Vitamin A (retinol) is one of the most commonly used dietary supplements. Retinoic acid, a derivative of vitamin A, has been demonstrated to play an important signalling role during prenatal development( Reference Duester 1 ). Vitamin A is also indispensable throughout the postnatal phase due to its effects on cell metabolism, retinal photo-pigment production, epithelial cell differentiation, bone growth, immune processes and reproduction( Reference Sporn, Roberts and Goodman 2 , Reference Boucheron-Houston, Canterel-Thouennon and Lee 3 ). A relatively low dose of vitamin A (about 8500 IU/kg per d) is administered to infants with a very low birth weight( Reference Mactier and Weaver 4 ), while high doses (about 50 000–100 000 IU/d) are used for therapeutic purposes in patients with leukaemia( Reference Fenaux, Chomienne and Degos 5 ). However, the dietary supplementation of vitamin A to healthy adult individuals is still a controversial issue. After documentation of its pro-oxidant properties when used at relatively high doses( Reference Zaidi and Banu 6 ), it has been suggested that vitamin A may act as a double-edged sword. The pro- and antioxidative actions of dietary supplements are especially important for the brain, which is known to be more susceptible to oxidative damage because of its high levels of oxygen consumption, rich content of easily oxidisable PUFA in cell membranes, relatively low contents of antioxidant enzymes such as catalase and superoxide dismutase as well as antioxidants such as α-tocopherol and glutathione, and eventually the presence of high concentrations of Fe when compared with other organs( Reference Elibol-Can, Jakubowska-Doğru and Severcan 7 , Reference Halliwell 8 ). In addition, certain regions of the central nervous system, such as the hippocampus and cerebellum, may be highly sensitive to oxidative stress because of their relatively low endogenous concentrations of vitamin E( Reference Wilson 9 ). The protective role of antioxidants and the potential role of vitamin A in the modulation of oxidative stress in the central nervous system have been reported previously( Reference Kashif, Zaidi and Banu 10 ).
Animal studies have also demonstrated the importance of vitamin A in the neurogenesis( Reference Bonnet, Touyarot and Alfos 11 ) and metaplasticity( Reference Misner, Jacobs and Shimizu 12 ) of the hippocampus, which is the brain structure crucial for learning and memory formation. A reduction in hippocampal Cornu Ammonis 1 (CA1) long-term potentiation and long-term depression have been observed in adult mice maintained on a vitamin A-deficient diet. Subsequent administration of vitamin A/retinoic acid has been reported to reverse deficits in hippocampal plasticity( Reference Misner, Jacobs and Shimizu 12 ). Parallel to these findings, hippocampus-dependent spatial learning has also been shown to deteriorate in vitamin A-deficient rats( Reference Bonnet, Touyarot and Alfos 11 , Reference Cocco, Diaz and Stancampiano 13 , Reference Mingaud, Mormede and Etchamendy 14 ). Considering the importance of vitamin A in many physiological processes, the high prevalence of vitamin A deficiency (VAD) occurring along with malnutrition in many African and Asian countries has been suggested to cause serious health problems( 15 ). Therefore, further studies on the biological role of vitamin A and the impact of VAD on biological systems are important from a clinical perspective. The effects of vitamin A supplementation and VAD at the organismal and cellular levels have been largely disclosed. However, VAD-related structural and functional changes occurring at the molecular level have not been elucidated yet. Therefore, the aim of the present study was to examine the effect of VAD on the structure, content and function of lipids, proteins and nucleic acids in the rat hippocampus, which, to the best of our knowledge, has not been investigated yet, and thus to contribute to the elucidation of the cellular mechanisms underlying VAD in the hippocampus. To achieve this, Fourier transform IR (FT-IR) spectroscopy was used as many studies have proved that it is a powerful and successful analytical technique that provides qualitative and/or quantitative information. This technique allows rapid, accurate, reproducible, cost-effective and operator-independent determination of global molecular alterations at the membrane, cellular and tissue levels( Reference Cakmak, Zorlu and Severcan 16 – Reference Severcan and Haris 23 ). FT-IR spectroscopy exhibits higher sensitivity in detecting even very small changes in the functional groups of biological molecules without the use of stains or probes. Using this method, comprehensive information about membrane fluidity, lipid order, the contents of saturated lipids, unsaturated lipids, cholesteryl ester, TAG, proteins, RNA, DNA and glycogen, and protein and nucleic acid conformation can be obtained rapidly and simultaneously from a single tissue sample( Reference Elibol-Can, Jakubowska-Doğru and Severcan 7 , Reference Siebert 21 – Reference Severcan and Haris 23 ).
Materials and methods
Experimental animals
Naive, male Wistar rats (n 23) aged 3 months and weighing 200–250 g were obtained from Animal Facilities at Gulhane Medical Military Academy, Ankara, Turkey. Rats were housed in a secluded room at an ambient temperature of 22°C, under a 12 h light–12 h dark cycle, and with free access to standard laboratory chow and tap water until the start of the experiment. The institutional and national guidelines for the care and use of animals were followed in the present study and all experimental procedures involving animals were approved by the Ethics Committee of the Middle East Technical University, Ankara, Turkey.
