Trichotillomania is an impulse control disorder characterised by repetitive hair-pulling that leads to noticeable hair loss and functional impairment (DSM–IV). 1 Unfortunately, the majority of sufferers have never been diagnosed and have never received treatment. Reference Cohen, Stein, Simeon, Spadaccini, Rosen, Aronowitz and Hollander2 Peak age at onset is 12–13 years, and hair-pulling most commonly affects the scalp and eyelashes/eyebrows. Reference Cohen, Stein, Simeon, Spadaccini, Rosen, Aronowitz and Hollander2 There have been no population-wide epidemiological studies of the condition. Based on a survey conducted in 2500 college students, lifetime prevalence has been estimated to be 0.6–2.5%. Reference Christenson, Pyle and Mitchell3 Trichotillomania has phenomenological overlap with obsessive–compulsive disorder and Tourette syndrome, and consequently represents a candidate member of the obsessive–compulsive spectrum. Reference Stein, Simeon, Cohen and Hollander4 Neurobiological models of these other conditions posit dysregulation of neural circuitry involved in habit formation and top-down inhibitory control, based on tiers of evidence from neuroimaging and elsewhere. Reference Chamberlain, Menzies, Sahakian and Fineberg5,Reference Singer and Minzer6 There have been no brain-wide structural studies of trichotillomania, which hampers our understanding of its neurobiological profile and overlap with other obsessive–compulsive disorders. Reference Chamberlain, Menzies, Sahakian and Fineberg5
In comparison with other related conditions where compulsivity is also a prominent component, trichotillomania offers advantages in terms of neuroscientific investigation. Studies of obsessive–compulsive disorder and Tourette syndrome have often been confounded by current medications. The lack of established treatment guidelines for trichotillomania, and lack of public/clinician awareness, facilitates recruitment of patients who are not medicated to avoid this confound. It is difficult to model intrusive obsessional thoughts or complex vocal tics in animals. By contrast, hair-pulling is a relatively specific behaviour that occurs across species and several promising animal models exist. Mice with disruption of the HoxB8 gene, which is involved in neuronal development, exhibit pathological grooming behaviour. Reference Greer and Capecchi7 The Hoxb8 gene is expressed in multiple brain regions, including the striatum and cingulate cortex. Mice with genetic deletion of a postsynaptic scaffolding protein expressed in the striatum (SAP90/PSD95-associated protein) exhibit compulsive grooming behaviour leading to hair-loss and skin lesions. Reference Welch, Lu, Rodriguiz, Trotta, Peca, Ding, Feliciano, Chen, Adams, Luo, Dudek, Weinberg, Calakos, Wetsel and Feng8
Although these translational models implicate developmental abnormalities of the striatum and cortex in pathological hair-pulling, and neurobiological overlap between trichotillomania and obsessive–compulsive disorder has been suggested, there have been no whole-brain studies of the disorder with which to validate these approaches. Extant trichotillomania neuroimaging studies have all used region-of-interest approaches, rather than exploring distributed abnormalities over the whole brain. Therefore, critically implicated regions may have been overlooked. This is particularly relevant when considering obsessive–compulsive-spectrum disorders, which are theoretically underpinned by abnormalities in large-scale brain systems, i.e. neurocognitive circuits, rather than lesions within a discrete region. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9,Reference Menzies, Achard, Chamberlain, Fineberg, Chen, del Campo, Sahakian, Robbins and Bullmore10
O'Sullivan et al Reference O'Sullivan, Rauch, Breiter, Grachev, Baer, Kennedy, Keuthen, Savage, Manzo, Caviness and Jenike11 reported reduced left putamen volumes in a sample of 10 patients with trichotillomania v. 10 healthy controls. However, these results were described by the authors as preliminary owing to the relatively small sample size and lack of correction for multiple comparisons. Stein et al found no evidence for caudate volume abnormalities in 7 patients with trichotillomania compared with 12 controls using magnetic resonance imaging (MRI) (putamen volumes were not assessed). Reference Stein, Coetzer, Lee, Davids and Bouwer12 Keuthen et al reported reduced cerebellar volumes in a sample of 14 patients with trichotillomania v. 12 controls, using MRI parcellation techniques. Reference Keuthen, Makris, Schlerf, Martis, Savage, McMullin, Seidman, Schmahmann, Kennedy, Hodge and Rauch13 Grachev investigated MRI abnormalities in 10 patients with trichotillomania v. 10 controls. Reference Grachev14 No significant abnormalities were detected in the initial analysis, although a broader analysis of 48 parcellated regions (without correction for multiple comparisons) identified reduced left inferior frontal gyrus volumes and increased right cuneal cortex volumes. Thus, there exists inconsistent evidence for structural abnormalities of the striatum, frontal regions and cerebellum in trichotillomania.
