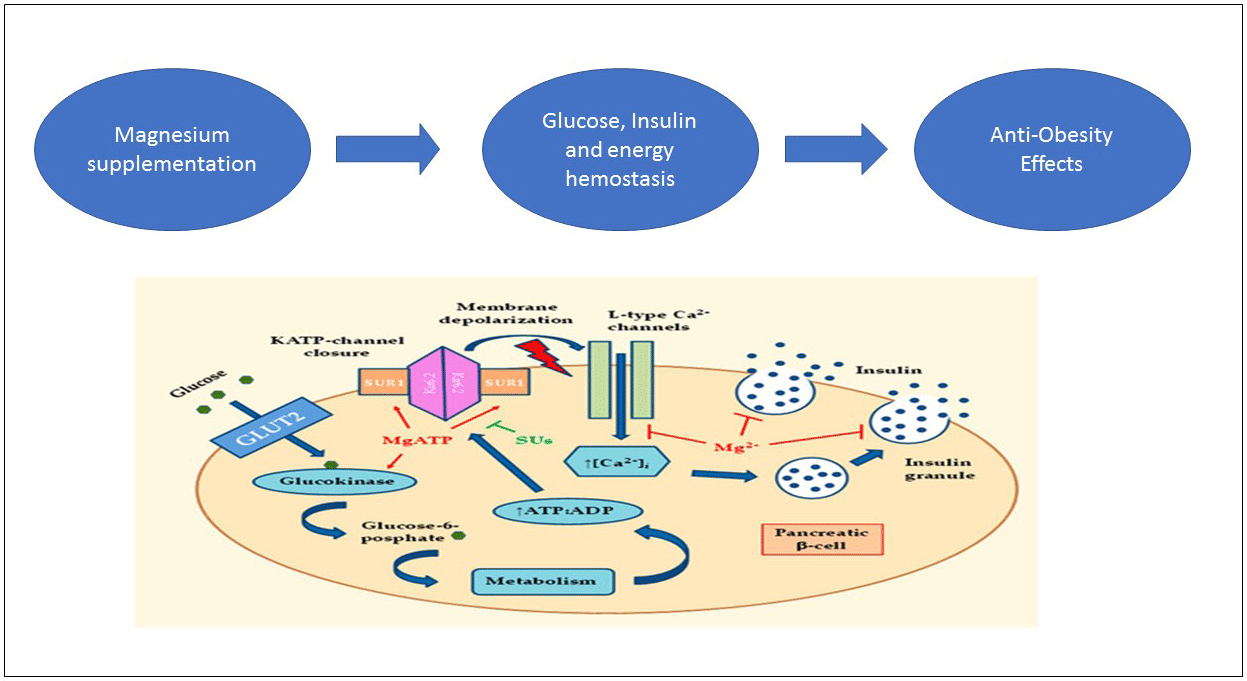
Obesity is a medical situation with abnormal or excessive adipose tissue accumulation(Reference Smith1). Obesity is characterised by imbalance between energy intake and energy expenditure(Reference Hubert, Feinleib and McNamara2). According to WHO report in 2016, the number of subjects with overweight and obesity in the world is approximately 1·9 billion and 650 million, respectively (almost 30 % of global population)(3). Over 3 million people each year die from obesity (5 % of the worldwide deaths)(4). Obesity is a major risk factor for range of chronic diseases including diabetes, CVD and cancer(Reference Barouki, Gluckman and Grandjean5). Also, it imposes plenty of economic burden into individuals, their families and nations. It is estimated that the burden costs 2·8 % of Global Gross Domestic Product (approximately 2 trillion USD)(Reference Dobbs, Sawers and Thompson6). All the aforementioned reasons emphasis on priority of obesity in public health in both developed and developing countries(Reference Çakmur7). The successful key for any weight loss is comprehensive lifestyle management including diet, physical activity and behaviour modification. Indeed, any safe and effective weight loss programme should include following components: healthy eating plans that reduce energy without ruling out specific foods or food groups, regular physical activity and/or exercise instruction and behaviour changes that are based on your cultural needs, but these are extremely challenging over a long time period(Reference Ramage, Farmer and Apps Eccles8–Reference Soeliman and Azadbakht11). Many natural materials have been surveyed for obesity treatment that develop as anti-obesity products such as drugs, natural dietary and herbal products(Reference Derosa and Maffioli12). Recently, complementary and alternative treatments such as weight loss supplements are common(Reference Kang and Park13). Mg supplement is one of them, which possess potential anti-obesity effects(Reference Jeong, Lee and Kim14). Mg is most abundant the second and fourth in intracellular and body, respectively(Reference Vormann15). Mg is an essential cofactor for numerous biological processes and is required for energy production, oxidative phosphorylation, glycolysis, protein and nucleic acid synthesis, ion transport and cell signalling(Reference Shils and Shike16). To produce energy from macronutrients, there are several metabolic pathways that are dependent on Mg. Furthermore, Mg and ATP make a complex (Mg-ATP) that provides energy for metabolic processes in mitochondria(Reference Buchachenko, Shchegoleva and Breslavskaya17). As a result of mentioned mechanisms, Mg deficiency has a role in aetiology of obesity. It is found that serum Mg has a correlation with obesity in several studies(Reference Guerrero-Romero and Rodriguez-Moran18). Thus, the serum Mg level was negatively correlated with BMI, systolic BP, diastolic BP, waist circumference (WC) and fasting insulin level(Reference Jose, Jain and Vikram19). It was hypothesised an anti-obesity effect of Mg due to capability of forming soaps with fatty acids in the intestine that can reduce the absorption of fat from the diet(Reference He, Liu and Daviglus20). Although, there are direct studies rarely about the effect of Mg supplement on obesity, but several studies have reported it as secondary outcome. Some clinical trials did not show any significant effect of Mg supplement on obesity parameters(Reference Talari, Zakizade and Soleimani21–Reference Solati, Ouspid and Hosseini42). However, Mg supplementation improved anthropometric indices in five randomised clinical trials (RCT)(Reference Toprak, Kurt and Sari43–Reference Hasan, Mshimesh and Khazaal47). Because of controversy of effects and also no existence of meta-analysis to date, the present study aimed to conduct a precise and vast systematic review and dose–response meta-analysis of all published reports on the effect of Mg supplementation on body weight (BW), BMI, WC and body fat percentage (BF%) in adults.
Methods
Protocol and registration
The reported items for systematic reviews and meta-analysis guidelines were followed in the conduct of the present study(Reference Moher, Liberati and Tetzlaff48). The Population, Intervention, Comparison, Outcome, Study design (PICOS) criteria included Population: adult, Intervention: oral Mg supplementation, Comparison: placebo, Outcomes: anthropometric indices including BW, WC, BMI and FM% and Study design: RCT were used for this systematic review and meta-analysis. Our study protocol was not registered on any website; however, it is available from the authors upon request.
Literature search and inclusion criteria
An online search was conducted using the databases including PubMed, The Cochrane Library, Scopus, Web of Science and Google Scholar up to February 2020. We used text words and medical subject headings to identify the potential interest articles. The search terms included the following combinations of keywords: (‘magnesium’ OR ‘magnesium’[tiab]) AND supplement*[Title/Abstract]) OR ‘magnesium’[Mesh]) OR ‘magnesium[tiab]) AND oral*’[Title/Abstract] AND (‘intervention studies’ OR ‘intervention’ OR ‘controlled trial’ OR ‘randomized’ OR ‘randomized’ OR ‘random’ OR ‘randomly’ OR ‘placebo’ OR ‘assignment’ OR ‘blind’). Our search is limited to studies that published randomised placebo-controlled trials (RCT). There is no restriction on language and time as per defined time frame. Because the anthropometric indices are secondary outcome, we used keywords of the intervention studies. Electronic database searches were completed along with reference list and citation hand searches.