Treatment
Rats were randomly divided into three groups: an experimental group maintained on a vitamin A-deficient liquid diet (VAD, n 7); a control group maintained on a vitamin A-supplemented liquid diet (CON, n 9); a pure control group maintained on standard solid laboratory chow (PC, n 7). The PC group was included in the study to ensure that the usage of liquid diet did not influence the outcomes of VAD. Rats form the VAD group were fed a 100 ml mixture of a modified liquid diet prepared with semi-skimmed milk (Pinar Co.) and sucrose (Merck) for 85 d daily as described previously( Reference Celik, Cakir and Kayir 24 ). Rats from the CON group were fed a liquid diet supplemented with 5000 IU/l vitamin A (Avicap; Kocak Farma). The mean total daily energy intake of each rat was 209 kJ (50 kcal). On the 86th day, rats were decapitated and their hippocampi were dissected and stored at − 80°C until the FT-IR analysis.
Sampling, data acquisition and Fourier transform IR spectroscopy
Hippocampal samples were dried in a Labconco freeze dryer (6 Liter Benchtop Freeze Dry System Model 77 520; Labconco FreeZone®) to remove free water content. Later, 1 mg of the samples was ground in agate mortar containing liquid N2 and mixed with 150 mg of KBr (Merck). This homogeneous mixture was used to prepare thin KBr discs by applying pressure of approximately 100 kg/cm2 in an evacuated die. IR spectra were obtained using a PerkinElmer Spectrum One FT-IR spectrometer (PerkinElmer, Inc.) equipped with a mid IR triglycine sulphate (MIR TGS) detector. The background spectrum was recorded using a transparent KBr disc without the sample and subtracted automatically using the Spectrum One software (PerkinElmer, Inc.). The FT-IR spectrum was recorded in the 4000–800/cm region by collecting 100 scans per sample with 4/cm resolution at room temperature. The average spectra of three replicates from each sample were used in detailed data analysis. The nine-point Savitzky–Golay smoothing function was applied to reduce noise( Reference Ozek, Sara and Onur 25 ). The band frequency, band area and bandwidth values of each spectrum were calculated from the spectral bands using the Spectrum One software. Baseline correction was performed as an input parameter in the band area calculations. The frequency and width values of specific bands were calculated as the centre of mass and width, respectively, at 0·80 × height position of the signal in terms of per cm where overlapping with the other bands was minimum. Normalisation processes were applied for visual demonstration of only spectral variations.
The general band assignment of the FT-IR spectra was done in accordance with previous studies( Reference Elibol-Can, Jakubowska-Doğru and Severcan 7 , Reference Ozek, Sara and Onur 25 – Reference Banyay, Sarkar and Graslund 27 ) and is summarised in Table 1. The area under the peaks gives information about the concentration of the related functional groups, and the changes in frequency correspond to the structural variations in macromolecules, while the width of the CH2 stretching bands provides information about lipid dynamics( Reference Ozek, Sara and Onur 25 , Reference Severcan, Sahin and Kazancı 28 ). Band area ratios were calculated by comparing the areas of specific spectral bands.
Table 1 General band assignment of the hippocampal tissue

Prediction of the secondary structure of proteins
The secondary structure of proteins was predicted using the artificial neural network (ANN) method based on the amide I band located between the 1700 and 1600/cm regions( Reference Garip, Yapici and Ozek 17 , Reference Akkas, Severcan and Yilmaz 19 , Reference Bozkurt, Severcan and Severcan 26 ). The amide I band was pre-processed by normalisation and discrete cosine transformation. The neural networks were first trained using a dataset containing the FT-IR spectra of eighteen water-soluble proteins recorded in water by Bayesian regularisation( Reference Severcan, Haris and Severcan 29 , Reference Severcan, Haris and Severcan 30 ). For each structure parameter, a separate ANN with the number of inputs, i.e. the number of discrete cosine transformation coefficients, and the number of hidden neurons optimised was trained. The secondary structure parameters of proteins were predicted by applying the pre-processed FT-IR data to the inputs of the trained ANN.
The secondary structure of proteins was determined by performing intensity reading of the second-derivative spectra using the amide I band (1700–1600 cm− 1) with the OPUSNT data collection software package (Bruker Optics GmbH) as described in detail previously( Reference Garip, Yapici and Ozek 17 ).
Hierarchical cluster analysis and principal component analysis
Hierarchical cluster analysis (HCA) and principal component analysis (PCA) were carried out to characterise the study groups based on spectral variations. HCA was performed using the cluster analysis module of the OPUS 5.5 software. The second-derivative and vector-normalised spectra of samples (in the 1700–1600 cm− 1 spectral region) were used in this analysis. Ward's algorithm was applied to construct dendrograms, using Pearson's product–correlation coefficient as a distance measure.
Specific software developed by the laboratory of Erik Goormaghtigh under MATLAB (MathWorks, Inc.) was used to carry out PCA. PCA was conducted using the average absorbance spectra utilising the first-derivative spectra in the 1700–1600/cm spectral region.
Statistical analyses
The results are presented as means with their standard errors. Spectral data were analysed using Student's t test with the SPSS Statistical Software version 15.0 program (SPSS, Inc.). A P value ≤ 0·05 was considered to be statistically significant.
Results
The FT-IR spectra of hippocampal tissue in the 3800–3030/cm, 3030–2800/cm (C–H) and 1800–400/cm (fingerprint) regions are shown in Fig. 1(a)–(c) for all the three groups. The main bands are labelled in the figures and the general band assignment is summarised in Table 1. Spectral calculation of the band area and frequency (Table 2) and bandwidth and band area ratio (Table 3) was performed to determine the VAD-induced alterations in the content, structure and function of molecules. Lastly, the secondary structure of proteins was determined (Table 4). Based on spectral variations, the VAD, CON and PC groups were successfully differentiated by HCA and PCA (Fig. 2(a) and (b)).

