, and is a prolific seed bearer (>40,000 seeds plant−1 and >1,000 seeds panicle−1) (Ngo et al. Reference Ngo, Boutsalis, Preston and Gill2014). The seeds of C. virgata have high wind dispersal ability due to their morphological features. They are light enough to travel up to 13 m from the mother plant in normal wind velocity (13 to 19 km h−1; (Osten Reference Osten2012). The seeds are pale white or dark gray, consisting of two significant hairlike appendages. The seeds can float on water, which allows them to be dispersed by water (hydrochory) (Fernando et al. Reference Fernando, Humphries, Florentine and Chauhan2016), and due to their hairy feature, they can stick to animal furs, agricultural machinery, and laborers and thus can easily be transported from one habitat to another (Desai and Chauhan Reference Desai and Chauhan2021b).
Weed management practices in Australia have shifted from heavy and frequent cultivation to zero or minimum tillage to preserve soil moisture compared with intensive-tillage conditions. However, these changes caused overreliance on glyphosate for fallow weed control. Owing to this, weed species that prefer no-till or minimum-tillage conditions have become established in Australian farming systems (Chauhan et al. Reference Chauhan, Manalil, Florentine and Jha2018), and one of the most important weeds is C. virgata (Figure 1). It is a troublesome weed in the large-scale farming systems of northern, central, and southern Queensland (specifically in the Darling Downs and Western Downs regions), and northern New South Wales, the Wheatbelt region of Western Australia, and the South Australian vineyards and orchards. Previously considered a roadside weed, it has now become a major weed in Australian farming systems, especially where zero and/or minimum tillage has been utilized for several years (Osten Reference Osten2012). Chloris virgata has been ranked as one of the top 20 national weeds in Australia, spanning an area of 117,512 ha, thus contributing to huge yield (39.329 kg) and revenue (A$7.7 million) losses per annum (Llewellyn et al. Reference Llewellyn, Ronning, Clarke, Mayfield, Walker and Ouzman2016).

Figure 1. Occurrence of Chloris virgata in Australia (Australasian Virtual Herbarium 2021)
The leaf blades are 5- to 25-cm long and 3- to 6-mm broad. Leaf blades possess a light green color at the early seedling stage and become brownish-green at maturity. The panicles are sometimes shiny white and/or dull white, consisting of 7 to 19 subspikes that are 3- to 9-mm long. Mature plants can occasionally produce roots from stem nodes if they directly contact the soil. Chloris virgata is often confused with junglerice [Echinochloa colona (L.) Link.] in its early seeding growth (Osten Reference Osten2012). This exotic weed species can act as a host for aphids (Aphididae), barley yellow dwarf and cereal yellow dwarf viruses (Tombusviridae), and some nematode species (Nematoda)(Osten Reference Osten2012).
Seed germination is considered one of the most essential criteria in deciding the successful establishment of any weed in an agroecosystem. Germination events are known to have major reliance on several environmental factors such as temperature, photoperiod, and pH, salinity, and soil moisture levels (Chachalis and Reddy Reference Chachalis and Reddy2000); however, temperature, light, and soil moisture are the most important environmental factors that regulate the germination and emergence of plant species (Baskin and Baskin Reference Baskin and Baskin1998). The time of germination is a critical period for annual plants in terms of survival (Saatkamp et al. Reference Saatkamp, Affre, Dutoit and Poschlod2009). Light and temperature are responsible for initiating the germination process by providing information to the seeds regarding their positions in the soil and level of soil disturbance (Batlla and Benech-Arnold Reference Batlla and Benech-Arnold2014). Seeds that possess plasticity in response to environmental factors are most likely to acclimatize rapidly in different agroecosystems (Boutsalis et al. Reference Boutsalis, Preston and Gill2017).
It is crucial to understand the effect of temperature on the germination behavior of different biotypes of C. virgata. This information can play an important role in the development of species- and area-specific weed control tactics by providing insights into the potential seasonal spread of C. virgata, allowing agronomic practices to be scheduled proactively according to the sites associated with the biotypes of interest.
