Article contents
Argentotetrahedrite-(Cd), Ag6(Cu4Cd2)Sb4S13, a new member of the tetrahedrite group from Rudno nad Hronom, Slovakia.
Published online by Cambridge University Press: 19 December 2022
Abstract
Argentotetrahedrite-(Cd), Ag6(Cu4Cd2)Sb4S13, has been approved as a new mineral species by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association using samples from Rudno nad Hronom, Slovak Republic. It occurs as anhedral grains up to 30 μm in size, steel-grey to black in colour, with a metallic lustre, in association with greenockite and other tetrahedrite-group minerals [argentotetrahedrite-(Zn) and tetrahedrite-(Zn)], earlier base-metal minerals, Ag sulfides and sulfosalts (acanthite, pyrargyrite and polybasite) and later galena. Argentotetrahedrite-(Cd) is isotropic, grey in colour, with a creamy tint and rapidly (tens of minutes) tarnishes to orange–brown. Reflectance data for Commission on Ore Mineralogy (COM) wavelengths in air are [λ (nm), R (%)]: 470, 30.4; 546, 30.3; 589, 30.3; and 650, 28.7. The chemical formula of the samples studied, recalculated on the basis of ΣMe = 16 atoms per formula unit, is: (Ag3.28Cu2.72)Ʃ6.00[Cu4(Cd1.68Fe0.27Zn0.16)]Ʃ6.11(Sb3.71As0.15)Ʃ3.86S12.79. Argentotetrahedrite-(Cd) is cubic, I$\bar{4}$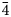 3m, with a = 10.65(2) Å, V = 1208(4) Å3 and Z = 2. Argentotetrahedrite-(Cd) is isotypic with other members of the tetrahedrite group. The structural relationship between argentotetrahedrite-(Cd) and other members of the freibergite series are discussed and previous findings of this species are briefly reviewed.
3m, with a = 10.65(2) Å, V = 1208(4) Å3 and Z = 2. Argentotetrahedrite-(Cd) is isotypic with other members of the tetrahedrite group. The structural relationship between argentotetrahedrite-(Cd) and other members of the freibergite series are discussed and previous findings of this species are briefly reviewed.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Oleg I Siidra
References
- 6
- Cited by


