The UK Food Standards Agency held a programme review in November 2006 to bring together Agency-funded researchers investigating the effects of diet and other lifestyle factors on bone health. The aim of the workshop was to share the results of these projects and to determine the progress made since a similarly focussed workshop held in 2002Reference Burns, Ashwell and Berry1 and a more general workshop held in 2005Reference Ashwell2. The Agency-funded projects were reviewed in the light of other pertinent research in the field of diet and bone health which has been reported elsewhereReference Francis3.
Background
Public health importance of prevention of osteoporosis and fracture
Bone health is a major public health issue. Osteoporosis is a disease that affects many millions of people around the world. It is characterised by (i) low bone mass and (ii) micro-architectural deterioration of bone tissue which may lead to enhanced bone fragility and consequent increase in fracture risk. Osteoporotic fractures are a major cause of morbidity and disability in the elderly and, in the case of hip fractures, can lead to premature death. Fragility fractures are most common at the wrist, spinal vertebrae and hip, although they can occur throughout the skeleton. Annually, 90 000 hip, 50 000 wrist and 130 000 vertebral fractures occur in BritainReference Torgerson and Bell-Syer4. Hip fracture numbers are projected to increase to 120 000 annually by the year 2020. The incidence of vertebral and hip fractures increases exponentially with advancing age while that of wrist fractures levels off after the age of 60 years. 90 % of hip fractures occur in those aged 65 years and over, and a quarter of these are in men.
Effect of fruit and vegetables on bone health
Based on the outcome of the previous review on bone healthReference Burns, Ashwell and Berry1, the Agency commissioned three projects, each using different study designs, to investigate the possible mechanism for a positive effect of the alkaline nature of fruit and vegetables based on the role that the skeleton plays in acid–base homeostasis.
Natural, pathologic and experimental states of acid loading or acidosis are associated with hypercalciuria and negative Ca balanceReference Barzel5. The high alkali salt content of fruit and vegetables could hence counteract the effects of acid-generating foods such as meat and cerealsReference Green and Kleeman6. Detrimental effects of ‘an acid environment’ on bone mineral density (BMD) have been shownReference Arnett, Burckhardt, Dawson-Hughes and Heaney7. The administration of alkali as potassium bicarbonate resulted in a decrease in urinary excretion of both Ca and P excretion and an improvement in overall Ca balance, which was accompanied by a decrease in urinary excretion of hydroxyproline (a marker of bone resorption) and an increase in serum osteocalcin (a marker of bone formation). This has been demonstrated both in short-term (18 d)Reference Sebastian, Harris, Ottaway, Todd and Morris8 and longer-term (3–6 month)Reference Sellmeyer, Schloetter and Sebastian9 studies. Alternatively, other, as yet unidentified ‘dietary’ components in fruit and vegetables might play a beneficial roleReference Lanham-New10.
Cross-sectional studies
A project based at Human Nutrition Research, Cambridge analysed a series of cross-sectional studies examining the association between fruit and vegetable consumption and bone mineral status in five cohorts selected by age and sex (132 boys and 125 girls aged 16–18 years, 107 premenopausal women aged 23–37 years, 70 men and 73 women aged 60–83 years). The datasets included bone mineral measurements of the whole-body, hip and spine by dual-energy X-ray absorptiometry, anthropometry, and dietary intakes as assessed by 7-d diet diaries. Stored plasma samples were also available. There were positive relationships between size-adjusted bone mineral content and fruit and vegetable intake, vitamin C intake, carotenoid status and vitamin E status in adolescents and older women, but not in young adult women or older men. If this does represent a causal relationship, then doubling the fruit intake would have resulted in a 5 % increment in spine size-adjusted bone mineral content. Significant inverse associations were observed between bone markers (osteocalcin, procollagen type I intact N-terminal propeptide (P1NP), C-terminal peptide and osteoprotegerin) and carotenoids and α-tocopherol suggestive of a modulatory effect of fruit and vegetable intake on bone turnover.