This study aims to expand on the sparse knowledge available on C. virgata germination in response to temperature. The major objective of this study is to understand the germination pattern of 10 geographically different biotypes of C. virgata under five different alternating temperature regimes. Such insights could contribute to developing area-specific control tactics for C. virgata.
Materials and Methods
Seed Collection and Production
Seeds were collected from five different locations over several field trips across southeastern Queensland, Australia, in March to April 2017 (Table 1). Unique codes were given to each biotype: D1 and D2 (Dalby), Ch (Chinchilla), CP1 and CP2 (Cecil Plains), SGM1 and SGM2 (St George), SGW1 and SGW2 (St George), and Ga (Gatton) (Figure 2). All biotypes are from different agroecosystems or climatic conditions. Seed collection was performed as described by Desai and Chauhan (Reference Desai, Chauhan and Chauhan2021a). The entire seed lot was then transported to the Queensland Alliance for Agriculture and Food and Innovation (QAAFI) weed science laboratory in Gatton, Australia (27.555°S, 152.334°E), where seeds were manually separated from panicles and kept in airtight containers to prevent contamination. All containers were stored in dark conditions at room temperature (25 ± 2 C). Seeds were gathered from multiple plants (at least 50 plants per biotype) for individual biotypes to attain a seed lot representative of the entire population. All collected seeds of each biotype were produced in a similar environment at Gatton as described by Desai et al. (Reference Desai, Thompson and Chauhan2020) and these seed batches were used to conduct this study. Thus, the seeds from the F2 generation were used in this study.
Table 1. Information for biotypes collected from different regions of Queensland (Desai et al. Reference Desai, Thompson and Chauhan2020).
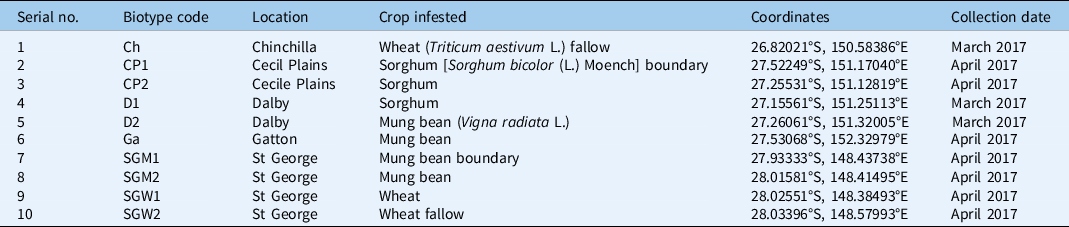

Figure 2. Biotype collection regions of southern Queensland, Australia: (A) Cecil Plains; (B) Chinchilla; (C) Dalby; (D) Gatton; and (E) Saint George. Note that point ‘A’ is overlapping point ‘C.’
Germination Experiment
Germination tests on 10 different biotypes were conducted at the QAAFI weed science laboratory from August 2019 to January 2020 (first run: August to October; second run: November to January). The experiment in each run was established using a randomized complete block design with three replications. Seeds were placed in petri dishes (92-mm diameter by 16-mm height) (Sarstedt, Mawson Lakes, South Australia, Australia) on top of a double layer of filter paper (Macherey-Nagel, Duren, Germany). In each petri dish, 25 seeds were placed uniformly on the filter paper via forceps, and the filter paper was moistened with 5 ml of deionized water using a micropipette (Boeco, Hamburg, Germany).
A desk-mounted LED laboratory magnifier lamp (model no. QM3546, White Label) was used for seed placement due to the small seed size of C. virgata. Only black-colored seeds were used (Fernando et al. Reference Fernando, Humphries, Florentine and Chauhan2016). The “seed pressing technique” was used to determine seed viability (Van Mourik et al. Reference Van Mourik, Stomph and Westerman2003): seeds were pressed with a pair of forceps, and if an endosperm emerged from seed testa, then the seed was classed as viable. If no endosperm emerged, then the seed was classed as nonviable. Petri dishes were placed in resealable plastic bags to reduce moisture loss due to evaporation.