The aim of the present study was to objectively investigate grey and white matter abnormalities over the whole brain in unmedicated patients with trichotillomania. We accomplished this by using cluster-based permutation analysis, which enabled automated, sensitive and unbiased analysis with correction for multiple comparisons. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer15,Reference Suckling and Bullmore16 In light of the above discussion, it was predicted that trichotillomania would be associated with structural abnormalities of large-scale brain networks, including the striatum and cortex.
Method
Participants
Patients with trichotillomania were recruited via support websites, and controls were recruited via newspaper advertisements in the UK. Prior to enrolment, all participants undertook an extended clinical interview conducted by a member of the study team using the Mini International Neuropsychiatric Inventory (MINI), a well-validated screening instrument for DSM–IV Axis I disorders. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar17 Twenty people with trichotillomania and 19 healthy volunteers were enrolled after providing written informed consent and meeting eligibility criteria. For trichotillomania, inclusion criteria were fulfillment of DSM–IV diagnostic criteria (as ratified by a consultant psychiatrist), no treatment for >6 months, and no prior history of neurological conditions such as tic-spectrum disorders and epilepsy. Further, we excluded patients with current depression (defined as meeting MINI criteria and/or having a Montgomery–Åsberg Depression Scale (MADRS) total score >10) Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar17,Reference Montgomery and Asberg18 and current obsessive–compulsive disorder (defined as meeting MINI criteria and/or having a Yale–Brown Obsessive Compulsive Scale total score >10). Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar17,Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney19 We measured IQ using the National Adult Reading Test (NART). Reference Nelson20 Trichotillomania disease severity was assessed using the Massachusetts General Hospital (MGH) Hair pulling Scale. Reference Keuthen, O'Sullivan, Ricciardi, Shera, Savage, Borgmann, Jenike and Baer21 Controls were included on the basis of no history of neurological or Axis I disorders. All volunteers were deemed safe for MRI by completion of a screening questionnaire and the study was approved by a local research ethics committee.
Neuroimaging
Structural MRI scans were acquired from all participants using a 1.5 T GE Signa system (General Electric, Milwaukee, USA) at the Department of Radiology, Addenbrooke's Hospital, Cambridge, UK. Axial three-dimensional (3-D) T1-weighted images were obtained using a spoiled gradient recall sequence with the parameters: 124 slices of thickness 2 mm, repetition time (TR)=33 ms, echo time (TE)=3 ms, flip angle 40°, matrix size 256 × 256 and in-plane voxel dimensions 0.94 mm2. Axial dual-echo fast spin echo images were also acquired with the parameters: 40 slices of thickness 4 mm, TR=5625 ms, TE=20 ms (proton density-weighted) and 102 ms (T2-weighted) with 8-echo train length, matrix size 256 × 256 and in-plane voxel dimensions 0.94 mm2. Structural scans were visually inspected by a consultant radiologist independent of the research team for clinically significant abnormalities.