Potential mechanisms for the effect of fruit and vegetables were also studied. To investigate the effect of fruit and vegetables on net renal acid load, two approaches based on dietary data were used: (a) calculation of net acid excretion by the Remer method and (b) calculation of net endogenous acid production by the Frasetto method which is the ratio of dietary protein to dietary KReference Lanham-New10. Estimates of net acid excretion or net endogenous acid production were not a significant inverse predictor of size-adusted bone mineral content in any of the age or sex groups; indeed estimates of net acid excretion were a positive predictor in older men and womenReference Prynne, Mishra, O'Connell, Muniz, Laskey and Yan11, Reference Ginty, Prynne, Muniz-Terrara, Prentice and O'Connell12.
Another research group from the University of Cambridge used cross-sectional analysis of the EPIC-Norfolk population study, examining diet (7-d diet diaries) and heel bone ultrasound attenuation (BUA) collected on more than 4000 men and women aged 40–79 years when first surveyed in 1993–1997Reference Jakes, Khaw and Day13, Reference Welch, Bingham, Camus, Dalzell, Reeve, Day and Khaw14. They used potential renal acid load (PRAL), as well as net endogenous acid production, to assess the acidity of the diet (A. Welch, S. Bingham and K. Khaw, unpublished results). PRAL is calculated by taking into account the mineral and protein composition of foods, the average intestinal absorption rates of the nutrients, S metabolism and urinary excretion of organic acids. The mean BUA in men was related positively to dietary Ca (P < 0·01) and vitamin K (P = 0·03). In women, BUA was significantly positively related to total fruit and vegetable intake (P = 0·002), dietary fibre (P < 0·001), dietary vitamin C (P < 0·001), and blood vitamin C (P < 0·001) and negatively related to dietary PRAL (P < 0·001). A similar (though non-significant) trend was seen for the relationship between PRAL and BUA in men. Differences, where significant, were of the order of about a 2–4 % difference in BUA between highest and lowest quartiles of intake (A. Welch, S. Bingham and K. Khaw, unpublished results). This magnitude is similar to those reported from other studiesReference Welch, Bingham, Camus, Dalzell, Reeve, Day and Khaw14.
Prospective studies
Data were also reported from the prospective EPIC-Norfolk population study examining diet and incident fractures ascertained through hospital record linkage and death certificationReference Khaw, Reeve, Luben, Bingham, Welch, Wareham, Oakes and Day15. Seven-day food diary information was available for 186 incident fractures in males and 6813 male controls, and for 431 incident fractures in females and 6851 female controls. Results were adjusted for age, BMI, cigarette smoking, physical activity, total energy intake and hormone use (in women). Although the direction of association with fracture risk was generally consistent with the relationship with BUA, fracture risk did not differ significantly by quartile of fruit and vegetable intake, PRAL, dietary vitamin K, dietary Ca, dietary fibre, or plasma vitamin C in men or womenReference Welch, Bingham, Reeve and Khaw16. This could mean there is no important association between dietary fruit and vegetable intake or PRAL and fracture risk, or that there was a lack of power to detect a weak association due to measurement errors both in assessment of diet and of fracturesReference Welch, Bingham, Reeve and Khaw16. Significant dietary associations with measures of bone health do not necessarily translate to significant relationships with fracture risk, the clinically relevant outcomeReference Welch, Bingham, Reeve and Khaw16. Some differences between men and women in this cohort, as well as those studies reported aboveReference Ginty, Prynne, Muniz-Terrara, Prentice and O'Connell12, suggest that it cannot necessarily be assumed that dietary factors have the same effects in men and women.