Petri dishes were arranged randomly under five different alternating temperature regimes (15/5, 20/10, 25/15, 30/20, and 35/25 C) in incubators (Labec Laboratory, Marrickville, Sydney, New South Wales,, Australia) with a 12-h light/12-h dark photoperiod. Temperature ranges were monitored by temperature controllers connected to a data logger (3216 PID temperature controller, Invensys Eurotherm, Itasca, IL, USA). All five alternating thermal schemes reflect the year-round day/night temperature of the biotypes’ source locations. The timing of temperature and photoperiod was regulated with the use of a flush-mounted time clock (45 series timers, no. UNI45TE, Grasslin Uni, Geogen, Germany), and light was provided by tube lights (Ultralamp ECO-T5, 28 W, 220 V/240 V, 1,170 mm) installed in all five incubators.
Seed germination was monitored daily throughout the experiment, and germinated seeds were removed using forceps. Seeds were identified as germinated after radicle emergence (>1 mm). In addition, a high evaporation rate was observed in high-temperature ranges (30/20 and 35/25 C). Consequently, 2 ml of deionized water was frequently added to petri dishes only for high-temperature regimes when required. The germination test was considered complete when no germination was observed for 5 consecutive days. This study lasted for 63 d, and the whole experiment was repeated 25 d after completion of the first run. The second run also took 63 d.
Statistical Analysis
Final cumulative germination (FCG) percentages were subjected to two-way ANOVA in GraphPad Prism v. 8.4.2 679 (GraphPad Software, San Diego, CA, USA) to determine the effect of biotype and temperature on germination. Nontransformed data were used for analysis, as data transformation did not improve the homogeneity of variance. The cumulative germination number on each day was obtained and converted to cumulative germination percent. To identify the cumulative germination pattern of all C. virgata biotypes over time, these sets of data were fit to a three-parameter sigmoidal-logistic model (Equation 1) using SigmaPlot v. 14.0 software (Systat Software, San Jose, CA, USA). No significant difference was observed, so data were pooled across the two runs.
where f is the percent cumulative germination over time, a is the maximum germination,
![]() $${x_0}$$
is the time taken to reach 50% germination, and b is the steepness of the slope.
$${x_0}$$
is the time taken to reach 50% germination, and b is the steepness of the slope.
Three germination parameters were estimated with the SeedCalc package in R software (Silva et al. Reference Silva, Medeiros and Oliveira2019): T 10 (time in hours taken to reach 10% germination; Equation 2), T 50 (time in hours taken to reach 50% germination; Equation 3), and T 90 (time in hours taken to reach 90% germination; Equation 4).
 $${T_{10}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {10}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
$${T_{10}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {10}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
 $${T_{50}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {50}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
$${T_{50}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {50}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
 $${T_{90}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {90}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
$${T_{90}} = {{{t_i} + \left\{ {\left[ {{N \over {{{100} \over {90}}}}} \right] - {n_i}} \right\}\left( {{t_j} - {t_i}} \right)} \over {\left( {{n_j} - {n_i}} \right)}}$$
In Equations 2–4, N is the final number of seeds germinated and n i and n j are the total number of seeds germinated in adjacent counts of time t i and t j , respectively.
The T parameters were further compared using the approximate t-test in GraphPad Prism v. 8.4.2 679 (GraphPad Software) to understand variations of T 10, T 50, and T 90 of all biotypes at all temperature ranges.
Results and Discussion
The interactions between biotypes and temperature regimes were significant (P = 0.001). Seeds of all biotypes germinated (11% to 76%) at wide alternating temperature regimes (from 15/5 to 35/25 C) (Table 2), suggesting that this weed species has the potential to germinate throughout the year, and growers across Queensland support this, as they have observed this species in winter months in their fields (Chauhan Reference Chauhan2019). However, in contrast, previous research on seed biology of this species revealed that the ideal temperature for optimum germination is 30/20 C, and seeds had a low germination rate at low-temperature ranges (15/5 and 20/10 C) (Fernando et al. Reference Fernando, Humphries, Florentine and Chauhan2016), indicating a summer annual life cycle. A comparison of the previous study (Fernando et al. Reference Fernando, Humphries, Florentine and Chauhan2016) and our study illustrates the differences between biotypes and the possibility of selection for different traits in different environments. Chloris virgata biotypes showed greater variations in germination timing that could be an indication of “bet-hedging” adaptation to variable climates (Cohen Reference Cohen1966). Many weed species have been shown to have the potential of germinating in a wide range of temperatures despite having annual life cycles, examples being horseweed [Conyza bonariensis (L.) Cronquist] (Loura et al. Reference Loura, Florentine and Chauhan2020), African turnipweed (Sisymbrium thellungii O. E. Schulz) (Mahajan et al. Reference Mahajan, Matloob, Walsh and Chauhan2018), and sowthistle (Sonchus oleraceus L.) (Manalil et al. Reference Manalil, Ali and Chauhan2018).