Images were preprocessed with tools from the FSL software package (www.fmrib.ox.ac.uk/fsl/). Non-brain tissues were first removed using an automated brain extraction procedure (Brain Extraction Tool). Reference Smith22 The resulting voxels were segmented using an automated tissue classification algorithm into probabilistic maps of grey matter, white matter, cerebrospinal fluid and ‘other’ using tissue-type segmentation and bias field correction (FSL Automated Segmentation Tool). Reference Zhang, Brady and Smith23 For each voxel, the partial volume coefficient was calculated, which represented the probability of that voxel belonging to each of the four tissue classes. The resulting segmented grey and white partial volume maps were then registered into standard space (Montreal Neurological Institute, MNI) using affine intermodal image registration (FSL Linear Image Registration Tool). Reference Jenkinson, Bannister, Brady and Smith24,Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak, Flitney, Niazy, Saunders, Vickers, Zhang, De Stefano, Brady and Matthews25 Prior to statistical inference, all segmented maps were smoothed via the Fourier domain, by a two-dimensional (2-D) Gaussian kernel with a standard deviation of 1.88 mm (2 voxels).
Statistical analyses
Between-group measurement of grey and white matter differences were performed using permutation tests implemented in Cambridge Brain Analysis (CAMBA) software in Linux (version 1.3.2; www-bmu.psychiatry.cam.ac.uk/software). An analysis of covariance (ANCOVA) model was fitted at each intracerebral voxel in standard space, with global grey matter density, age and MADRS scores as covariates. We tested the null hypothesis of no differences in brain structure between the two groups by permutation at the level of spatially contiguous 3-D voxel clusters, as described in detail elsewhere. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer15,Reference Pantelis, Velakoulis, McGorry, Wood, Suckling, Phillips, Yung, Bullmore, Brewer, Soulsby, Desmond and McGuire26 This non-parametric method of analysis incorporates spatial information and is generally more powerful than other tests, such as those informed only by data at the single voxel level. Reference Pantelis, Velakoulis, McGorry, Wood, Suckling, Phillips, Yung, Bullmore, Brewer, Soulsby, Desmond and McGuire26 For between-group comparisons, we used probability thresholds for cluster testing so that the average number of false-positive clusters expected per map was less than one (equivalent P<0.004), with the voxel threshold set to P<0.05. Clusters showing significant between-group differences were then described in terms of their peak coordinates, and the automated anatomical labelling template image regions contained therein. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot27 An additional permutation analysis was conducted to explore relationships between grey/white matter densities and symptom scores in patients, again such that the expected number of false-positive clusters per map was less than one, and the voxel threshold was set to P<0.05. Correlational analysis was performed in patients between mean grey/white densities in those clusters showing group differences and symptom scores in patients (Pearson's r).
Results
Routine clinical screening of MRI scans identified significant structural abnormalities in two patients, whose data were excluded from the subsequent analysis. One patient's scans showed a basal ganglia signal hyperintensity; this was deemed of uncertain clinical significance and the patient was asked to monitor for worsening of symptoms or onset of new symptoms. The other patient's scans showed evidence of a brainstem event, leading to urgent clinic follow-up. Thus, the final sample size was 18 patients and 19 controls. As can be seen in Table 1, the two study groups did not differ significantly in terms of age, NART IQ, male:female ratio, and handedness. Total MADRS scores for both groups were well below threshold for clinically significant depression; as anticipated, patients showed significantly higher scores than healthy controls. The mean total MGH score at the time of study participation for the patients was 15.11 (s.d.=4.54), consistent with mild to moderate disease severity. One patient met DSM criteria for current panic disorder and agoraphobia; the others were free from current Axis I comorbidities according to MINI screen. The mean age of symptom onset reported by patients was 11.89 years (s.d.=6.85).