Randomised controlled trials
Previous results from the Aberdeen Prospective Osteoporosis Screening Study showed a positive relationship between fruit and vegetable intake and markers of bone health and suggested that this could be due to their role in correcting the acid–base balance of the dietReference Macdonald, New, Golden, Campbell and Reid17, Reference Macdonald, New, Fraser, Campbell and Reid18. The ultimate test of a relationship between a diet component and a health outcome is a randomised controlled trial (RCT). Women were therefore recruited from the Aberdeen Prospective Osteoporosis Survey cohort into a RCT to test whether, in the long term (2 years), the beneficial effects of fruit and vegetables on bone health are because of the organic salts of potassium they provide which can help neutralise the excess acidity generated through consuming a Westernised dietReference Macdonald, Black, Sandison, Aucott, Hardcastle, Lanham-New, Fraser and Reid19. Women (n 276) were randomised to receive 55·6 or 18·5 mEq potassium citrate (equivalent to 900 g and 300 g fruit and vegetables, respectively) per day, or placebo, or an extra 300 g fruit and vegetables per day. Although the type of fruit and vegetables was not controlled for, the study was designed so that the 300 g provided the same alkaline load as the lower dose potassium citrate group. The four intervention groups were matched using minimisation criteria for smoking, vitamin D receptor genotype and apo E genotype. Despite good reported compliance, there was no significant change in bone formation or resorption markers (serum P1NP, serum C-terminal peptide and urinary pyridinoline–deoxypyridinoline cross-links) during the intervention in any of the four groups. Bone loss at the spine was 1·6 % in 2 years in the placebo group and 2·1 % in the fruit and vegetable and potassium citrate groups and was not significantly different among groups. There was no difference in bone loss at the hip between the placebo and the fruit and vegetable group (H. Macdonald et al, unpublished results). Although the low dose potassium citrate group, with which it was matched, had significantly greater bone loss than placebo this probably reflects the marginally elevated bone turnover in the former groupReference Macdonald, Black, Sandison, Aucott, Hardcastle, Lanham-New, Fraser and Reid19 (H. Macdonald et al, unpublished results). The data are compatible with the theory that changing to an alkaline generating diet may reduce Ca excretion, but less Ca will be absorbed from the gut to compensateReference Rafferty, Davies and Heaney20. The results of the RCT suggest that any long-term benefits of fruit and vegetable intake on bone are not due to the provision of alkaline salts which improve the acid–base balance in a normal healthy postmenopausal population.
Relevance to public health of fruit and vegetables and bone health
These studies support the recommendation to the general public, and to young people in particular, to increase their consumption of fruit and vegetables. They have provided cross-sectional evidence that people who eat more fruit and vegetables are more likely to have a higher bone mineral content for their size, which is also associated with healthier bones. The prospective study linking increased fruit and vegetable consumption to prevention of fracture did not produce statistically significant results with the current rates of fractures in the study, but longer term follow up with more fractures may produce more definitive results in the future. The results of the RCT did not support the theory that fruit and vegetable consumption changes acid–base balance in a beneficial way. However, it is important to note that both cross-sectional and longitudinal studies do indicate a beneficial effect of high alkalinity-excess (achieved by diet) on markers of bone healthReference Welch, Bingham, Camus, Dalzell, Reeve, Day and Khaw14, Reference Macdonald, New, Golden, Campbell and Reid17. The balance between acidity and alkalinity (dietary-induced or otherwise) may be important for those with severe kidney or respiratory disease where acid–base homeostasis is compromisedReference Jehle, Zanetti, Muser, Hulter and Krapf21. Furthermore, diets which emphasise particularly high levels of potassium (and Ca) such as the Dietary Approaches to Stopping Hypertension (DASH) have shown beneficial effects on bone in the short termReference Lin, Ginty, Appel, Aickin, Bohannon, Garnero, Barclay and Svetkey22. Longer- term trials using DASH-style diets or specific types of fruit and vegetables on indices of bone health are now urgently requiredReference Rafferty23, Reference Lanham-New24. Other constituents of fruit and vegetables (e.g. Mg, potassium, vitamin C and fibre) may be involved in the mechanism for improved bone health. It remains to be determined how much and what type would be most beneficial.