Study selection and data extraction
First, electronic and manual search results were exported to End-Note software, version X7 (Thomson Reuters) and duplicate publications were removed. Then, eligible articles were sorted by title, abstract and related full texts of publications. Finally, all human RCT (either parallel or cross-over designs) that examine the effects of Mg supplementation on anthropometric indices were included. If studies (1) Mg was administrated in combination with other components, minerals or botanicals (unless a separate arm controlled the effect of the mixed substance); (2) were publications with duplicate data; (3) without a placebo group; (4) without sufficient data and (5) unavailable full text were excluded. The following data were extracted: author’s first name, year of publication, country of origin, study design, sex, mean age and BMI of participants, total sample size, study duration and dosage of Mg supplementation. When the data were reported at multiple measurements, only the outcomes at the end of the intervention were included in the analysis. All processes from systematic search to the data extraction were followed independently by two research experts (M. R. and A. Gh.) (kappa statistic for agreement for quality assessment; 0·91). Probable discrepancy was resolved under the supervision of the third expert’s opinion (Gh. A.).
Assessment of methodological quality
The Cochrane Risk of Bias tool was used for quality assessment as follows: sequence generation, allocation and concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. According to the Cochrane guideline handbook, the words ‘yes’, ‘no’ and ‘unclear’ corresponded to low, high and unknown risk of bias, respectively. According to the mentioned domains, the overall quality of study was considered as good (low risk for all items), fair (low risk more than three items) and poor (low risk for equivalent and less than three items)(Reference Higgins, Altman and Gøtzsche49). Also, quality assessment was also undertaken by two authors (M. R. and A. Gh.) separately.
Statistical analysis
All analyses were performed using STATA software version 12 (STATACorp.). The pooled weighted mean difference (WMD) and its 95 % CI was used to assess the effects of Mg supplementation on anthropometric indices. Mean change and its sd in anthropometric indices including weight (kg), WC (cm), BMI (kg/m2) and body fat (%) within the intervention and placebo groups was used to calculate the effect size for meta-analysis. In studies in which mean change was not directly reported in intervention and control groups, it was calculated by the minus of the post-intervention data from the baseline value. Besides, if only sd for the baseline and final values were provided, the sd for the net changes was imputed according to the method of Borenstein et al.(Reference Borenstein, Hedges and Higgins50) using a correlation coefficient of 0·5. Due to the fact that included RCT were performed in different settings, random-effects models were used to conduct all meta-analyses. The heterogeneity between studies was examined by the I-squared (I 2) index. Heterogeneity was considered statistically significant if P < 0·05 or I 2 > 50 %(Reference Higgins and Green51). We conducted subgroup analysis according to the median Mg supplement dose and the mean of duration of treatment, BMI, age and sex patients to assess the impact of this variable on outcomes. Rather, sensitivity analysis was performed to explore the extent to which inferences might depend on a particular study or group of studies. Begg’s rank correlation test and Egger’s weighted regression test were used to investigate any possible bias. A P value of < 0·05 was considered as statistically significant.
Results
Literature search
Our search was based on 2954 articles through databases searching. Two thousand nine hundred seventeen records were excluded due to irrelevance to the inclusion criteria and duplication. Among thirty-seven remaining articles, nine records were excluded. Finally, twenty-eight articles (with thirty arms treatment)(Reference Talari, Zakizade and Soleimani21–Reference Hasan, Mshimesh and Khazaal47,Reference Moslehi, Vafa and Rahimi-Foroushani52) were calibrated in the present systematic review and meta-analysis. The process of study identification is presented in Fig. 1.
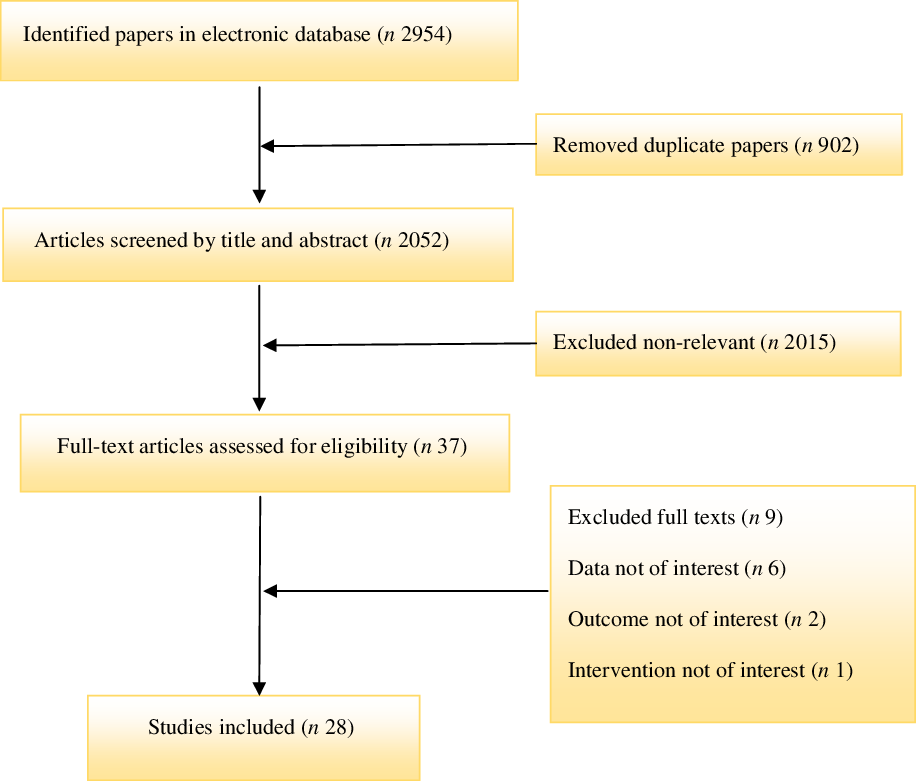
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram of the study selection process.
Study characteristics
Included studies have been published between 1994 and 2019. A total of 2013 subjects (1033 cases and 980 controls) were included in the analysis. All studies had parallel design. Out of twenty-eight included studies, four studies performed in Europe(Reference Mooren, Krüger and Völker34,Reference Zorbas, Kakurin and Afonin39,Reference Witteman, Grobbee and Derkx41,Reference Toprak, Kurt and Sari43) , ten in America(Reference Rodríguez-Morán, Simental-Mendía and Gamboa-Gómez26,Reference Rodríguez-Ramírez, Rodríguez-Morán and Reyes-Romero28,Reference Simental-Mendía, Rodríguez-Morán and Guerrero-Romero30–Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero32,Reference Rodriguez-Hernandez, Cervantes-Huerta and Rodriguez-Moran35,Reference Guerrero-Romero and Rodríguez-Morán37,Reference Rodriguez-Moran and Guerrero-Romero38,Reference Guerrero-Romero, Simental-Mendía and Hernández-Ronquillo44,Reference de Souza45) and fourteen studies in Asia(Reference Talari, Zakizade and Soleimani21–Reference Rashvand, Mobasseri and Tarighat-Esfanjani24,Reference Razzaghi, Pidar and Momen-Heravi27,Reference Rajizadeh, Mozaffari-Khosravi and Yassini-Ardakani29,Reference Abbasi, Kimiagar and Shirazi33,Reference Day, Liauw and Tozer36,Reference Itoh, Kawasaki and Nakamura40,Reference Solati, Ouspid and Hosseini42,Reference Karandish, Tamimi and Shayesteh46,Reference Hasan, Mshimesh and Khazaal47,Reference Moslehi, Vafa and Rahimi-Foroushani52) . The intervention period ranged between 4 and 48 weeks. The supplementation dose of Mg ranged from 168 to 625 mg/d. All of included studies were conducted on both sexes except six studies that were conducted in women(Reference Rodriguez-Hernandez, Cervantes-Huerta and Rodriguez-Moran35,Reference Day, Liauw and Tozer36,Reference Witteman, Grobbee and Derkx41,Reference de Souza45,Reference Hasan, Mshimesh and Khazaal47,Reference Moslehi, Vafa and Rahimi-Foroushani52) and one study on men(Reference Zorbas, Kakurin and Afonin39). Included studies were carried out in cases with hypertension(Reference Rodríguez-Ramírez, Rodríguez-Morán and Reyes-Romero28,Reference Witteman, Grobbee and Derkx41) , diabetes and prediabetes(Reference Talari, Zakizade and Soleimani21,Reference Sadeghian, Azadbakht and Khalili23–Reference Zghoul, Alam-Eldin and Mak25,Reference Razzaghi, Pidar and Momen-Heravi27,Reference Simental-Mendía, Rodríguez-Morán and Guerrero-Romero30,Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero32,Reference Guerrero-Romero and Rodríguez-Morán37,Reference Rodriguez-Moran and Guerrero-Romero38,Reference Solati, Ouspid and Hosseini42–Reference Guerrero-Romero, Simental-Mendía and Hernández-Ronquillo44) , obesity(Reference Solati, Kazemi and Majd22,Reference Abbasi, Kimiagar and Shirazi33,Reference Mooren, Krüger and Völker34,Reference Moslehi, Vafa and Rahimi-Foroushani52) , the metabolic syndrome(Reference Rodríguez-Morán, Simental-Mendía and Gamboa-Gómez26,Reference de Souza45,Reference Hasan, Mshimesh and Khazaal47), non-alcoholic fatty liver disease(Reference Karandish, Tamimi and Shayesteh46), insulin resistance(Reference Mooren, Krüger and Völker34,Reference Guerrero-Romero and Rodríguez-Morán37 ), depression(Reference Rajizadeh, Mozaffari-Khosravi and Yassini-Ardakani29) and healthy volunteers(Reference Day, Liauw and Tozer36,Reference Itoh, Kawasaki and Nakamura40) . Detailed characteristics of included trial were presented in Table 1.