Fig. 1 IR spectra of hippocampal tissue in the (a) 3800–3030/cm region normalised with respect to the amide A mode at 3306/cm, (b) 3030–2800/cm region normalised with respect to the CH2 asymmetric stretching mode at 2924/cm, and (c) 1800–400/cm region normalised with respect to the amide I mode at 1654/cm in the pure control (![]() ), control (
), control (![]() ) and vitamin A-deficient (
) and vitamin A-deficient (![]() ) groups. Description of numerals above the bands is given in Table 1.
) groups. Description of numerals above the bands is given in Table 1.
Table 2 Detailed numerical summary of the differences in the frequency and area values of spectral bands in each group (Mean values with their standard errors)

PC group, pure control rats maintained on standard solid laboratory chow; CON group, control rats maintained on a vitamin A-supplemented liquid diet; VAD group, experimental rats maintained on a vitamin A-deficient liquid diet; ↓ , decrease in the band frequency and band area in the VAD group with respect to the CON group; ↑ , increase in the band frequency and band area in the VAD group with respect to the CON group.
Mean value was significantly different from that of the CON group: * P< 0·05, ** P< 0·01, *** P< 0·001.
Table 3 Detailed numerical summary of the differences in the ratio of spectral bands in each group (Mean values with their standard errors)
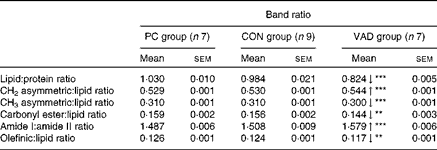
PC group, pure control rats maintained on standard solid laboratory chow; CON group, control rats maintained on a vitamin A-supplemented liquid diet; VAD group, experimental rats maintained on a vitamin A-deficient liquid diet; ↓ , decrease in the band frequency and band area in the VAD group with respect to the CON group; ↑ , increase in the band frequency and band area in the VAD group with respect to the CON group.
Mean value was significantly different from that of the CON group: **P< 0·01, ***P< 0·001.
Table 4 Changes in the secondary structure of proteins predicted using the artificial neural network method (Mean values with their standard errors)
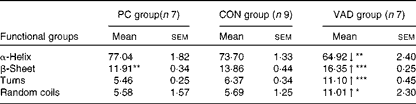
PC group, pure control rats maintained on standard solid laboratory chow; CON group, control rats maintained on a vitamin A-supplemented liquid diet; VAD group, experimental rats maintained on a vitamin A-deficient liquid diet; ↓ , decrease in the band frequency and band area in the VAD group with respect to the CON group; ↑ , increase in the band frequency and band area in the VAD group with respect to the CON group.
Mean value was significantly different from that of the CON group: * P< 0·05, ** P< 0·01, *** P< 0·001.

Fig. 2 Results of the (a) hierarchical cluster analysis and (b) principal component (CP) analysis of the Fourier transform IR spectra in the fingerprint region of the spectral range in the pure control (PC), control (CON) and vitamin A-deficient (VAD) groups. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
As can be seen from Fig. 1(a)–(c) and Tables 2 and 3, there were no significant differences in the areas and area ratios of all the studied bands in the CON and PC groups. Therefore, the VAD group was compared with the CON group with regard to hippocampal molecule content. The areas of spectral bands in the CH region were used to determine alterations in the lipid content among the study groups. The olefinic band located in the 3014/cm region was analysed to obtain information about unsaturated lipids, while the bands located in the 2924, 2872 and 2852/cm regions were used to obtain information about saturated lipids( Reference Cakmak, Togan and Severcan 31 – Reference Melin, Perromat and Deleris 33 ). These data indicated a significant decrease in the contents of both saturated and unsaturated lipids in the VAD group when compared with the CON group (P≤ 0·05; Fig. 1(b) and Table 2). This decrease in lipid content was also supported by the decrease in the area of another lipid band located in the 1459/cm region (P≤ 0·05; Fig. 1(c) and Table 2). The decrease in the area of the olefinic band (Fig. 1(b) and Table 2) and thus the decrease in unsaturation of the system were additionally supported by the decrease in the olefinic–CH:lipid ratio (Table 3) in the VAD group. In addition, the ratios of some specific lipid functional groups to the sum of the CH2 asymmetric and symmetric stretching bands and CH3 asymmetric stretching band were investigated to determine the changes in lipid composition. A significant increase (P≤ 0·001) was observed in the band area ratio of CH2 asymmetric stretching to total lipids in the VAD group when compared with the CON group. In contrast, a significant decrease was observed in the band area ratio of CH3 asymmetric stretching and carbonyl esters to total lipids (P≤ 0·001 and P≤ 0·01, respectively) in the VAD group (Table 3). In addition to changes in these ratios, the lipid:protein ratio and the lipid ester:protein ratio were significantly reduced and the saturated:unsaturated lipid ratio was significantly elevated in the VAD group when compared with the CON group (P≤ 0·001; Table 3).