Vitamin K
The previous Agency review of bone health projectsReference Burns, Ashwell and Berry1 stated that vitamin K, mainly found as phylloquinone in the diet, was increasingly being considered as an important nutrient for bone health. Vitamin K mediates the γ-carboxylation of glutamyl residues on several bone proteins, in particular osteocalcin, to produce γ-carboxyglutamyl (Gla) residues. This protein, which is formed by the osteoblasts, is often used as a marker of bone formation and is incorporated into bone due to the high binding specificity of the Gla residues for the Ca ion of the hydroxyapatite molecule. At the previous reviewReference Burns, Ashwell and Berry1, preliminary results were reported for the possible synergy between vitamins D and K in a group of Dundee women (aged 60–85 years) in a 2-year randomised placebo-controlled parallel group study. The four intervention groups were: placebo; 200 μg phylloquinone; 10 μg vitamin D and 1 g Ca; 200 μg phylloquinone, 10 μg vitamin D and 1 g Ca. The rationale for the choice of supplementation was as follows: 10 μg is the recommended amount of vitamin D for supplementing the elderly and is easily obtainable from a portion of oil-rich fish; 200 μg vitamin K is easily obtained from a 50 g portion of leafy green vegetables; 1 g Ca was to ensure adequacy. BMD was measured by dual X-ray absorptiometry at the hip (three sites) and at the wrist (two sites). Dietary and other lifestyle information, including activity and sunlight exposure, were also collected at 6-month intervals. The final results from the trial were presentedReference Bolton-Smith, McMurdo and Paterson25. Significant bone mineral loss was seen only at the mid-distal radius, but with no significant difference between the four groups. Women who took combined phylloquinone and vitamin D plus Ca showed a significant and sustained increase in both BMD and bone mineral content at the site of the ultra-distal radius when compared with baseline values. However, no supplementation effect was seen on markers of bone turnover. Indicators of status and function of vitamin K and of vitamin D all responded significantly to respective supplementation with the vitamins. The greatest changes in glutamyl osteocalcin and 25(OH) vitamin D (25-OHD) occurred in women who at baseline had the poorest status of vitamin K and D respectively.
A follow up study at St Thomas' Hospital, London, using samples from the Dundee trial to assess new markers of status for vitamin K, showed that regular intakes of foods providing 200 μg phylloquinone/d substantially improves the γ-carboxylation status of osteocalcin and probably of other non-coagulation Gla-proteins. Previous reports that a high circulating concentration of glutamyl osteocalcin is an independent determinant of low BMD in elderly women were not supported, despite using two independent methodologies to determine γ-carboxylation status.
The purpose of the project undertaken at Human Nutrition Research, Cambridge was to provide information on vitamin K absorption in the context of the sources of phylloquinone in British foods. Dietary and biochemical status (plasma phylloquinone concentration) were assessed in adults aged 19–64 years from the 2000–2001 National Diet and Nutrition Survey (NDNS)Reference Henderson, Gregory and Swan26 in comparison with previous NDNS and other British data. More than 50 % of the subjects in the latest NDNS survey had phylloquinone intakes below the UK guideline for adequacy ( ≥ 1 μg/kg body weight per d)27, Reference Thane, Bolton-Smith and Coward28. Decreases in intake compared to NDNS 1986–1987 dataReference Gregory, Foster, Tyler and Wiseman29 were mainly due to lower consumption of cooked leafy green vegetablesReference Thane, Bolton-Smith and Coward28. Variation of only 8 % of plasma phylloquinone concentration was explained by phylloquinone intake without correction for possible variation in bioavailabilityReference Thane, Wang and Coward30. Plasma phylloquinone concentration is therefore a poor status marker for vitamin K if it is measured in uncontrolled conditions. The diets consumed by this population were then categorised using dietary pattern analysis. Using conditional Gaussian mixture modelling, three major clusters were common to men and womenReference Fahey, Thane, Bramwell and Coward31. These were a ‘convenience’ diet (cluster 1) with relatively low consumption of plant and unrefined foods, pasta/rice, fish, skimmed milk, alcoholic drinks and water, a ‘cosmopolitan’ diet (cluster 2) had a relatively high consumption of plant and unrefined foods, pasta/rice, skimmed milk, fish, dairy foods, coffee/tea, alcoholic drinks (particularly wine) and water, and an ‘animal-oriented’ diet (cluster 3) with the greatest median energy intakes and a preference for animal products and refined cereals. There were significant differences in phylloquinone intake between clusters, with women in cluster 2 having the highest intakes, but these differences were, surprisingly, not fully reflected in differences in plasma concentration.