Table 2. Final cumulative germination and estimated parameters of Chloris virgata biotypes obtained from the three-parameter logistic sigmoidal model.

a FCG, final cumulative germination. b is the slope of a curve indicating the germination rate of biotypes over time.
b Ch, CP, D, Ga, and SG biotypes were collected from Chinchilla, Cecil Plains, Dalby, Gatton, and Saint George, respectively. CP1 and CP2 are the biotypes collected from the sorghum boundary and sorghum field of the Cecil Plains region of Queensland, respectively. SGM1, SGM2, SGW1, and SGW2 are the biotypes collected from the mung bean boundary, mung bean field, wheat field, and wheat fallow of the Saint George region of Queensland, respectively.
c Values ± standard errors of the mean.
Biotypes SGM1, SGW1, SGM2, and SGW2 displayed noticeable variation in germination (from 29% to 76%) under all temperature regimes tested regardless of the similar origin and/or environmental conditions. Similarly, biotypes CP1 and CP2 and D1 and D2 exhibited an observable difference in germination under all temperature regimes (from 11% to 60% and from 16% to 48% for Cecil Plains and Dalby biotypes, respectively). The differences in the germination behavior of biotypes from similar regions could be due to different crop associations, long-term exposure to a variety of agronomic operations, and/or herbicide resistance status (Kumar and Jha Reference Kumar and Jha2017).
The maximum germination of the biotype from Gatton (Ga) was observed at low-temperature ranges (15/5 and 20/10 C) (Table 2), indicating that this biotype could be problematic in winter-season crops grown in the Gatton region, such as broccoli (Brassica oleracea var. italica Plenck), beetroot (Beta vulgaris L.), lettuce (Lactuca sativa L.), cabbage (Brassica oleracea L. var. capitata), cauliflower (Brassica oleracea L. var. botrytis), and many other Brassicaceae crops, if it survives frost conditions during winter months. The biotype from Chinchilla (Ch) also exhibited high germination at low-temperature ranges (15/5 and 20/10 C), demonstrating the possibility of germination in the winter months. It could affect the pastureland of the Chinchilla region by decreasing pasture quality. Therefore, this weed species could be problematic for the beef and pork industries, the most dominant agricultural industries of the Chinchilla region. In addition, high FCGs of 76%, 57%, 53%, 51%, and 49% of SGM2, SGW2, Ch, CP2, and SGM1, respectively, were observed at the lowest alternating temperature regime (15/5 C) (Table 2), revealing the potential of C. virgata to grow throughout the year despite being a summer annual weed species.
Biotypes demonstrated high germination percentages at 30/20 C, for example, 59%, 51%, and 40% for SGM2, SGM1, and CP1, respectively (Table 2), suggesting that this weed species could become considerably challenging to control in summer months, because C. virgata is a high-yielding species (600 g m−2 dry matter plant−1) and a prolific seed producer (>40,000 seeds plant−1) in summer months (Ngo et al. Reference Ngo, Boutsalis, Preston and Gill2014). The slope (b) obtained from the three-parameter sigmoidal-logistic model estimated that all the biotypes took a longer time to achieve the highest FCGs at high-temperature ranges (30/20 and 35/25 C) compared with low alternating temperature regimes (15/5 and 20/10 C). Larger values of the slope (b) at 15/5 C than at 30/20 and 35/25 C (Table 2) suggest rapid germination of all biotypes at 15/5 C compared with 30/20 and 35/25 C. This suggests that all of these biotypes could exhibit extended germination in the summer season and could germinate rapidly in the winter months, hampering the yield of winter grain crops. Extended germination at higher temperatures could aid in seedling recruitment in years having a dry fall and winter (Mummey et al. Reference Mummey, Herget, Hufford and Shreading2016).