Table 1. Characteristics of eligible studies
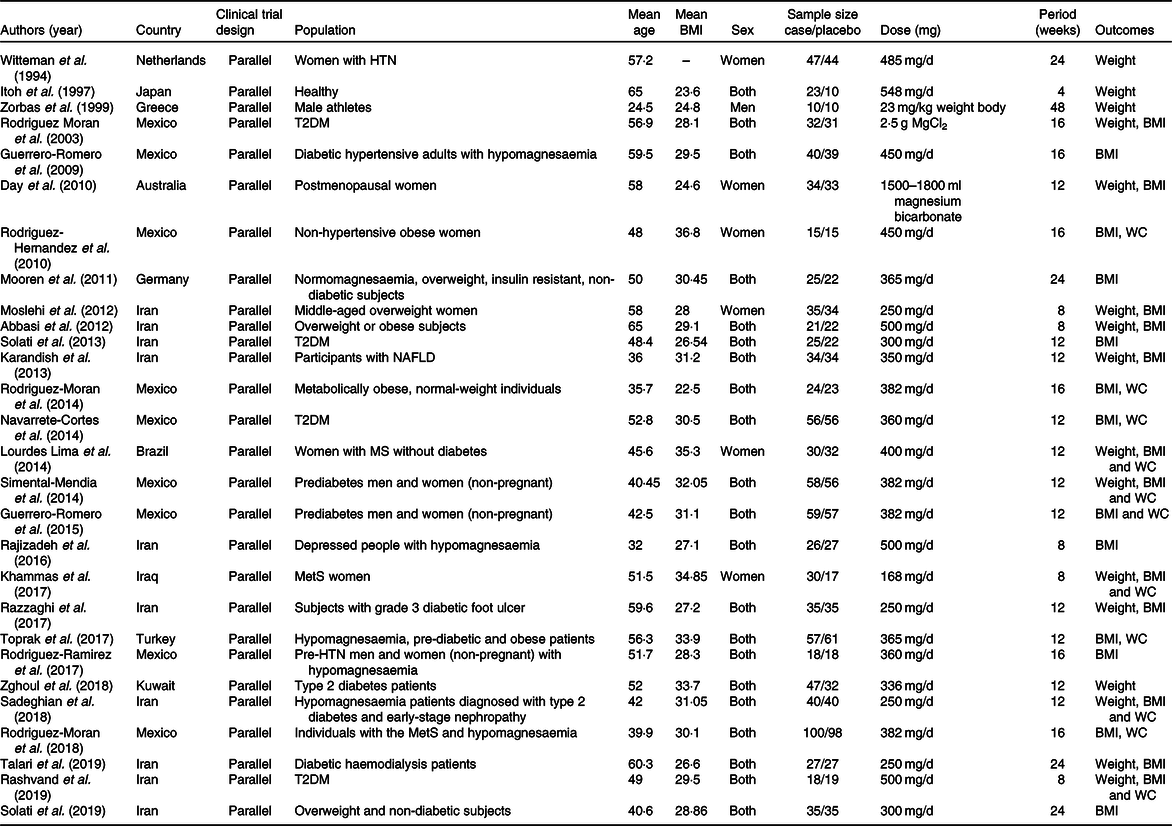
HTN, hypertension; T2DM, type 2 diabetes mellitus; WC, waist circumference; NAFLD, non-alcoholic fatty liver disease; MetS, metabolic syndrome.
Quality assessment
Present quality assessment of included studies was based on the Cochrane Collaboration Risk of Bias tool. Among twenty-eight included RCT, the quality of twelve, fifteen and one study was poor, fair and good, respectively. All studies showed low risk of bias based on selective reporting. The details of the risk of bias in individual studies according to the domains used by the Cochrane Collaboration’s tool are provided in Table 2.
Table 2. Risk of bias assessment for included randomised controlled clinical trials
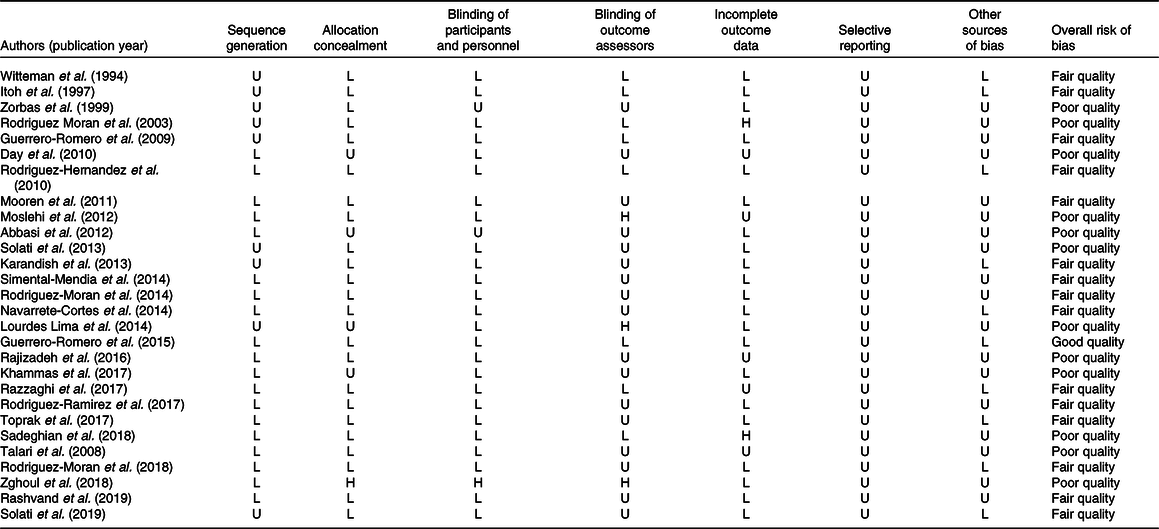
H, high risk of bias; L, low risk of bias; U, unclear risk of bias.