Reductions were also observed in the cholesteryl ester (1744 cm− 1) and TAG (1170 cm− 1) contents in the hippocampus of the VAD group when compared with the CON group (P≤ 0·01; Table 2 and Fig. 1(c)). In addition, a significant shift to lower levels was observed in the frequency of the ester band in the VAD group, while no differences were observed in the frequency when the control groups were compared with each other (P≤ 0·05; Table 2).
The bandwidth and band frequency analyses of the CH2 asymmetric and symmetric stretching bands give information about lipid fluidity and lipid order, respectively( Reference Severcan, Sahin and Kazancı 28 , Reference Severcan, Toyran and Kaptan 34 ). In the present study, a significant decrease from 12·41 (sem 0·05) to 5·90 (sem 0·02) arbitrary units in the CON group, from 12·47 (sem 0·05) to 5·89 (sem 0·03) arbitrary units in the PC group, and from 12·08 (sem 0·03) to 5·74 (sem 0·02) arbitrary units in the VAD group was observed in the bandwidths of the CH2 asymmetric and symmetric stretching bands, which monitor lipid fluidity (P≤ 0·001). In addition, a significant shift to lower levels was observed in the frequencies of both bands in the VAD group (P≤ 0·001) when compared with the CON group (Table 2). Conversely, a significant shift to higher levels was observed in the frequency of the CH2 symmetric stretching band in the CON group when compared with the PC group (P≤ 0·05; Table 2).
Alterations in the areas and frequencies of the amide I and II bands and the amide I:amide II ratio are commonly analysed to understand the changes in protein content and structure( Reference Cakmak, Zorlu and Severcan 16 , Reference Melin, Perromat and Deleris 33 ). In the present study, a significant decrease in the frequency of the amide I band (P≤ 0·001) and a significant increase in the frequency of the amide II band (P≤ 0·001) were observed in the VAD group when compared with the CON group. However, no differences were observed in these frequencies when the control groups were compared with each other (Table 2). Moreover, the amide I:amide II ratio was significantly higher in the VAD group (P< 0·001; Table 3). Significant shifts to lower levels (P≤ 0·05) were observed in the frequencies of the amide A and CH3 symmetric stretching and amide III bands, which also mainly originate from the functional groups of proteins, in the VAD group when compared with the CON group (Table 2).
To obtain more detailed information, the secondary structure of proteins was determined from the amide I band in the FT-IR spectra for all groups, using the ANN method and the measurement of the intensity of the second-derivative spectra. The ANN results (Table 4) revealed a significant decrease in the α-helix structure content (P≤ 0·01) and a significant increase in the β-sheet (P≤ 0·001), turn (P≤ 0·001) and random coil (P≤ 0·05) structure contents in the VAD group when compared with the CON group. As the increase in the random coil structure content indicates protein denaturation, this band was further investigated using a second approach, namely the measurement of the intensity of the second-derivative spectra. Using this approach, supporting neural network results, a significant increase in the random coil structure content was detected in the VAD group when compared with the CON group (VAD: − 0·113 (sem 0·01), CON: − 0·079 (sem 0·02), and PC: − 0·096 (sem 0·01); P< 0·05), which also confirms protein denaturation. Conversely, a significant increase was observed in the β-sheet structure content in the CON group (P≤ 0·01) when compared with the PC group.
It has previously been shown that absorption in the 1300–900/cm range occurs due to several macromolecules including polysaccharides and phosphorylated compounds such as phospholipids and nucleic acids( Reference Ozek, Sara and Onur 25 ). In the VAD group, when compared with the CON group, a significant increase in the frequency of the COO− symmetric stretching band (1394 cm− 1) due to fatty acids and in the frequency of the C–O stretching band of the phosphodiester and ribose (1066 cm− 1) due to nucleic acids was observed (P≤ 0·05 and P≤ 0·001, respectively; Table 2). Moreover, the frequency of the PO2 asymmetric stretching band (1236 cm− 1) was significantly shifted to lower levels in the VAD group (P≤ 0·01). In addition, in this group, the frequency of the N-type sugar (876 cm− 1) band representing RNA and DNA significantly shifted to lower levels and the frequency of Z-type DNA (925 cm− 1) bands significantly shifted to higher levels (P≤ 0·001 and P≤ 0·01, respectively). However, both vitamin A supplementation and VAD, compared with PC, led to a significant shift in the frequencies of the RNA bands centred at 990/cm (P≤ 0·001). Moreover, significant decreases in the areas of these bands were observed in the VAD group when compared with the CON group (P≤ 0·05).
Discussion
In the present study, the effect of VAD on the content, structure and function of molecules in the hippocampus, the brain structure crucial for learning and memory formation( Reference Jarrard 35 ), was investigated. Although the effects of VAD in the hippocampus have been studied extensively as well as the effects of VAD in other regions of the brain such as the striatum, cerebellum, hindbrain and cortex have been investigated in some studies( Reference Misner, Jacobs and Shimizu 12 , Reference McCaffery, Adams and Maden 36 – Reference Zetterstrom, Lindqvist and Mata de Urquiza 40 ), none of these studies has investigated molecular changes in these brain structures. Therefore, the present study is the first to investigate the effect of VAD on hippocampal macromolecules.