Each line (slope b) in Figure 3 represents the time taken by each biotype to complete the germination cycle (-smaller values of b denote the rapid completion of the germination cycle). It is evident that at low-temperature ranges (15/5 and 20/10 C), all biotypes achieved highest germination faster than at other temperature ranges (25/15, 30/20, and 35/25 C). The SGW2 biotype revealed extended germination at higher alternating temperature regimes, as it required 57 d and 63 d at 30/20 and 35/25 C, respectively (Figure 3), to attain maximum FCG percentage (42% at 30/20 C and 31% at 35/25 C). These kinds of dissimilarities in germination between biotypes might occur due to different dormancy levels, herbicide-resistance status, crop associations, and/or long-term exposure to various agronomic practices at the sites of their original generation. Previous studies showed that differential herbicide-resistance status could cause differences in the germination behavior in many weed species, such as kochia [Bassia scoparia (L.) A.J. Scott] (Kumar et al. Reference Kumar, Jha, Lim and Stahlman2018, Kumar and Jha Reference Kumar and Jha2017) and E. colona (Mutti et al. Reference Mutti, Mahajan and Chauhan2019).

Figure 3. Germination behavior of 10 biotypes of Chloris virgata (collected from regions of southern Queensland, Australia) at different temperature regimes over time. Symbols represent cumulative germination percentage for each day, and slope (b) represents the time course of germination. (A) 15/5 C, (B) 20/10 C, (C) 25/15 C, (D) 30/20 C, and (E) 35/25 C. Abbreviations for key: Ch, CP, D, Ga, and SG biotypes were collected from Chinchilla, Cecil Plains, Dalby, Gatton, and Saint George, respectively. CP1 and CP2 are the biotypes collected from the sorghum boundary and sorghum field of the Cecil Plains region of Queensland, respectively. SGM1, SGM2, SGW1, and SGW2 are the biotypes collected from the mung bean boundary, mung bean field, wheat field, and wheat fallow of the Saint George region of Queensland, respectively.
The computations of T 10, T 50, and T 90 provide in-depth insights into germination patterns of C. virgata biotypes in response to different thermal conditions and allow comprehensive inferences to be drawn. All the biotypes required a longer time to achieve T 10 (170 to 173 h) at the lowest alternating temperature regime (15/5 C). T 10 was achieved more quickly, even though all biotypes took more time to achieve T 90 at higher temperatures (Table 3). All biotypes required less time to reach 10% germination (T 10) (ranging from 28 to 40 h) at higher temperature ranges (30/20 and 35/25 C) compared with other temperature ranges, consequently signaling rapid germination initiation in summer months. This characteristic of C. virgata could increase its potential to establish colonies by producing more dry matter and seeds, thus enriching the seedbank in the summer months.
Table 3. Three biological parameters of Chloris virgata obtained from R software.

a T 10, incubation period required to reach 10% germination; T 50, incubation period required to reach 50% germination; T 90, incubation period required to reach 90% germination.
b Ch, CP, D, Ga, and SG biotypes were collected from Chinchilla, Cecil Plains, Dalby, Gatton, and Saint George, respectively. CP1 and CP2 are the biotypes collected from the sorghum boundary and sorghum field of the Cecil Plains region of Queensland, respectively. SGM1, SGM2, SGW1, and SGW2 are the biotypes collected from the mung bean boundary, mung bean field, wheat field, and wheat fallow of the Saint George region of Queensland, respectively.
c Values ± standard errors of the mean.