Publication bias
Neither Begg’s test nor Egger’s test revealed any significant evidence for publication bias on anthropometric indices (P values for Begg’s test for BW = 0·73, WC = 0·89, BMI = 0·10 and BF% = 0·34 and Egger’s test for BW = 0·66, WC = 0·34, BMI = 0·88 and BF% = 0·66). The removing of studies, one by one, did not substantially change the effect of Mg supplementation on BW, BMI, WC and BF%.
Effect of magnesium supplementation on body weight
Totally, sixteen eligible studies(Reference Talari, Zakizade and Soleimani21,Reference Sadeghian, Azadbakht and Khalili23–Reference Zghoul, Alam-Eldin and Mak25,Reference Razzaghi, Pidar and Momen-Heravi27,Reference Simental-Mendía, Rodríguez-Morán and Guerrero-Romero30,Reference Abbasi, Kimiagar and Shirazi33,Reference Day, Liauw and Tozer36,Reference Rodriguez-Moran and Guerrero-Romero38–Reference Witteman, Grobbee and Derkx41,Reference de Souza45–Reference Hasan, Mshimesh and Khazaal47,Reference Moslehi, Vafa and Rahimi-Foroushani52) with eighteen treatment arms, including a total of 1079 participants, examined the effect of Mg supplementation on BW. Combining their findings based on random-effects model, data analysis did not show any significant effect of Mg supplementation on BW (WMD 0·16 kg, 95 % CI −1·19, 1·51, P = 0·810) compared with the control group, also there were no significant heterogeneity for weight (I 2 = 0·0 %, P = 0·97), Fig. 2.
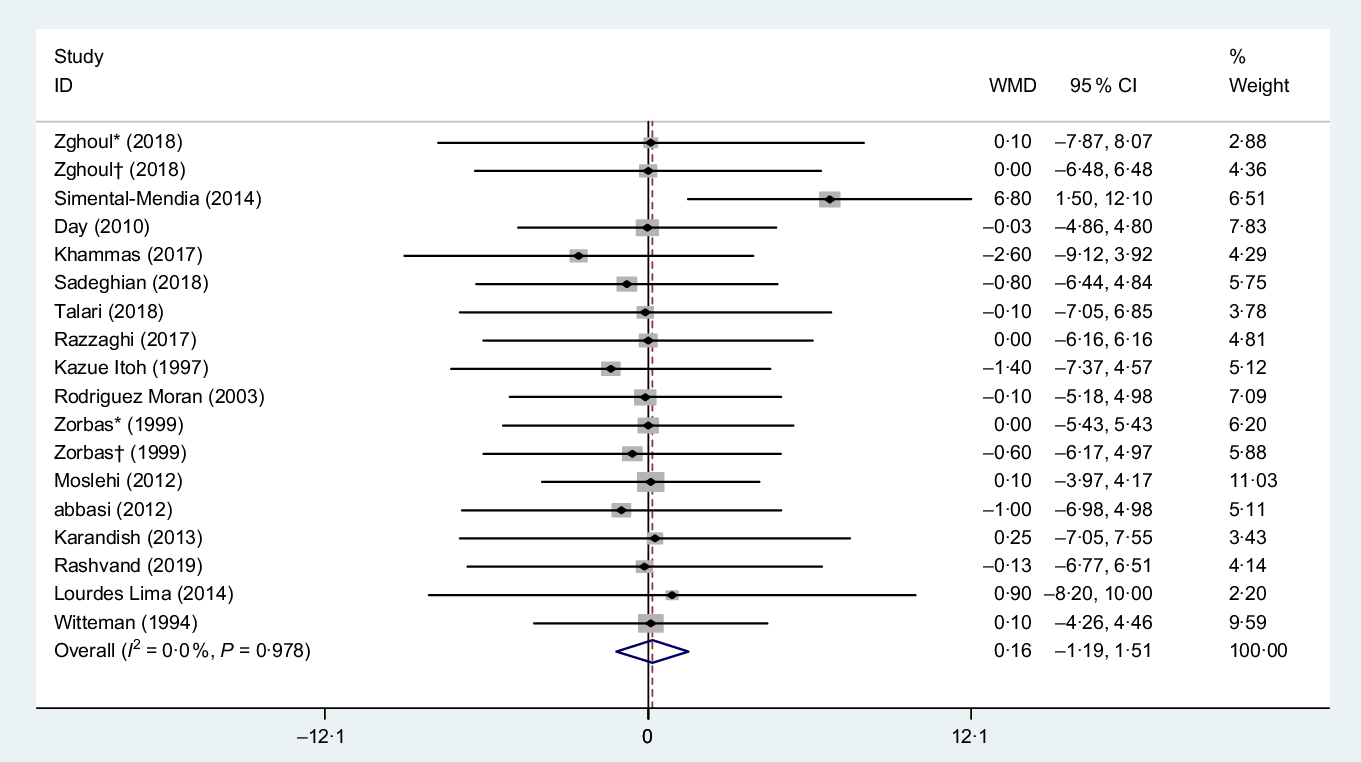
Fig. 2. Forest plot of the effect of magnesium supplementation on body weight. WMD, weighted mean difference. * Placebo group was obese; † placebo group was non-obese.
Subgroup analysis based on the median dose of intervention (>360 and ≤360 mg/d), duration of supplementation (>12 and ≤12 weeks), mean BMI of subjects (BMI > 28 and ≤28 kg/m2), mean age of participants (age of >50 and ≤50 years) and sex (men, women and both) effect remained non-significant in all of subgroup analysis as outlined in Table 3.
Table 3. Result of subgroup analysis on anthropometrics indices of included studies in the meta-analysis*
(Weighted mean differences (WMD) and 95 % confidence intervals)
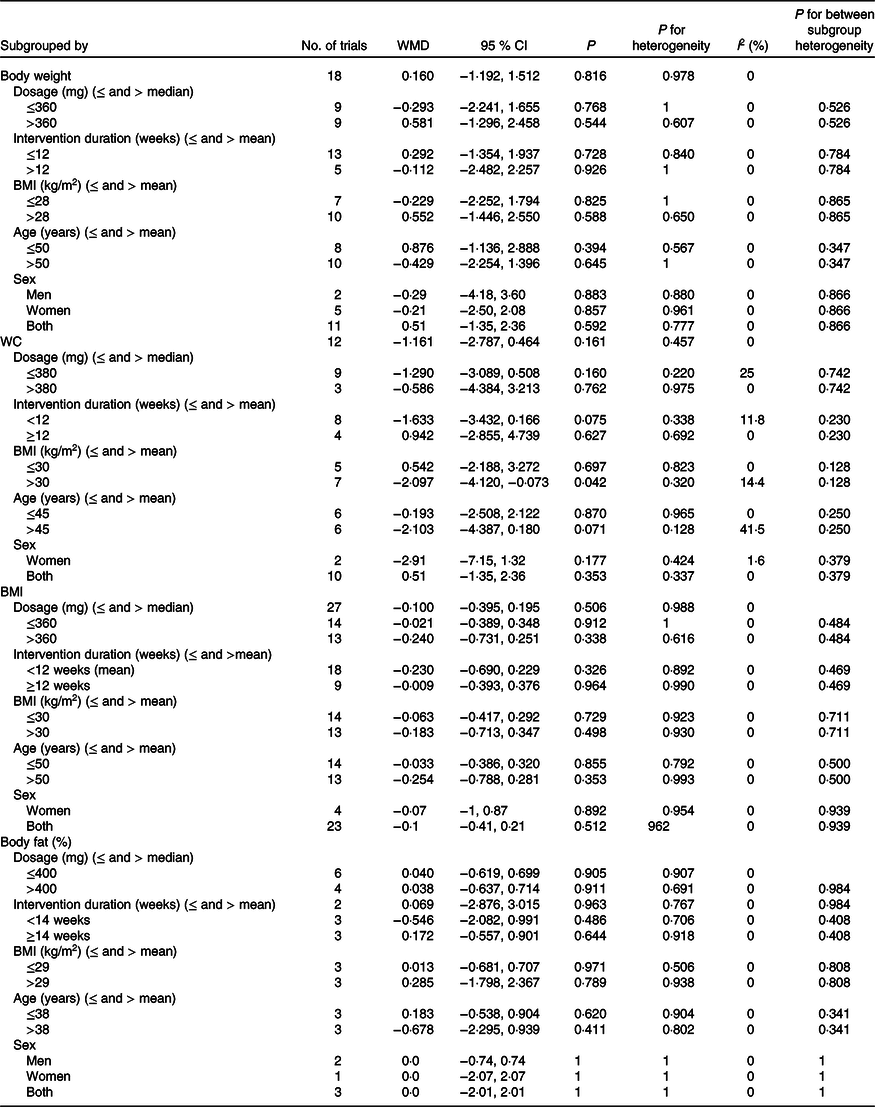
WC, waist circumference.