Table 1 Demographic and clinical characteristics of controls and patients with trichotillomania

| Variable | Trichotillomania (n=18) | Controls (n=19) | F (d.f.=1,36) | P |
|---|---|---|---|---|
| Age, years: mean (s.d.) | 37.39 (11.65) | 33.05 (9.09) | 1.61 | 0.21 |
| NART IQ score, mean (s.d.) | 115.52 (7.11) | 111.73 (9.20) | 1.95 | 0.17 |
| Males:females | 1:17 | 3:16 | 1.00 | 0.32a |
| Left:right handedness | 3:15 | 2:17 | 0.30 | 0.59a |
| MADRS score, mean (s.d.) | 3.17 (4.71) | 0.21 (0.63) | 7.37 | 0.01* |
| MGH total score, mean (s.d.) | 15.11 (4.54) | |||
| Age at onset, years: mean (s.d.) | 11.89 (6.85) |
The patients did not differ significantly from controls in terms of global grey matter density (trichotillomania 9.26 arbitrary units (s.d.=0.47) v. controls 9.14 (s.d.=0.66); P=0.51). In comparison with the healthy volunteers, patients with trichotillomania showed grey matter density excesses in three clusters (see Fig. 1 and Table 2 for anatomical details and peak MNI coordinates). These comprised: (a) a mean density increase of 18% in the striatum (left putamen) and limbic system (left amygdalo-hippocampal complex); (b) a mean density increase of 23% in bilateral frontal regions (cingulate, supplemental motor, and superior cortices); and (c) a mean density increase of 21% in left occipital and parietal regions (Fig. 2). These results were not dependent on entering age as a covariate, since when the analysis was rerun without age as a covariate, the core results were still evident (i.e. increased grey matter in the left amygdalo-hippocampal formation, bilateral cingulate, and right middle/superior frontal cortices). There were no significant regions of relative grey matter reductions or changes in white matter (increases or decreases) in the patients. No clusters were found in which density covaried significantly with symptom severity in the patients. No significant correlations were found between mean grey matter densities and patient disease severity scores, in those clusters identified in the between-group analysis (P>0.10).
Table 2 Regions of increased grey matter in patients with trichotillomania (n=18) compared with controls (n=19)

| Automated anatomical labelling regions within each cluster | MNI coordinates of peak (x,y,z) | Voxels |
|---|---|---|
| Left hippocampus, left amygdala, left putamen | -22, -10, 14 | 912 |
| Bilateral anterior/middle cingulate, bilateral supplemental motor area, bilateral frontal superior cortex, bilateral frontal superior medial cortex | -4, 34, 10 | 2464 |
| Left superior occipital cortex, left middle occipital cortex, left superior parietal cortex, left inferior parietal cortex | -24, -74, 44 | 414 |

Fig. 1 Map of grey matter volume excesses (red) in patients with trichotillomania compared with controls in (Montreal Neurological Institute (MNI) space, superimposed onto a standard template. Representative slices with z-coordinates indicated. Expected number of false positive cluster tests <1 over the whole map (equivalent P<0.004).
Discussion
The key finding of this study was that unmedicated patients with trichotillomania exhibited abnormally increased grey matter densities compared with matched healthy controls in the left amygdalo-hippocampal complex, left striatum and multiple cortical regions. These distributed structural abnormalities occurred in the absence of medication confounds and appeared to be unrelated to disease severity.
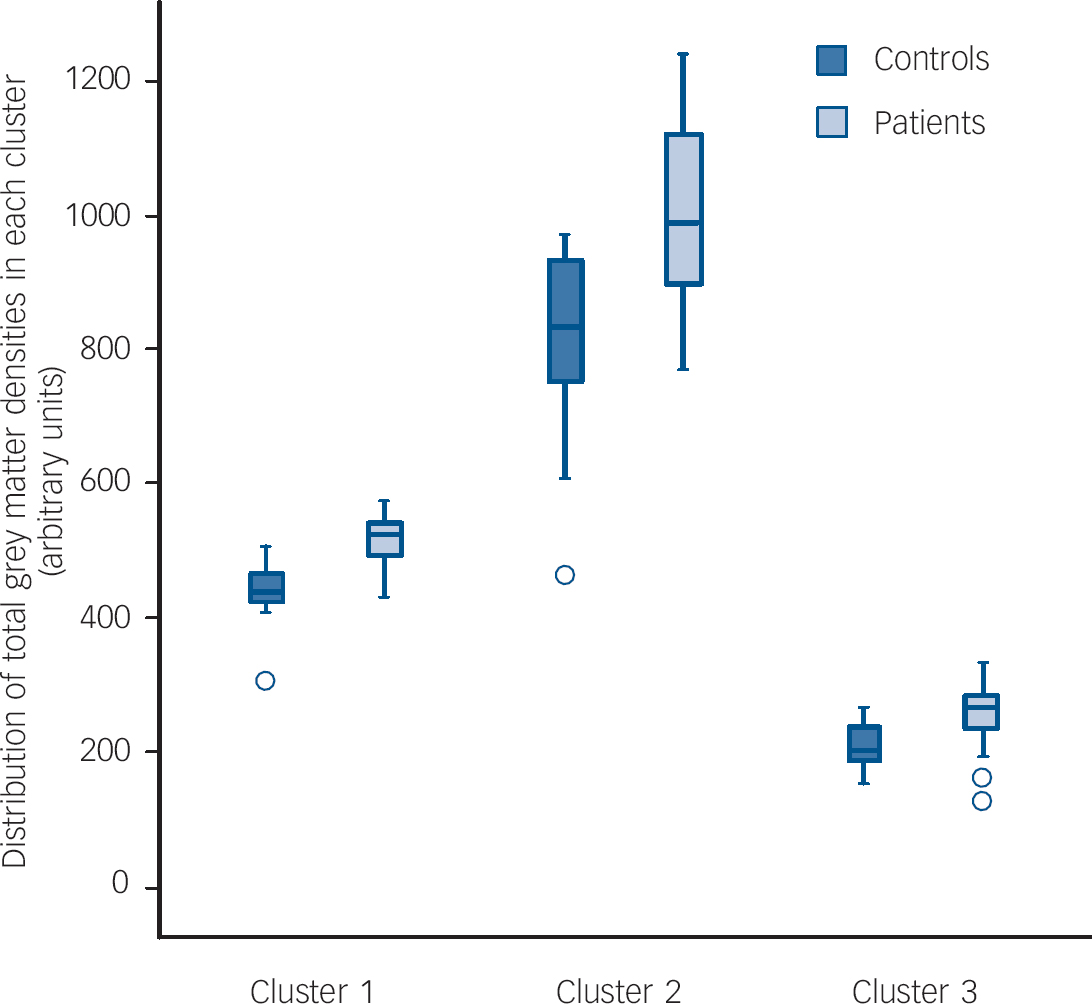
Fig. 2 Grey matter densities in healthy controls and patients with trichotillomania for each of the three clusters identified in the permutation analysis.