Experiments to compare relative bioavailability of phylloquinone from meals that are representative of the three clusters emerging from analysis of data from NDNS adults have been completed in twelve subjects in a cross-over study designReference Jones, Bluck and Coward32 (K. Jones, L. Bluck and L. Wang, unpublished results). Phylloquinone was more bioavailable from a meal containing more of the vitamin in oil-based foods (representative of the ‘convenience diet’) than from one where the phylloquinone was in leafy green vegetables (representative of the ‘cosmopolitan’ diet) or from a meal based on the ‘animal-oriented’ dietary cluster. However, the differences in bioavailability could not explain the relationship between phylloquinone intake and status in the different clusters (K. Jones, L. Bluck and L. Wang, unpublished results).
Importance to public health of the effect of vitamin K on bone health
The Agency-funded RCT represents the first ever randomised, medium-term, intervention study with an amount of vitamin K1 that is potentially attainable from increased dietary intakes. One other RCT which has reported in the meantimeReference Braam, Knapen, Geusens, Brouns, Hamulyak, Gerichhausen and Vermeer33 has shown a positive effect for vitamin K (in combination with vitamin D and selected minerals) but a dose of 1 mg was used, which could not be achieved in the diet. Other, mainly Japanese, studies indicate a strong positive link between supra-nutritional doses of menaquinone-4 (a member of the vitamin K2 series) and markers of bone health, including fracture riskReference Cockayne, Adamson, Lanham-New, Shearer, Gilbody and Torgerson34, Reference Knapen, Schurgers and Vermeer35. There is evidence that putative protective effects on fracture of supra-nutritional doses of vitamin K may operate by a different mechanism from simply increasing γ-carboxylationReference Shearer, Torgerson, Cockayne, Adamson, Lanham-New and Gilbody36.
The Agency-funded projects also facilitated the study of the possible nutritional interaction of vitamins K and D. Without evidence that increased dietary intakes of vitamin K can prevent fractures, it is too early to translate the results into advice for public health. However, in the light of previous evidence linking osteocalcin undercarboxylation to fracture risk, it is possible that the substantial and sustained increase in the γ-carboxylated fraction of osteocalcin in response to high dietary vitamin K may realise long-term benefits to bone health.
The effect of early nutrition on bone health
The previous review of Agency funded projects on bone healthReference Burns, Ashwell and Berry1 concluded that evidence is needed on the effect of lifestyle factors on the accretion of bone mass in young adults and children and the interplay of these factors in utero. Evidence that the risk of osteoporosis might be determined during intrauterine and early postnatal development stems from different types of studies. Retrospective epidemiological studies confirm that subjects who were born light, and whose growth falters in the first year of postnatal life, have significantly lower bone size and mineral content at age 60–75 years. Cohort studies have demonstrated that subsequent lower trajectories of childhood growth are associated with an increased risk of hip fracture among men and women. Detailed physiological studies have revealed candidate endocrine systems which might be modified by environmental influences during intrauterine life. Finally, studies characterising the nutrition, body build and lifestyle of pregnant women show that these relate to the bone mass of their newborn offspring. This last group of studies has identified a number of important determinants of reduced fetal mineral accrual (maternal smoking, low maternal fat stores, maternal vitamin D deficiency, and intense levels of weight-bearing physical activity during late pregnancy)Reference Cooper, Westlake, Harvey, Javaid, Dennison and Hanson37, Reference Cooper, Javaid, Westlake, Harvey and Dennison38. Consequently, data from the Southampton Women's Survey has been used to investigate the influence of diet in infancy on early growth, and bone health and cognitive function at 4 years of age. All 12 500 non-pregnant women in the survey, aged 20–34 years, were targeted between 1998 and 2002, to provide a sample of 3000 women for study before conception and in pregnancy. The Agency funding has supported the processing and analysis of the dietary data of these infants, and the follow-up of a sub-sample of 467 children at the age of 4 years for assessment of bone status and cognitive function. Diet was assessed at 6 months and 12 months using administered FFQ, to record the frequency and amounts of foods that were consumed by the infant during the week (6 months) or month (12 months) preceding the interview. Principal component analysis has been used to identify diet patterns in mothers and infants. No results are available yet on bone health, but an analysis of bone mass in 9-year-old children from a different cohort of mothers showed that reduced levels of 25-OHD in mothers during late pregnancy was associated with significantly reduced bone-mineral content in whole-body and lumbar-spine in children at age 9 yearsReference Javaid, Crozier and Harvey39. In this cohort, the mothers' prudent diet score was also related positively to the bone mass of their children at 9 years of ageReference Cole, Javaid and Taylor40.