* Effect size was calculated by random-effects model.
Effect of magnesium supplementation on waist circumference
Overall, twelve clinical trials(Reference Sadeghian, Azadbakht and Khalili23,Reference Rashvand, Mobasseri and Tarighat-Esfanjani24,Reference Rodríguez-Morán, Simental-Mendía and Gamboa-Gómez26,Reference Rodríguez-Ramírez, Rodríguez-Morán and Reyes-Romero28,Reference Simental-Mendía, Rodríguez-Morán and Guerrero-Romero30–Reference Navarrete-Cortes, Ble-Castillo and Guerrero-Romero32,Reference Rodriguez-Hernandez, Cervantes-Huerta and Rodriguez-Moran35,Reference Toprak, Kurt and Sari43–Reference de Souza45,Reference Hasan, Mshimesh and Khazaal47) including a total of 997 subjects reported the effect of Mg consumption on WC. Pooled effect size did not show any significant effect of Mg supplementation on WC (WMD −1·161 cm, 95 % CI −2·78, 0·46, P = 0·16). Also, between-study heterogeneity was NS (I 2 = 0·0 %, P = 0·450), Fig. 3.
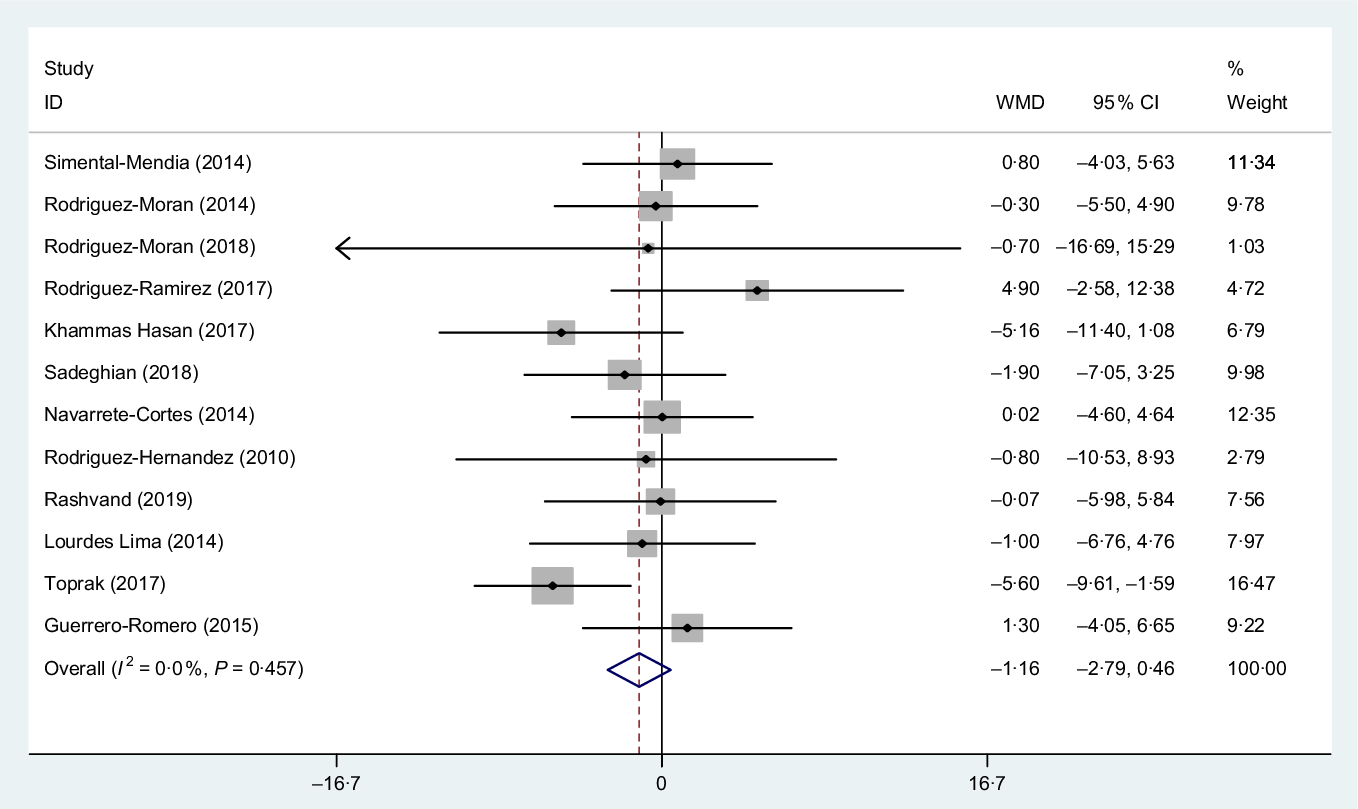
Fig. 3. Forest plot of the effect of magnesium supplementation on waist circumference. WMD, weighted mean difference.
Subgroup analysis based on the median dose of supplementation (dose > 380 and ≤380 mg/d), mean BMI ( > 30 and < 30), mean age (≤45 and >45 years), mean duration of intervention (≤12 and <12 weeks) and sex (women and both) showed only a significant effect in mean BMI > 30 kg/m2 subset (WMD −2·09 cm, 95 % CI −4·12, −0·07, P = 0·040) (Fig. 4) and also a similar reduction in WC was observed in the mean age >45 subset, but this is NS (WMD −2·10 cm, 95 % CI −4·38, 0·18, P = 0·070) as outlined in Table 3.