Cluster 1, left hippocampus, left amygdala, left putamen; Cluster 2, bilateral anterior/middle cingulate, bilateral supplemental motor area, bilateral fronto-superior cortices; Cluster 3, left superior/middle occupital cortex, left superior/inferior parietal cortex.
Increased grey matter has also been reported in studies of focal dystonia, Tourette syndrome and obsessive–compulsive disorder Reference Kim, Lee, Kim, Kim, Kim, Han, Chang and Kwon28–Reference Garraux, Goldfine, Bohlhalter, Lerner, Hanakawa and Hallett31 albeit not consistently. Reference Chamberlain, Menzies, Sahakian and Fineberg5 These novel findings support the notion that trichotillomania shares some neurobiological overlap with other putative obsessive–compulsive-spectrum disorders, with implications for upcoming diagnostic revisions. Peak onset of trichotillomania is at 12–13 years of age. Adolescence is a critical time for neurodevelopment and multiple studies have reported intense reductions of grey matter tissue in the pubescent period. Reference Pfefferbaum, Mathalon, Sullivan, Rawles, Zipursky and Lim32–Reference Whitford, Rennie, Grieve, Clark, Gordon and Williams35 These grey matter excesses detected in patients with trichotillomania could reflect deviation from normal developmental trajectories. Alternatively, they may reflect neuro-plastic changes occurring through use of brain regions involved in grooming and habit learning, there being some evidence that increases in grey matter can occur through motor-skill training. Reference Draganski, Gaser, Busch, Schuierer, Bogdahn and May36
The striatum is thought to play a critical role in habit learning and in the chunking of automated action sequences, according to tiers of evidence from animals and humans. Reference Graybiel37 Striatal damage in rats has been shown to disrupt the ability to perform choreographed grooming sequences. Reference Cromwell and Berridge38 In humans, clinical data suggest that the striatum is responsible for the gradual incremental learning of associations typical of habit learning. Reference Knowlton, Mangels and Squire39 Striatal involvement in trichotillomania is reminiscent of obsessive–compulsive disorder, and supports a similar conceptualisation emphasising the role of the basal ganglia in the development of pathological habits. Reference Chamberlain, Menzies, Sahakian and Fineberg5,Reference Graybiel and Rauch40
Cortical regions such as the cingulate and prefrontal lobes are involved in multiple high-level cognitive processes. Reference Robbins41 The structural abnormalities detected in these regions in trichotillomania could mediate cognitive problems previously identified in patients, which likely impede quality of life. In neuropsychological studies, patients with trichotillomania showed deficits on tests of divided attention, Reference Stanley, Hannay and Breckenridge42 response inhibition and working memory. Reference Chamberlain, Fineberg, Blackwell, Robbins and Sahakian43,Reference Bohne, Savage, Deckersbach, Keuthen and Wilhelm44 The relationship between grey matter excesses and cognitive deficits in patients with trichotillomania merits follow-up, since these neural regions and their dependent cognitive functions represent targets for novel cognitive enhancers. Reference Chamberlain and Sahakian45