VAD is caused due to inadequate intake of vitamin A. This condition can be caused by either total depletion or reduction of vitamin A in the diet. Both these mechanisms can lead to a similar effect at the organismal level. For example, Antipatis et al. ( Reference Antipatis, Grant and Ashworth 41 ) have previously found that pregnant rats fed a vitamin A-deficient diet and a vitamin A-low diet have plasma retinol concentrations 50 and 30 % lower than those of pregnant rats fed a vitamin A-sufficient diet. Lower plasma retinol concentrations result in many disorders such as liver disease( Reference Newsome, Beldon and Moussa 42 ) and lung disease( Reference Spears, Cheney and Zerzan 43 ). For example, chronic hepatitis C patients exhibit a reduction of 30–35 % in plasma retinol concentrations when compared with normal patients( Reference Newsome, Beldon and Moussa 42 ). In another study, rats fed a diet with reduced vitamin A content accidentally have been found to exhibit an increase in mortality from urinary tract infection in contrast to that observed in studies in rats fed vitamin A-free diets( Reference Munday, McKinnon and Aberdein 44 ). Furthermore, both vitamin A-depleted animals and animals fed a vitamin A-low diet have been found to exhibit significantly reduced weight gain( Reference Munday, McKinnon and Aberdein 44 ). Therefore, feeding animals with a vitamin A-depleted diet either totally or partially may cause significant health problems.
In the present study, the spectral analysis of hippocampal tissue using HCA and PCA clearly showed that both the CON and PC groups differed significantly from the VAD group with respect to spectral data, while no significant differences were observed in the spectral data of the control groups.
As has been mentioned earlier, brain tissue is highly sensitive to free radicals because of its high rate of oxidative metabolic activity and high concentrations of oxidative substances (PUFA and metals such as Fe) and low concentrations of antioxidants( Reference Zwart, Meerman and Commandeur 45 ). When molecules possessing antioxidant activity such as vitamin A are removed from biological systems, brain becomes highly prone to lipid peroxidation. In the present study, as has been suggested previously, information about lipid peroxidation was obtained by analysing the integrated area of the olefinic band (3014 cm− 1) arising from the CH stretching mode of HC–CH groups as these groups are highly sensitive to oxidative attack and highly prone to lipid peroxidation because of their double bond content( Reference Cakmak, Zorlu and Severcan 16 , Reference Sills, Moore and Mendelsohn 46 – Reference Reiter 48 ). Furthermore, a significant decrease in the area of the olefinic band and thus a decrease in the number of double bonds in fatty acids and a decrease in the olefinic–CH:lipid ratio were observed in the VAD group. These findings indicate a loss of olefinic bonds( Reference Sills, Moore and Mendelsohn 46 ) and thus a decrease in the content of unsaturated lipids resulting from the increased lipid peroxidation( Reference Cakmak, Togan and Severcan 31 – Reference Melin, Perromat and Deleris 33 , Reference Zwart, Meerman and Commandeur 45 ). Moreover, Fonzo et al. ( Reference Fonzo, Golini and Delgado 49 ) demonstrated increased lipid peroxidation in the hippocampus of vitamin A-deficient rats, which also confirms our findings.
Lipid peroxidation leads to the oxidative degradation of lipids due to the fragmentation of long chains resulting in a decrease in lipid content. In the present study, a significant decrease in the areas of lipid bands (CH2 asymmetric and symmetric stretching, CH3 asymmetric stretching and CH2 bending), indicating an overall decrease in lipid content, was observed in the VAD group. In line with the results of the present study, Khanna & Reddy( Reference Khanna and Reddy 50 ) reported a decrease in hepatic phospholipid content in rats maintained on a vitamin A-deficient diet. To better reveal the spectroscopic changes in lipid content, and thus variations in lipid synthesis, the band ratios of some specific lipids to total lipids were evaluated( Reference Cakmak, Zorlu and Severcan 16 , Reference Cakmak, Miller and Zorlu 51 ). The phospholipids of cell membranes in all living organisms are strongly affected by either excessive loss or insufficient dietary supply of macro- and micronutrients, leading to pathophysiological conditions( Reference Gimenez, Oliveros and Gomez 52 ). In the present study, a significant decrease in the carbonyl:lipid ratio (decrease in carbonyl content) and the CH3 asymmetric stretching:lipid ratio (decrease in methyl concentration) and an increase in the CH2 asymmetric stretching:lipid ratio (increase in the acyl chain length( Reference Cakmak, Miller and Zorlu 51 )), indicating a relative increase in the saturation levels of lipids in the hippocampus of vitamin A-deficient rats occurring parallel to the decrease in the area of the olefinic band, were observed. These findings point to the protective antioxidant effects of vitamin A in biological systems. Changes in lipid content and composition strongly affect the structure and functions of the cell membrane. It has been demonstrated that the degree of fatty acid unsaturation in the cell membrane determines its biophysical properties, which in turn influence many critical membrane-associated functions including the activity of membrane-bound enzymes( Reference Horrocks and Farooqui 53 ).
Information about the contents of cholesteryl esters and TAG is generally acquired from the areas of spectral bands located in the 1740 and 1170/cm regions, which are due to the C–O stretching functional groups. The C–O ester band gives valuable information about the interfacial region of lipid assemblies in addition to information about lipid accumulation( Reference Kneipp, Lasch and Baldauf 54 ). The frequency of the C–O ester carbonyl band near the aqueous part of membrane lipids, in the interfacial region, is an important marker for lipid–lipid and lipid–protein interactions via hydrogen bonds because of their proton-accepting ability( Reference Severcan, Sahin and Kazancı 28 , Reference Wong, Wong and Caputo 55 ). In the present study, the frequency of this band significantly shifted to lower levels in the VAD group, suggesting an increase in the hydrogen-bonding ability of lipids( Reference Gorgulu, Dogan and Severcan 56 ). This indicates the re-orientation of the lipid head groups with a decrease in free carbonyl groups( Reference López-García, Micol and Villalaín 57 ).