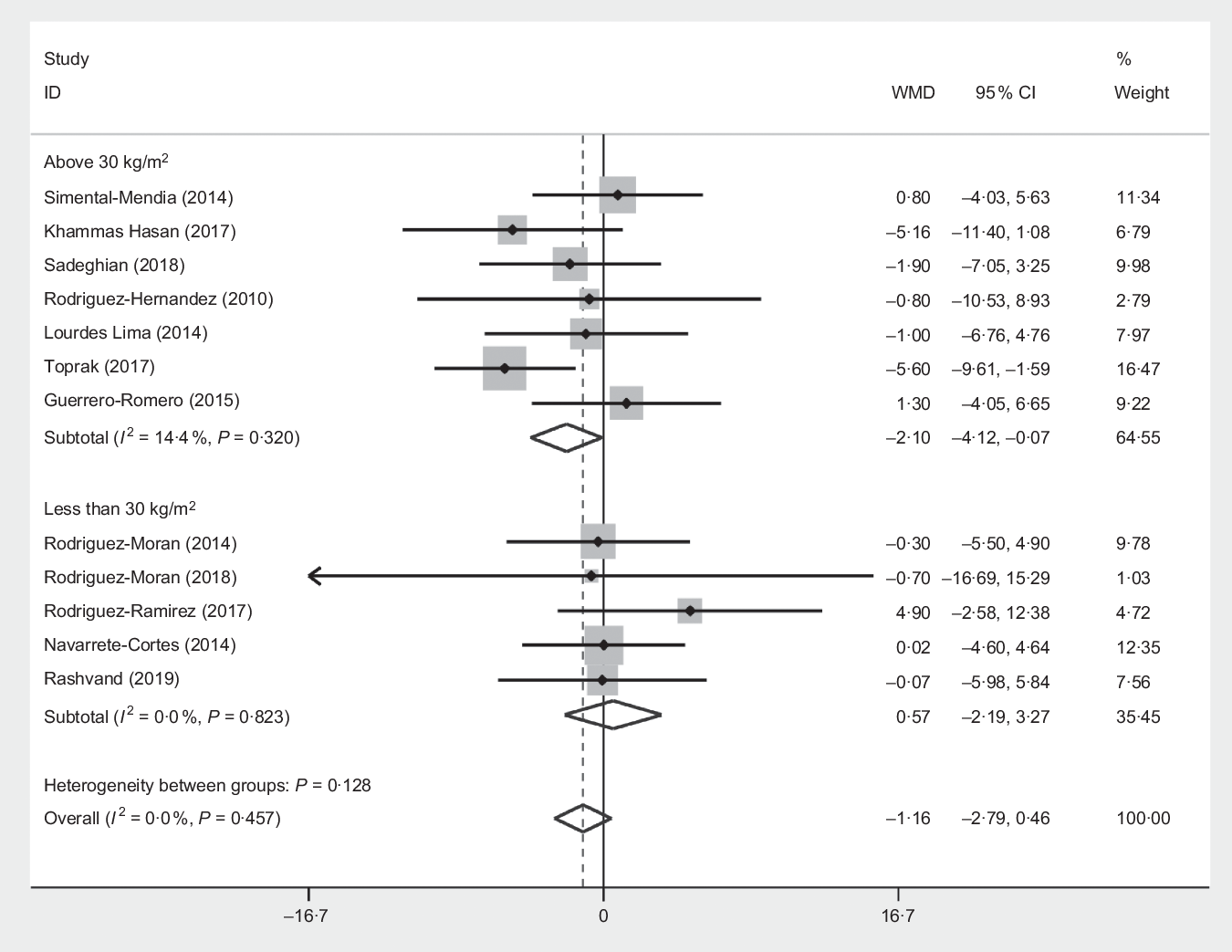
Fig. 4. Forest plot of the effect of magnesium supplementation sub grouped by BMI > 30 and ≤30 kg/m2 on waist circumference. WMD, weighted mean difference.
Effect of magnesium supplementation on BMI
The pooled estimation from the random-effects model that performed on twenty-five studies(Reference Talari, Zakizade and Soleimani21–Reference Rodriguez-Moran and Guerrero-Romero38,Reference Solati, Ouspid and Hosseini42–Reference Hasan, Mshimesh and Khazaal47,Reference Moslehi, Vafa and Rahimi-Foroushani52) (twenty-six treatment arms) including 1931 participants showed that Mg had no significant effect on BMI (WMD −0·10 kg/m2, 95 % CI −0·39, 0·19, P = 0·500). Also, between-study heterogeneity was NS (I 2 = 0·0 %, P = 0·980), Fig. 5.
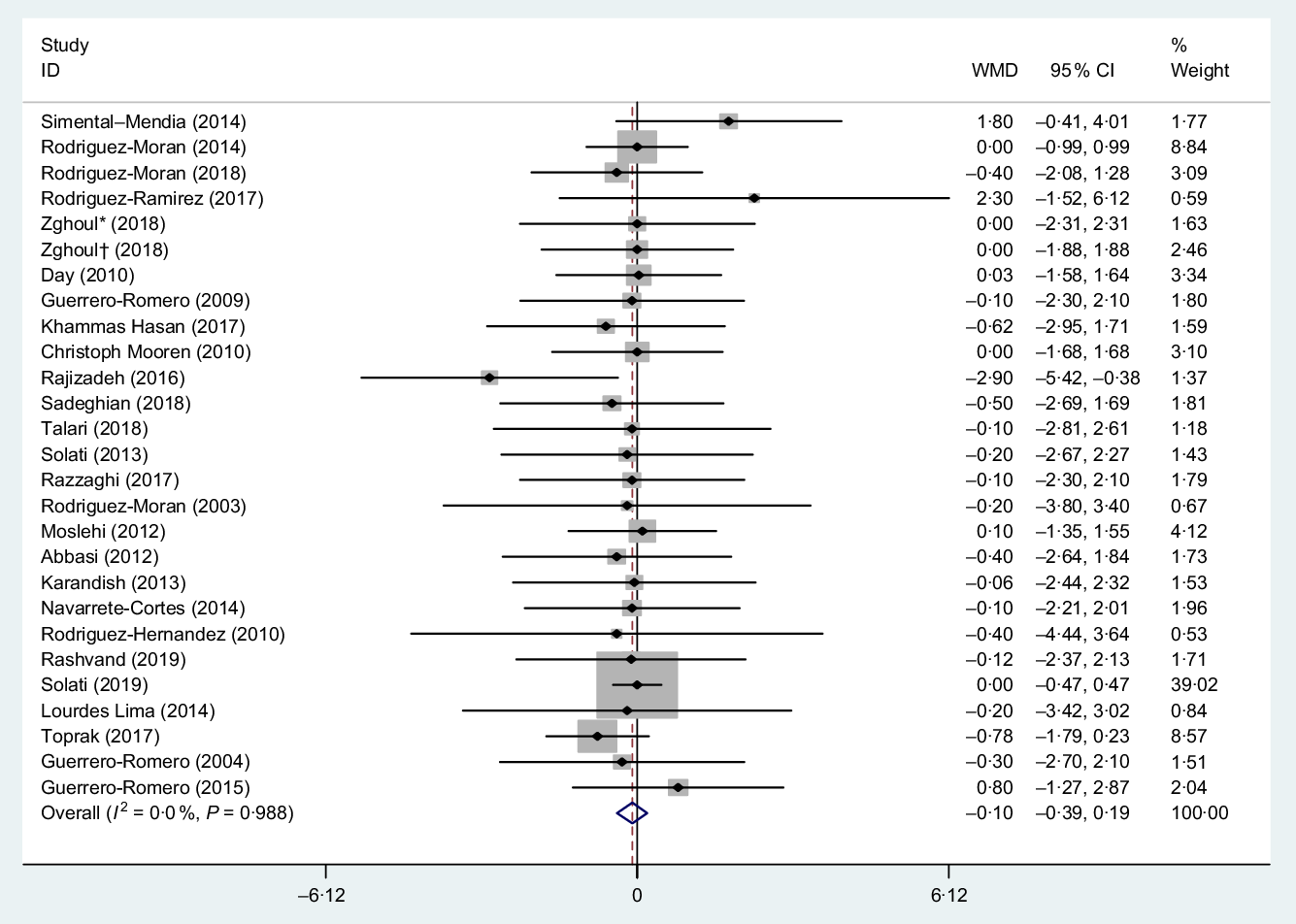
Fig. 5. Forest plot of the effect of magnesium supplementation on BMI. WMD, weighted mean difference. * Placebo group was obese; † placebo group was non-obese.
Subgroup analysis based on the median dose of supplementation (>360 and ≤360 mg/d), mean duration of intervention (>12 and ≤12 weeks), mean BMI (BMI > 30 and ≤30 kg/m2), mean age (>50 and ≤50 years) and sex (women and both) showed non-significant effect of Mg supplementation on BMI as outlined in Table 3.