The finding of amygdalo-hippocampal abnormalities was not predicted a priori. The amygdalo-hippocampal formation constitutes part of the limbic system, which regulates arousal and emotional learning processes. Reference McGaugh46 Several studies support a causal relationship between increased or decreased arousal and hair-pulling episodes, and between negative affect and hair-pulling episodes. Reference Christenson, Mackenzie and Mitchell47–Reference du Toit, van Kradenburg, Niehaus and Stein49 Furthermore, trichotillomania has been associated with childhood trauma and post-traumatic stress disorder. In one survey, the majority of patients reported physical abuse and/or emotional neglect as a trigger in childhood. Reference Boughn and Holdom50
Positive features of this study include the use of permutation cluster analysis to maximise power while facilitating corrected whole-brain analysis, and the inclusion of patients who were untreated for at least 6 months prior to scanning. None the less, several caveats must be considered. Details of past treatments (beyond 6 months) received by patients were not available, to explore whether past treatment-seekers differed from non-treatment-seekers. It remains to be seen whether the present findings generalise to other groups of patients with trichotillomania who differ in terms of their clinical characteristics (past treatments, disease severity, comorbidities). Another potential limitation is that patients showed significantly higher MADRS (dysphoric mood) scores than controls, despite being free from DSM–IV depression. However, we controlled for this by entering MADRS scores as a covariate into the imaging analysis. Finally, voxel-based morphometry techniques carry potential limitations such as confounds due to choice of smoothing kernel size and potential misalignment of brain structures during normalisation. On the other hand, such techniques enable objective and sensitive analysis, with stringent correction for false positives over the whole brain. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer15,Reference Suckling and Bullmore16
This study, using a cluster-level technique to investigate trichotillomania, provides an objective whole-brain-based analysis that directs researchers to areas that are abnormal in this disorder, namely cortical regions, the amygdalo-hippocampal formation and the striatum. These results suggest overlap with other putative obsessive–compulsive-spectrum disorders in terms of neurobiology. Reference Singer and Minzer6,Reference Graybiel and Rauch40 The functional significance of these grey matter abnormalities requires clarification in follow-up longitudinal studies, and in studies of unaffected first-degree relatives (asymptomatic individuals at increased genetic risk). The further application of translational neuroscientific techniques to the study of trichotillomania will extend these findings and provide new insights into its neurobiology, with implications for diagnosis and treatment of trichotillomania and related obsessive–compulsive-spectrum disorders.
Acknowledgements
The Behavioural and Clinical Neuroscience Institute is supported by a joint award from the Medical Research Council and Wellcome Trust. This research was supported by a grant from the Wellcome Trust (076274/Z/04/Z awarded to T.W.R. and B.J.S.). Software development was supported by a Human Brain Project grant from the National Institute of Biomedical Imaging & Bioengineering and the National Institute of Mental Health, USA. S.R.C was supported by a priority studentship from the Medical Research Council. L.A.M. was supported by the Harnett Fund, University of Cambridge. The authors wish to thank the study participants and radiographers at the Magnetic Resonance Imaging Service, Addenbrooke's Hospital, Cambridge, UK.







eLetters
No eLetters have been published for this article.