Relative contributions of diet and sunlight to vitamin D status
Currently, there is no UK dietary reference value for vitamin D for adults, except for those with restricted sunshine exposure27. In USA, the Institute of Medicine decided there was insufficient scientific information to establish an RDA for vitamin D. Instead, they listed an Adequate Intake, which represents the daily vitamin D intake that should maintain bone health and normal Ca metabolism in healthy people41. It is clear that it is assumed that, in the general population, exposure of the skin to UV B-rays in summer sunlight is sufficient to maintain adequate vitamin D status. However this assumption is based on limited scientific evidence42.
Vitamin D is derived from two sources, the skin (endogenous) and the diet (exogenous), but the relative contribution of these sources to vitamin D status in the UK is not known42. This question needed to be addressed given the findings of NDNS surveys (4–18 years; 19–64 years and > 65 years)Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke43–Reference Gregory and Lowe45and, more recently, the 1958 British Cohort studyReference Hypponen and Power46 showing that low vitamin D status (as indicated by a plasma 25-OHD of < 25 nmol/l) is a concern in several population groups. Levels below this threshold are associated with an increased risk of clinical vitamin D deficiency (rickets and osteomalacia). Furthermore, there has been a move in recent years to redefine the criteria of vitamin D adequacy, and hence dietary reference values for vitamin D, based upon health outcomes other than rickets and osteomalaciaReference Bischoff-Ferrari, Giovannucci, Willett, Dietrich and Dawson-Hughes47. Three Agency-funded projects are currently addressing these issues, with the novel approach of comparing different latitudes within the British Isles with differing habitual sunshine exposure.
Prospective studies
The Aberdeen Prospective Osteoporosis Screening Study database has been used to recruit 360 women to see whether the seasonal differences in vitamin D status can be seen longitudinally. The proportion of women with low plasma 25-OHD concentrations was higher in winter and spring than in summer and autumn. This study will see whether diet and previous summer's sunlight exposure can maintain 25-OHD levels even at northerly latitudes (57°). It will also determine if there is an association between vitamin D status, markers of bone health, muscle strength and falls.
In a study run by the University of Surrey, 270 women aged 19–70 years of Caucasian origin and 78 similarly aged women of Asian origin have been recruited. This project will also determine the percentage contribution of diet and sunlight exposure on vitamin D status and functional markers of Ca metabolism in each of the four seasons in pre- and postmenopausal women, as well as investigating the effect of ethnicity. Diet may play a more significant role in determining vitamin D status in Asian than in Caucasian populationsReference Roy, Berry, Pye, Adams, Swarbrick, King, Silman and O'Neill48. The study will also investigate whether poor vitamin D status has a detrimental effect on functional markers of bone health.