Effect of magnesium supplementation on body fat percentage
The pooled estimation from five studies(Reference Rashvand, Mobasseri and Tarighat-Esfanjani24,Reference Rodriguez-Hernandez, Cervantes-Huerta and Rodriguez-Moran35,Reference Zorbas, Kakurin and Afonin39,Reference Karandish, Tamimi and Shayesteh46,Reference Moslehi, Vafa and Rahimi-Foroushani52) (six treatment arms) including 244 participants showed that Mg had no significant effect on body fat (WMD 0·40 %, 95 % CI −0·61, 0·69, P = 0·90). Also, between-study heterogeneity was NS (I 2 = 0·0 %, P = 0·900), Fig. 6. Subgroup analysis based on the median dose of supplementation (>400 and ≤400 mg/d), mean duration of intervention (>14 and ≤14 weeks), mean BMI (BMI > 29 and ≤ 29 kg/m2), mean age (>38 and ≤38 years) and sex (men, women and both) showed a non-significant effect in short duration (≤14 weeks) subset (WMD −0·54 %, 95 % CI −2·08, 0·99, P = 0·480) as outlined in Table 3.
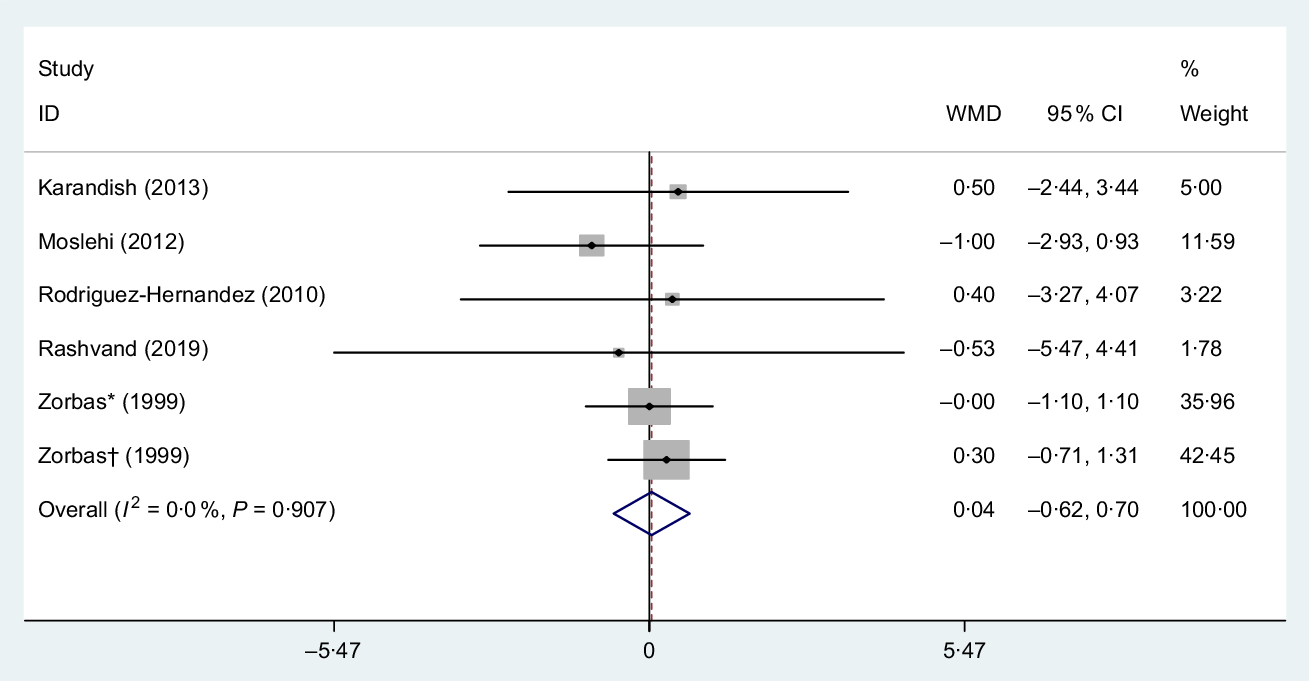
Fig. 6. Forest plot of the effect of magnesium supplementation on body fat percentage. WMD, weighted mean difference. * Placebo group was obese; † placebo group was non-obese.
Dose–response meta-analysis
Dose–response analysis not revealed a significant effect of Mg supplementation dosage on anthropometric indices including BW, BMI and WC (r 0·028, P-non-linearity = 0·543, r 0·2·88, P-non-linearity = 0·129, r 82·04, P-non-linearity = 0·246, respectively) and based on duration of intervention on BW, BMI and WC (r −0·20, P-non-linearity= 0·218, r 0·015, P-non-linearity = 0·776, r −1017·7, P-non-linearity = 0·552, respectively) (Fig. 7).
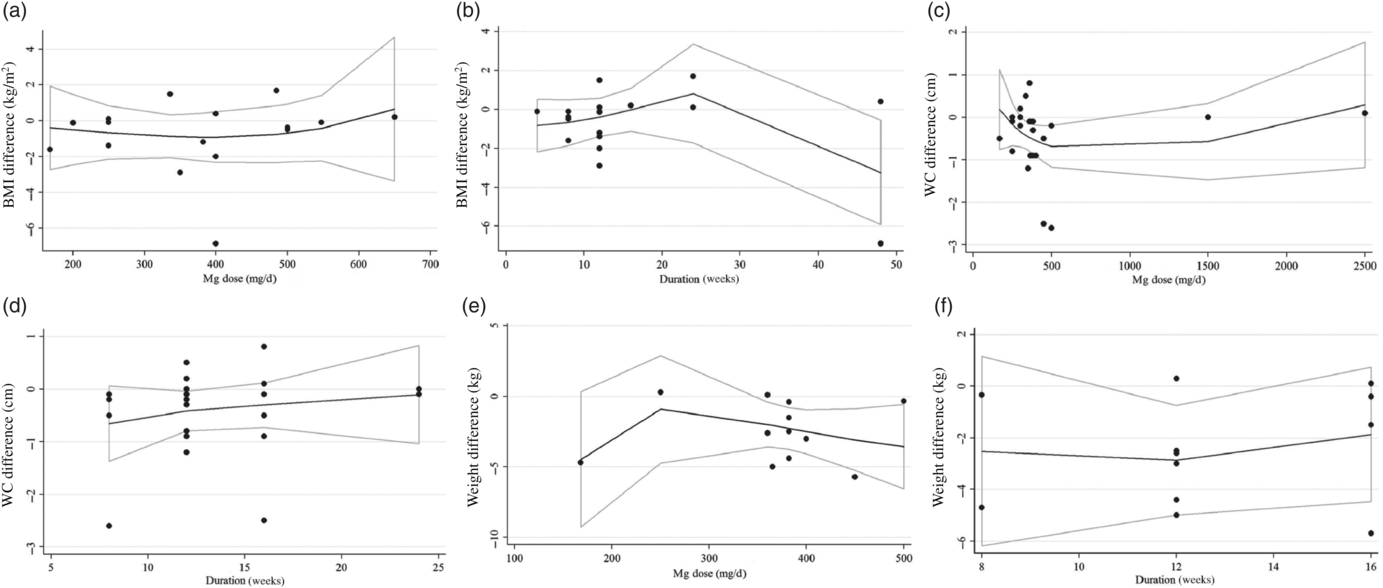
Fig. 7. Non-linear dose–response associations between magnesium supplementation and absolute mean differences. Dose–response relationships between magnesium supplementation and absolute mean differences in BMI (kg/m2 – twenty-five trials) (a and b), waist circumference (WC) (cm – twelve trials) (c and d) and weight (kg – sixteen trials) (e and f) based on dose of magnesium (mg/d) are depicted.