T 90 gradually increased with increasing temperature ranges for all the biotypes. It should be noted that all the biotypes took more time to initiate the germination process (T 10) at the lowest alternating temperature regime (15/5 C). Yet they attained T 90 in a shorter period. In contrast, all the biotypes initiated the germination process (T 10) more quickly at higher temperature regimes (25/15, 30/20, and 35/25 C) than at 15/5 C, yet they required more time to achieve T 90 germination. These results further indicated a longer germination cycle of C. virgata in summer than in winter. Rapid germination initiation in the summer months indicates the possibility of higher seed production from early summer flushes due to the plant’s summer annual life cycle in combination with favorable growing conditions, thus enhancing the seedbank during the crop-growing season.
The reduced FCG percentages, extended germination, and slow germination rates of Ch, D2, SGM1, SGW2, and Ga, especially at higher temperature regimes (30/20 and 35/25 C) (Table 2), could be a sign of escape mechanisms in summer fallow conditions securing survival against preplant weed management operations (e.g., burndown spraying tactics or tillage). In this way, these biotypes could be more challenging to tackle in winter months (if they tolerate frost conditions), as FCG percentages of some biotypes were higher at the lowest alternating temperature regime (15/5 C).
Our previous research revealed that biotype SGW2 is highly resistant to glyphosate (inhibitor of 5-enolpyruvylshikimate-3-phosphate synthase enzyme) (Desai et al. Reference Desai, Thompson and Chauhan2020). Biotype SGW2 exhibited a higher level of dormancy, as it required comparatively more time to achieve T 90 under all alternating temperature regimes, except the lowest temperature range of 15/5 C (Table 3). These results consolidate the aforementioned discussion that different herbicide-resistance levels could be a causal factor for different germination characteristics (Kumar et al. Reference Kumar, Jha, Lim and Stahlman2018). The no-till system has been followed in the Australian farming system for a long time; therefore, herbicide application is the most common way of controlling weed species in fallow land. Intensive weed management operations, such as the frequent use of selective or nonselective herbicides, support the survival of weed biotypes that possess late emergence or delayed germination (Fleet and Gill Reference Fleet and Gill2012). Additionally, in the case of herbicide resistance, this kind of extended germination behavior facilitates the subsequent survival of herbicide-resistant biotypes against in-crop herbicide application, enhancing the opportunity to develop a higher level of herbicide resistance (Kumar et al. Reference Kumar, Jha, Lim and Stahlman2018). The results suggest the need for more effective and aggressive weed management tactics to combat this problematic weed species.
The results of this study strongly suggest the need for area-specific control tactics for C. virgata in the Australian farming systems and have direct implications for choosing tillage schedules and planting calendars to help control this problematic weed species. Our results showed that C. virgata is not simply a summer annual but can germinate and grow throughout the calendar year. Nonselective herbicide application (e.g., glyphosate) in fallow conditions would not be sufficient to achieve season-long C. virgata control. Therefore, novel in-crop herbicide programs should be developed to manage this weed species efficiently. More biotypes should be collected from different regions of Australia, and germination characteristics in response to temperature and other environmental factors should be thoroughly examined, because the germination ecology of C. virgata has not yet been explored to the required extent, considering its potential to hinder agricultural production. Despite C. virgata being a summer annual weed species, the ability of all the biotypes to express germination at low alternating temperature regimes (15/5 and 20/10 C) suggests that it could also germinate in the winter months in eastern regions of Australia. However, successful germination under controlled conditions (i.e., in a laboratory) does not guarantee successful survival in field conditions. Therefore, further research should be conducted on C. virgata’s phenology, with seeds planted every month throughout the year so that emergence and growth can be recorded and survival probabilities in the winter months can be studied.
The high level of adaptability across a wide range of temperatures, distinctive germination characteristics, different dormancy levels between biotypes, delayed and extended germination patterns, and the indication of escape mechanisms against preplant weed control tactics put C. virgata in the hard-to-control weed category. Therefore, it should be managed by adopting region-specific control tactics and integrated management approaches. Results from the current study could aid in developing sustainable weed management protocols and simulation models to understand the population dynamics of C. virgata biotypes, selection of tillage timings, and scheduling of crop calendars.
Acknowledgment
The authors acknowledge funding from the Grains Research and Development Corporation. No conflicts of interest have been declared.