These two projects will measure similar health outcomes (25-OHD, parathyroid hormone, bone turnover markers and whole body, lumbar spine and total hip BMD by dual X-ray absorptiometry in the autumn and spring) which will enable data at northerly and more southerly latitudes (where the contribution of sunshine is expected to be higher) to be compared.
Randomised controlled trials
In a collaborative project between the Universities of Cork and Ulster, the intake of vitamin D needed to prevent seasonal vitamin D deficiency as well as the relative importance of diet and sunlight on vitamin D status will be determined. Two RCT will be conducted in 240 adults (aged 20–40 years) and 240 elderly subjects (aged > 65 years) using supplemental levels of 0, 5, 10 and 15 μg vitamin D3/d throughout winter.
Importance to public health of the effect of diet on vitamin D status
By conducting these studies across the extremes of latitudes in the British Isles and across ethnic groups, the worst-case scenario for the contribution of diet to maintaining vitamin D status will be ascertained. This information should help provide evidence for dietary recommendations across a wide age range, and help with conflicting advice with respect to sun exposure for avoiding skin cancer.
Frameworks for discussion at review meeting
The discussion at the Review Meeting focussed on how the results of the Agency-funded bone health projects would alter the following two frameworks which both relate to the research objectives of the Nutrient Status and Function programme. Table 1 is a modified version of a consensus table, first compiled in 2004Reference Ashwell2, on functional markers for micronutrients related to bone health. It is proposed that plasma vitamin K concentration be regarded as a marker of vitamin K status, but that care needs to be taken in its interpretation because of its known association with TAG-rich lipoproteins. Although some workers have expressed vitamin K concentration as a vitamin K:lipid ratio, this has not seen widespread acceptance or use. At the present time the best compromise is to take plasma samples in the fasting state. There are still no markers of Ca exposure or status, but the participants at the Workshop agreed that markers of bone turnover, as well as plasma parathyroid hormone concentration, reflected Ca function. Undercarboxylated osteocalcin, with suitable adjustment for total osteocalcin, remains the best marker of vitamin K bone-related function. In the Agency-funded project, the measurement of free urinary Gla showed too much variability to be included in the list as a sensitive indicator of vitamin K function. Measurements of a potentially new vitamin K function marker, matrix Gla protein, did not reflect vitamin K status but at that time only total protein concentrations could be measured. Future measurements of the carboxylation status of matrix Gla protein should be more informative. Confirmatory evidence was available for plasma 25-OHD concentration as a marker of vitamin D status, but no Agency-funded studies had yet investigated what levels should denote sufficiency, and insufficiency. This is an important area for future research, as vitamin D insufficiency has been linked to increased risk of osteoporosis but also of a number of chronic diseases, such as CVD, hypertension, diabetes, inflammatory and autoimmune diseases, and certain cancersReference Holick49. Evidence from changes in functional markers of vitamin K (osteocalcin carboxylation) and vitamin D (parathyroid hormone) suggested that suboptimal status was common among the subjects in all studies, even though their plasma vitamin K and 25-OHD concentrations were adequate according to generally accepted criteria.
Table 1 Functional markers for micronutrients related to bone health

PTH, parathyroid hormone.
* Markers are more speculative.
Table 2 summarises the consensus reached at the Workshop on functional outcomes related to bone health. Using the ultimate endpoint of fracture, the only recognised markers are BUA and BMD, but these appear to be applicable only in large populations of older people. Other markers, including bone quality as measured by peripheral quantitative computed tomography, can only be regarded as providing contributory evidence of likelihood of fracture in this sub-group of the population. Focussing on markers of bone health, rather than markers of reduced risk of disease, BMD and BUA are again better predictors than the other markers.
Table 2 Markers of functional outcomes related to bone health
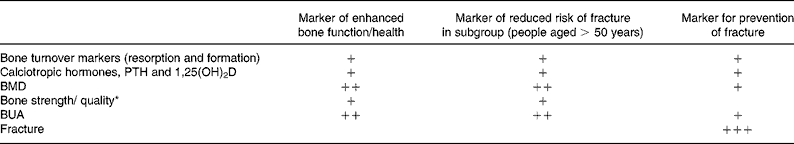
PTH, parathyroid hormone; BMD, bone mineral density; BUA, bone ultrasound attenuation.