Discussion
The results of our meta-analysis of twenty-eight RCT studying the impact of Mg supplementation on anthropometric measurements in adults showed a reduction in WC measurement in obese (BMI > 30 kg/m2). However, there was no significant association between Mg supplement and others anthropometric parameters. Our meta-analysis verified the effectiveness of anti-obesity effect of Mg supplementation just in subjects with obesity of the population. The outcome that Mg supplement may have a decreasing effect on WC in certain subgroups (subjects with obesity) is supported by Mg capability of forming soaps with fatty acids in the intestine (and so reducing the digestible energy content of the diet)(Reference Drenick53). On the other hand, Mg is a cofactor of enzyme cholesterol acyltransferase and lipoprotein lipase, which take part in lipoproteins metabolism(Reference Sales, dos Santos and Cintra54). Numerous experimental studies have confirmed that Mg deficiency cause an increase in the TAG serum levels and abdominal obesity due to decrease in chylomicron clearance and activity of lipoprotein lipase(Reference Rayssiguier55). With regard to all possible mechanisms of Mg anti-obesity effect, there is no an exact mechanism for it yet. Also, several human studies show that hypomagnesaemia is significantly associated with high risk of obesity especially abdominal obesity(Reference Johansson, Zethelius and Öhrvall56–Reference Guerrero-Romero, Flores-García and Saldaña-Guerrero59). To the best of our knowledge, our study is the first meta-analysis on this topic, and the findings show that Mg supplementation may significantly reduce WC only in subjects with obesity but has no effect in other subjects and measurements related to obesity. Since the baseline BMI mean was more than 30 in most studies, one of the reasons for justification our results may be associated with BMI status. Another reason for explaining this finding may be related to healthy condition of participants. It is worth mentioning that there is no significant change after adjusting for duration of intervention, dose and age in all anthropometric indices. There are several limitations that should be mentioned when interpreting the findings in the present meta-analysis. First, there are a few studies on the topic ‘the effect of magnesium supplementation on anthropometric indices in healthy subjects’ and also the number of included studies in this meta-analysis was not adequate to reach a firm conclusion. Second, the most of the included studies were organised in the two countries, Mexico and Iran, which though strengthen internal validity of the findings but decrease their overall generalisability. Third, the magnitude of changes in anthropometric indices may not be equal and reliable during the intervention. Finally, the data analysis in most of studies was not adjusted for confounding variables such as lifestyle behaviours including diet, physical activity and tobacco use. Therefore, it is obvious that conducting well-designed randomised controlled trials with different racial populations are essential in future. Despite the above limitations, this is the first study to investigate systematically and meta-analytically the effect of Mg supplementation on anthropometric indices. So, our study can make a good perspective to the researcher in the future. Several studies have demonstrated that hypomagnesaemia was associated with the pathogenesis of obesity(Reference Zaakouk, Hassan and Tolba57,Reference Demerdash60,Reference Kokot, Malczyk and Wilemska-Kucharzewska61) . On the other hand, the anti-obesity effects of Mg supplements are not supported by firm evidence(Reference López-López, Rojas-Sobarzo and Arredondo-Olguín62). It is valuable to note that, based on Recommended Dietary Allowances, Mg deficiency is a common global complication. The Institute of Medicine (USA) Food and Nutrition Board set estimated average requirement of Mg at level of 255–265 mg/d for females and 330–350 mg/d for males(Reference Nielsen63). Mg is a fundamental divalent metal ion and a critical cofactor for numerous enzymes involved in the metabolism of fats, proteins and carbohydrates and also helps insulin to act better(Reference Nielsen63,Reference Rosolova, Mayer and Reaven64) . As well as, it is essential for the activity of lecithin cholesterol acyltransferase and lipoprotein lipase enzyme, which has an important role in increasing level of serum HDL and decreasing TAG levels, respectively. Moreover, Mg is a regulatory factor in cholesterol biosynthesis(Reference Inoue65). Mg-ATP complex is also a main factor in glucose and insulin metabolism, mainly through its impact on kinase tyrosine activity, by transferring phosphate from ATP to protein. In addition, Mg may directly affect GLUT-4 and help to uptake glucose into cells(Reference Volpe66). Eventually, Mg deficiency may promote pathogenesis of obesity by altering the aforementioned functions. In this regard, studies have found that low intake of Mg and hypomagnesaemia is strongly associated with obesity and insulin resistance(Reference Bertinato, Xiao and Ratnayake67–Reference Huerta, Roemmich and Kington70). Present meta-analysis showed that Mg supplementation caused reduction in WC in subjects with obesity. A high prevalence of Mg deficiency has been previously indicated in this population(Reference ul Hassan, Ahmed and Nasrullah58,Reference Kokot, Malczyk and Wilemska-Kucharzewska61) . Hirschler et al. (Reference Hirschler, Esteban and Gonzalez71) have showed that hypomagnesaemia was associated with central obesity, defined as WC more 90 cm. Also, numerous studies indicated that patients with central obesity and hypomagnesaemia are more susceptible to show abnormal parameters of fasting blood glucose and lipid profile(Reference Zaakouk, Hassan and Tolba57,Reference Hadjistavri, Sarafidis and Georgianos72) . The low serum level of Mg in patients with diabetes was principally caused by reduced insulin sensitivity, which leads to enhancement of Mg excretion in urine(Reference Wei, Zeng and Li73). Impacts of insulin resistance could illustrate a strong association between central obesity and low concentration of Mg(Reference Watson, Preedy and Zibadi74,Reference Mooren, Krüger and Völker75) . We observed that Mg supplementation had no significant impact on BW, BMI and BF% in all subgroups. However, the Coronary Artery Risk Development study in 5115 American young adults, aged 18–30 years, after 30-year follow-up found that Mg intake was negatively associated with incidence of obesity. According to this follow-up, intakes of foods rich in Mg, including whole grains, nuts and seeds, legumes, and dark-green vegetables, can decrease obesity risk(Reference Lu, Chen and Yang76). In the present study, we found that Mg supplementation intake has a negative association with WC, which supports this hypothesis. It is important to note that most of included studies in this meta-analysis have reported anthropometrics indices as secondary outcomes. Hence, difference in anthropometrics measurement between intervention and placebo groups may be due to estimations bias. Our dose–response analysis showed that neither dosage nor duration of Mg supplementation led to significant changes in BMI, BW and WC. Further, this hypothesis ‘the high dose of supplementation Mg and/or long duration of intervention can have an effect to decrease anthropometric measurements’ seems to be not effective. The weakness of significant association between Mg supplementation and anthropometric indices may be related to several factors. First, the effect of Mg supplementation on anthropometric indices may depend on Mg status at the beginning of the study and variation of serum level of Mg at baseline. Second, diets with high content of Mg such as whole grains, nuts, fruits and vegetables may have more strong anti-obesity effects rather than the single nutrient intake as supplement. In addition to Mg, many other components of foods including fibres, vitamin E, several B-vitamins and lignans may contribute to the beneficial effects on obesity(Reference Liu, Willett and Manson77). Finally, the small number of trials led to decline our ability to diminish subgroup and publication bias analysis for BW, WC, BMI and BF%. Our study has several advantages. First of all, our study is the first meta-analysis of RCT to review systematically the effects of Mg supplementation on anthropometric indices in adults. Second, our results were firm and strong because all included studies were RCT. Finally, analysis was based on subgrouping conducted to assess the effects of various subgroups and confounder variables.
In conclusion, the present meta-analysis on clinical trials revealed that supplementation with Mg significantly decreased WC in subjects with obesity (BMI > 30 kg/m2), and also it had no effect on BMI, BW and BF% in all subgroups. Although Mg supplementation generally had no statistically significant effects on anthropometric indices, but it may be effective on subjects with obesity. High-quality, long-time, large population studies and further investigation and assessment of the efficacy and effective dose of Mg supplementation on anthropometric indices in future research are required.
Acknowledgements
The authors are thankful to the student research committee of Isfahan University of Medical Sciences.
The present study with code 198210 was supported by the Nutrition Research Committee of School of Nutrition and Food Science Isfahan University of Medical Sciences.
M. R., A. G. H., S. H. and G. H. A. designed the research; M. R. and A. G. H. conducted the research; M. R. analysed the data; M. R. and A. G. H. wrote the paper. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.
The present study was approved by the Ethical Committee of Isfahan University of Medical Sciences.