As lipids constitute the main component of the cell membrane, their order parameters and fluidity are definitely important for the functioning of the membrane. The order information can be obtained from the frequencies of the CH2 asymmetric and symmetric stretching bands( Reference Severcan, Sahin and Kazancı 28 , Reference Severcan 58 ). The frequencies of these bands are conformation sensitive and respond to changes in the trans:gauche ratio in acyl chains. A lower frequency indicates lower acyl chain flexibility, which implies lipid ordering( Reference Severcan 58 ). In the present study, the frequencies of these bands significantly shifted to lower levels in the VAD group, indicating that VAD leads to an increase in lipid order and thus a decrease in acyl chain flexibility. The significant shift to higher levels observed in the frequency of the CH2 symmetric stretching band in the CON group when compared with the PC group additionally supports the lipid-disordering capacity and -recovery effect of vitamin A.
Lipid fluidity or dynamics is detected by monitoring the bandwidths of the CH2 stretching bands( Reference Severcan, Kazanci and Zorlu 59 ). An increase in the bandwidth corresponds to an increase in lipid content and thus membrane fluidity. In the present study, the bandwidths of the CH2 asymmetric and symmetric stretching bands decreased significantly in the VAD group, indicating a decrease in lipid dynamics and membrane fluidity. The decrease in lipid dynamics due to VAD has also been reported previously( Reference Hamm, Chan and Wolf 60 ). The decrease in membrane fluidity may be associated with lipid peroxidation( Reference Cakmak, Zorlu and Severcan 16 ). The loss of membrane motion (decrease in membrane fluidity) due to oxidative stress and the restoration of membrane fluidity after the administration of melatonin (a potent antioxidant) in biological and liposomal membranes have been reported previously( Reference Garcia, Reiter and Guerrero 61 ). These findings again point to the protective antioxidant effects of vitamin A in the brain and the importance of vitamin A for maintaining the integrity of cell membranes.
Vitamin A-induced alterations in membrane fluidity may also be associated with alterations in the contents of saturated and unsaturated lipids, proteins and cholesterol as well as with the change in the lipid:protein ratio( Reference Bozkurt, Severcan and Severcan 26 , Reference Gimenez, Oliveros and Gomez 52 ), as has been observed in the present study. Changes in phospholipid metabolism in different tissues of vitamin A-deficient rats have also been reported previously( Reference Gimenez, Oliveros and Gomez 52 ).
As has been mentioned earlier, FT-IR spectroscopy provides a greater opportunity to simultaneously examine proteins and lipids in biological tissues( Reference Cakmak, Zorlu and Severcan 16 , Reference Szalontai, Nishiyama and Gombos 62 ). In the present study, a significant increase was observed in the amide I:amide II ratio in the VAD group, which is in accordance with the results of previous studies indicating an alteration in the composition of cellular proteins( Reference Cakmak, Zorlu and Severcan 16 , Reference Yu, Doiron and Liu 63 ). It is known that this ratio changes due to diseased states, as has been reported previously( Reference Bozkurt, Haman Bayari and Severcan 20 , Reference Szczerbowska-Boruchowska, Dumas and Kastyak 64 ). In addition to this, in the present study, shifts were observed in the frequencies of the amide I and II bands in the VAD group, indicating conformational alterations in the structure of proteins due to VAD. The variations in the frequencies of the amide A, CH3 symmetric stretching and amide III bands, mainly originating from the functional groups of proteins, also support the conformational changes in proteins occurring under dietary VAD. To further investigate alterations in the secondary structure of proteins, the amide I band region, corresponding to absorption values between 1600 and 1700/cm, was analysed using the ANN and intensity measurement methods. Recently, this approach has been successfully applied to tissue and membrane proteins by our group( Reference Elibol-Can, Jakubowska-Doğru and Severcan 7 , Reference Cakmak, Zorlu and Severcan 16 , Reference Bozkurt, Severcan and Severcan 26 ). In the present study, a significant decrease in the α-helix structure content and a significant increase in the turn and random coil structure contents were observed, revealing an alteration in the secondary structure of proteins due to VAD. The increase in the random coil structure content indicates protein denaturation, which may be due to oxidative stress and free radical formation( Reference Davies 65 ). As can be seen from Table 4, no significant changes were observed in the turn and random coil structure contents in the control groups, supporting the protective effect of vitamin A on the secondary structure of proteins. Based on the protein denaturation findings in the VAD group, it can be proposed that VAD induces protein structural/conformational changes and thus alterations in protein function. There are only a few studies that have been carried out on the role of vitamins or micronutrients in the protein structure in the literature. In one of these studies, it has been demonstrated that vitamin E prevents the oxidative stress-induced denaturation of nerve proteins( Reference Kaneai, Arai and Takatsu 66 ). Moreover, Toyran et al. ( Reference Toyran, Severcan and Severcan 18 , Reference Toyran, Turan and Severcan 67 ) investigated the effect of Se supplementation on the secondary structure of proteins in rat heart, heart apex and right ventricle and reported significant structural alterations in these proteins. In addition to structural studies, there are a few studies that have investigated vitamin deficiency-induced proteome alterations. For example, Zhang et al. ( Reference Zhang, Huang and Zhang 38 ) demonstrated vitamin A depletion-induced protein alterations in cortical and hippocampal tissues. Moreover, an association between cobalamin (vitamin B12) deficiency and alterations in the cerebrospinal fluid proteome has been demonstrated( Reference Gianazza, Veber and Eberini 68 ). The role of vitamin D deficiency in protein expression alterations in the brain has been demonstrated by Almeras et al. ( Reference Almeras, Eyles and Benech 69 ). However, these studies are based on only protein expression analysis, i.e. quantitative, but not on structural analysis. Therefore, further studies are required to elucidate the association between vitamin deficiencies and protein structural/conformational changes.