* As measured by peripheral quantitative computed tomography.
+ Evidence based on the use of this marker would provide contributory information but, on its own, cannot be used to relate to the functional outcome.
++ Evidence based on the use of this marker is probably sufficient to relate to the functional outcome.
+++ The clinically relevant functional outcome.
Progress against global context for the effect of diet on bone health
The 2003 WHO Expert Report50 attempted to categorise the evidence for the effect of diet on bone health into three categories (decreased risk of fracture, no relationship and increased risk). It also provided consensus assessment on three levels of certainty of the evidence base (convincing, probable and possible). The roles of Ca, vitamin D and physical activity in decreasing risk of fracture were classified as being based on convincing evidence, but only in studies of older people. Having a low body weight and having a high alcohol intake were judged to be factors for increased risk of fracture based on convincing evidence. The evidence relating to fruit and vegetable consumption and decreased risk of fracture was given a possible rating. In terms of a mechanism, the report stated that several components of fruit and vegetables are possibly positively linked at levels within the normal range of consumption (e.g. alkalinity, vitamin E, vitamin C, carotenoids, vitamin K, phytoestrogens, potassium, Mg, B).
The consensus at the Agency Workshop was that, on the basis of Agency-funded projects, no more convincing evidence had accrued for the role of fruit and vegetables in bone health and that the alkalinity provided by fruit and vegetables was unlikely to be among the possible important mechanisms. The evidence for vitamin K (at levels found in the diet) would add to the existing evidence base for its possible positive role for bone health.
Suggestions for future research
The Workshop participants considered the research priorities suggested at the previous reviewReference Burns, Ashwell and Berry1 and agreed that many had been, or were being, undertaken. They concluded that it was important to explore other mechanisms by which fruit and vegetables might be protective for bone health since the acid–base balance hypothesis had not been supported by these Agency-funded projects. They agreed that the overview of other RCT, to investigate the role of Ca and vitamin D in populations which would be thought to benefit, showed that RCT were challenging in this areaReference Francis3, probably because such long-term studies are needed to show effects on BMD, let alone fracture. The participants agreed that the major research gap was the need to investigate vitamin D status to define deficiency, insufficiency, and depletion across age and ethnic groups so that a reference nutrient intake, if any, could be considered for these different population groups. Vulnerable population groups suggested included preterm babies, low income groups and adolescents. Other important issues to investigate are the relationship between low body weight and poor bone health in older people and the interaction between physical activity and bone health. However, since aspects of these topics might be outside the scope of Agency policy objectives, there is an opportunity for coordination of research with other fund providers.
Participants
Participants at the Workshop were: Dr Margaret Ashwell, Ashwell Associates; Dr Jacqueline Berry, University of Manchester; Professor Stephen Barnes, University of Alabama at Birmingham, USA; Professor Juliet Compston, University of Cambridge School of Clinical Medicine; Ms Rachel Elsom, Food Standards Agency; Professor Roger Francis, Newcastle University; Professor Tim Key, University of Oxford; Professor Kevin Cashman, University College, Cork; Professor Cyrus Cooper, University of Southampton; Professor Kay Tee Khaw, University of Cambridge; Dr Susan Lanham-New, University of Surrey; Dr Helen Macdonald, University of Aberdeen; Professor John Mathers, Newcastle University; Ms Emma Peacock, Food Standards Agency; Dr Ann Prentice, Dr Celia Prynne and Dr Alison Stephen, MRC Human Nutrition Research, Cambridge; Professor David Reid, University of Aberdeen; Dr Sian Robinson, University of Southampton, Dr Martin Shearer, St Thomas' Hospital London; Dr Elaine Stone, Ms Rachel Stratton and Dr Alison Tedstone, Food Standards Agency.