It can be inferred that protein denaturation leads to alterations in hippocampal cell function, as cell structure is closely associated with function. Cocco et al. ( Reference Cocco, Diaz and Stancampiano 13 ) suggested that VAD leads to the impairment of cholinergic neurotransmission and a damaged structure of CA1 neurons in the hippocampus of rats. Moreover, the decrease in hippocampal neuron number under VAD has also been reported( Reference Guo, Bryant and Sultana 70 ). Therefore, the damaged neurons and/or decreased number of neurons and thus the impairment of neurotransmission prove the loss of cell activity.
These structural changes in proteins may also lead to alterations in hippocampus-dependent behaviour such as spatial memory impairments, as it has previously been reported that VAD impairs spatial learning and memory due to lower levels of histone acetylation of retinoic acid nuclear receptor-α( Reference Hou, Ren and Gong 71 ).
Any variation in the lipid:protein ratio gives information about the variation in the lipid and/or protein asymmetry, which is also closely associated with membrane function( Reference Ozek, Sara and Onur 25 , Reference Bozkurt, Severcan and Severcan 26 ). In the present study, with the decrease in the total lipid content and no change in the protein content, the lipid:protein ratio decreased significantly in the VAD group, suggesting an alteration in the lipid asymmetry in the cell membranes of vitamin A-deficient rats. It has been reported that the disruption of lipid asymmetry leads to a change in the intra- and intercellular ion concentrations and thus membrane functions( Reference Becker, Kleinsmith, Hardin and Wilbur 72 ).
The 1300–1000/cm spectral range is commonly coupled with the IR bands of the stretching modes of the P–O bond present in the
![]() $$PO _{2}^{ - } $$
moieties of phospholipids and nucleic acids and with distinct ring vibrations of carbohydrates (C–O, C–C and C–O–C) such as the sugars in the backbone of DNA and RNA(
Reference Ozek, Sara and Onur
25
,
Reference Mendelsohn, Mantsch, Watts and De Pont
73
). The phosphate stretching vibrations are believed to provide valuable information about the head groups of the phospholipids in the polar–non-polar interface of membranous structures(
Reference Bozkurt, Severcan and Severcan
26
). Furthermore, these IR bands can also help to monitor alterations in the quantity, conformational state, and degree and position of phosphorylation of molecules in DNA and RNA(
Reference Kneipp, Lasch and Baldauf
54
). The spectral region between 995 and 990/cm is assigned to the ribose-phosphate main chain vibrations of RNA(
Reference Banyay, Sarkar and Graslund
27
). In the present study, a significant decrease was observed in RNA content in the VAD group, indicating a suppression of gene transcription. The vibration of N-type sugars in the sugar phosphate backbone of nucleic acids is centred at 876/cm(
Reference Severcan, Sahin and Kazancı
28
). In the present study, a significant increase in the area of the N-type sugar band and a significant decrease in the areas of other DNA bands (925 and 1065 cm− 1) were observed in the VAD group, indicating that VAD causes substantial alterations in the content of hippocampal nucleic acids, as has been reported previously for other organ systems(
Reference Feng, Zhao and Hong
74
). The shift in the frequencies of specific RNA and DNA bands located in the 995 and 925/cm regions can be attributed to conformational or structural changes in nucleic acids(
Reference Turker, Ilbay and Severcan
75
,
Reference Whelan, Bambery and Heraud
76
). Some studies have reported an association between nucleic acid bands and gene expression. For example, one of these studies has demonstrated an association between gene expression and DNA conformation(
Reference Kohwi and Kohwi-Shigematsu
77
). Moreover, McClintick et al.
(
Reference McClintick, Crabb and Tian
78
) reported that VAD induces alterations in the hepatic expression of genes involved in fatty acid metabolism, peroxisomal function, glycolysis, and glutamate metabolism, which supports our findings. Recent studies have shown that deficiencies of individual nutrients can alter DNA methylation status and subsequently alter gene expression(
Reference Choi and Friso
79
). These findings are consistent with the decrease in the overall RNA content in the hippocampus of vitamin A-deficient rats observed in the present study, because of the oxidative stress-related inhibition of RNA synthesis as reported previously(
Reference Ding, Dimayuga and Keller
80
).
$$PO _{2}^{ - } $$
moieties of phospholipids and nucleic acids and with distinct ring vibrations of carbohydrates (C–O, C–C and C–O–C) such as the sugars in the backbone of DNA and RNA(
Reference Ozek, Sara and Onur
25
,
Reference Mendelsohn, Mantsch, Watts and De Pont
73
). The phosphate stretching vibrations are believed to provide valuable information about the head groups of the phospholipids in the polar–non-polar interface of membranous structures(
Reference Bozkurt, Severcan and Severcan
26
). Furthermore, these IR bands can also help to monitor alterations in the quantity, conformational state, and degree and position of phosphorylation of molecules in DNA and RNA(
Reference Kneipp, Lasch and Baldauf
54
). The spectral region between 995 and 990/cm is assigned to the ribose-phosphate main chain vibrations of RNA(
Reference Banyay, Sarkar and Graslund
27
). In the present study, a significant decrease was observed in RNA content in the VAD group, indicating a suppression of gene transcription. The vibration of N-type sugars in the sugar phosphate backbone of nucleic acids is centred at 876/cm(
Reference Severcan, Sahin and Kazancı
28
). In the present study, a significant increase in the area of the N-type sugar band and a significant decrease in the areas of other DNA bands (925 and 1065 cm− 1) were observed in the VAD group, indicating that VAD causes substantial alterations in the content of hippocampal nucleic acids, as has been reported previously for other organ systems(
Reference Feng, Zhao and Hong
74
). The shift in the frequencies of specific RNA and DNA bands located in the 995 and 925/cm regions can be attributed to conformational or structural changes in nucleic acids(
Reference Turker, Ilbay and Severcan
75
,
Reference Whelan, Bambery and Heraud
76
). Some studies have reported an association between nucleic acid bands and gene expression. For example, one of these studies has demonstrated an association between gene expression and DNA conformation(
Reference Kohwi and Kohwi-Shigematsu
77
). Moreover, McClintick et al.
(
Reference McClintick, Crabb and Tian
78
) reported that VAD induces alterations in the hepatic expression of genes involved in fatty acid metabolism, peroxisomal function, glycolysis, and glutamate metabolism, which supports our findings. Recent studies have shown that deficiencies of individual nutrients can alter DNA methylation status and subsequently alter gene expression(
Reference Choi and Friso
79
). These findings are consistent with the decrease in the overall RNA content in the hippocampus of vitamin A-deficient rats observed in the present study, because of the oxidative stress-related inhibition of RNA synthesis as reported previously(
Reference Ding, Dimayuga and Keller
80
).
As has been mentioned earlier, the frequency of the PO2 asymmetric stretching band is between 1220 and 1260/cm and indicates the degree of hydrogen bonding(
Reference Wong, Wong and Caputo
55
,
Reference Rigas, Morgellot and Goldman
81
). It has been shown that the frequency of the PO2 asymmetric stretching vibration band shifts to lower levels as hydrogen bonding increases(
Reference Severcan, Kaptan and Turan
32
). In the absorption spectra of hippocampal tissue, in the region of the PO2 asymmetric stretching band, two bands located in the 1262 and 1236/cm regions were observed. The band located in the 1262/cm region is due to non-hydrogen-bonded
![]() $$PO _{2}^{ - } $$
groups and the band located in the 1236/cm region is due to fully hydrogen-bonded
$$PO _{2}^{ - } $$
groups and the band located in the 1236/cm region is due to fully hydrogen-bonded
![]() $$PO _{2}^{ - } $$
groups. In the spectra, a significant shift to lower levels in the frequency of the band located in the 1236/cm region can result from an increase in the hydrogen-bonding capacity of nucleic acids, which is the same as that observed for lipids. These data suggest that structural changes in DNA and RNA can also be associated with changes in hydrogen bonding in DNA and RNA backbones due to increasing nucleic acid hydration under VAD(
Reference Dovbeshko, Gridina and Kruglova
82
).
$$PO _{2}^{ - } $$
groups. In the spectra, a significant shift to lower levels in the frequency of the band located in the 1236/cm region can result from an increase in the hydrogen-bonding capacity of nucleic acids, which is the same as that observed for lipids. These data suggest that structural changes in DNA and RNA can also be associated with changes in hydrogen bonding in DNA and RNA backbones due to increasing nucleic acid hydration under VAD(
Reference Dovbeshko, Gridina and Kruglova
82
).
The results of the present study demonstrate that vitamin A, which at higher doses has been reported to act as a pro-oxidant adversely affecting living tissues( Reference Zaidi and Banu 6 ), at low-to-moderate doses exhibits beneficial effects, many of which arise from its antioxidant properties. Dietary exclusion of vitamin A for 3 months led to significant alterations in the composition and structure of hippocampal lipids with a dramatic decrease in lipid content, an increase in lipid order, and a significant decrease in membrane dynamics. Moreover, the composition and secondary structure of proteins and nucleic acids were altered. The molecular changes induced by VAD may attenuate gene expression and disturb optimal membrane fluidity and lipid–protein as well as protein–protein interactions, which are important for normal membrane functions especially in the nervous system. Therefore, further investigations should be conducted to justify the structural and functional alterations in the molecular constituents of tissues due to VAD.
Acknowledgements
The present study was supported by a grant from the Turkish Scientific and Technical Council (TÜBITAK), SBAG-110S083, provided to E. J.-D. and by the METU Scientific Research Foundation; B. E.-C. was a recipient of the PhD scholarship from the TÜBITAK.
The authors’ contributions are as follows: E. J.-D. and F. S. designed the study; B. E.-C. conducted the study; B. E.-C. and N. S.-O. analysed the data; M. S. conducted the PCA and ANN analysis; B. E.-C., N. S.-O., E. J.-D. and F. S. wrote the article; F. S. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.









