- BAT
brown adipose tissue
- CLS
crown-like structures
- WAT
white adipose tissue
Organs of adult animals are dissectable structures composed mainly of differentiated cells responsible for several different coordinated functions usually converging toward a single finalistic role. The main function of an organ is usually performed by a leader cell that is termed the parenchymal element of the organ. From a quantitative point of view the parenchymal cell usually represents the majority of the organ's elements, often comprising ≥60–70% of the total number of cells present in the organ. Thus, for example, 60–80% of the parenchymal cells in the liver are hepatocytes (responsible for most of the physiological activity of liver)(Reference Ramadori and Saile1).
Parenchymal cells are therefore differentiated cells with a phenotype corresponding to a specific series of functions that together are compatible with the main function of their organ.
Parenchymal cells usually have a specific half-life that is strictly dependent on the intrinsic characteristics of the cells forming the organ and on the intrinsic characteristics of the organ itself. After a variable period of time, which is characteristic for any organ, most of the parenchymal cells are substituted by new elements derived from the differentiation of stem cells. This process requires a stem cell compartment in each organ as well as provision for continual ‘cleaning’ of cell debris derived from the programmed death of parenchymal cells. This ‘cleaning’ function is performed by the macrophages, which are bone marrow cells attracted into organs by cell debris and specific chemo-attractants. Foreign bodies are also removed by macrophages that often form multinucleated giant cells for special requirements such as the removal of large structures (i.e. larger than a single macrophage). This inflammatory picture is also known in histopathology as the ‘foreign body reaction’(Reference Luttikhuizen, Harmsen and Van Luyn2). The non-parenchymal cells form organized structures or isolated cells supporting the function of the organ. Blood vessels and nerves are examples of organized structures, while fibroblasts and macrophages are examples of isolated elements.
For several years the author's group has been developing the concept that adipose tissues (white (WAT) and brown (BAT)) are organized into a true organ with all the anatomical and physiological characteristics described earlier for other well-recognized organs of mammals.
The concept of an adipose organ
It has been shown that almost all white and brown adipocytes are contained within dissectable structures with a specific gross anatomy that is maintained at different ages and in different strains in small rodents(Reference Cinti3–Reference Cinti6). In mice and rats it comprises two main subcutaneous depots, anterior and posterior (Fig. 1). The former depot is the more complex; it has a central body with a pyramidal shape with the superficial base and deep apex located in the interscapular area. From the central body lateral symmetrical projections mainly located beneath the scapulae and among the skeletal muscles reach the cervical and axillary regions and the large vessels at the base of the neck. The posterior subcutaneous depot comprises a single strand surrounding the posterior part of the trunk of the animal at the base of the posterior limbs. In rats and mice subcutaneous depots account for about 60–70% of the adipose organ.

Fig. 1. Gross anatomy of the adipose organ of the adult female 129Sv mouse. The subcutaneous and visceral depots were dissected and positioned on a template of the mouse to show their location in the organism: (a) mouse was maintained in warm conditions (28°C for 10 d); (b) mouse maintained in cold conditions (6°C for 10 d). There is a visually-evident transformation of the colour of the organ as the result of an increase in brown adipose tissue and decrease in white adipose tissue contained in the organ. The organ is made up of two subcutaneous depots: A+B, anterior (deep cervical (A), superficial cervical, interscapular, subscapular, axillo-thoracic); G, posterior (dorso-lumbar, inguinal, gluteal). There are several visceral depots: B, mediastinal; C, mesenteric; D, retroperitoneal; E, retroperitoneal; F, abdomino-pelvic (perirenal, periovarian, parametrial, perivesical).
The remaining parts of the organ form several visceral depots in the thorax, abdomen and limbs. The thoracic visceral depot is located around the aorta and its main collaterals. In the abdomen several depots are surrounded by peritoneum (mesenteric, omental, perigonadal, parametrial, perirenal and perivescical) or located behind the peritoneum (retroperitoneum). In the anterior limbs (in addition to the axillary part of the anterior subcutaneous depot described earlier) a small depot is visible at the ventral part of the elbows and in the posterior limbs there are distinct depots at the base of the thigh and in the popliteal fossa at the posterior part of the knee. In the cervical region two symmetrical depots are located among the muscles of the posterior part of the neck (deep cervical depots). Adipocytes are also present as normal constituents of bone marrow, parotid glands, parathyroid glands (human subjects), pancreas and synovial membranes.
The major differences in human subjects are the extensions of the subcutaneous depots that are present in all parts of the body. In females they are more developed in the mammary glands and in the gluteo-femoral areas.
Each depot is provided with vessels and nerves. The major murine subcutaneous depots are provided with specific vessels and nerves, all other depots receive collaterals of vessels and nerves dedicated to the organs of the anatomical area.
Adipose tissues
A fundamental factor supporting the concept of the adipose organ is that it is composed of two different tissues (WAT and BAT) that cooperate for the best use of highly-energetic molecules (fatty acids) in order to accommodate the variable energetic needs of the organism.
WAT is composed of white adipocytes, large spherical cells (60–80 μm in diameter) characterized by a single lipid droplet that accounts for about 90% of the cell volume (Fig. 2). The nucleus is compressed and a variable number of elongated thin mitochondria with short and randomly-oriented cristae and numerous caveolae are visible in the thin rim of cytoplasm (Fig. 3). The number of mitochondria seems to be correlated with the size of the cell; smaller cells have more numerous mitochondria. Other organelles are poorly developed (Golgi complex, rough and smooth endoplasmic reticulum, lysosomes). Glycogen particles are often visible in small (probably developing) cells. The main function of this cell is to store and release highly-energetic molecules (fatty acids) in order to allow animals to have intervals between meals. Furthermore, these cells are able to inform the brain about the nutritional condition of the organism through the secretion of the hormone leptin(Reference Zhang, Proenca and Maffei7). The absence of leptin induces a strong stimulus for a specific behaviour of food search and intake(Reference Friedman8). Other endocrine functions include secretion of adiponectin, which favours insulin sensitivity of organs. For both hormones secretion is related to the size of adipocytes, but leptin secretion is positively correlated with size while adiponectin is negatively correlated, at least in obesity(Reference Skurk, Alberti-Huber and Herder9, Reference Shetty, Kusminski and Scherer10). Several other adipokines are involved in numerous metabolic pathways (for review, see Trayhurn & Beattie(Reference Trayhurn and Beattie11)).
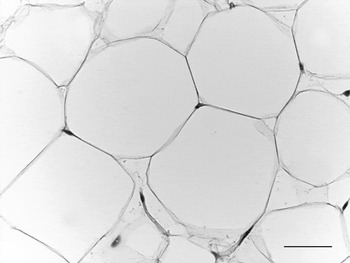
Fig. 2. Adult mouse white adipose tissue (subcutaneous depot). Light microscopy; stain, haematoxylin and eosin; —, 50 μm.
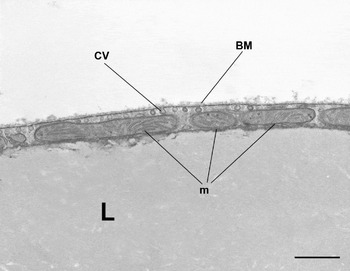
Fig. 3. Mouse subcutaneous white adipose tissue showing part of a white adipocyte. Mitochondria (m) are small and elongated with randomly-oriented cristae. CV, caveolae; BM, basal membrane; L, lipid droplet. Transmission electron microscopy; —, 1·4 μm.
BAT is composed of brown adipocytes. They are polygonal cells (30–50 μm in diameter) with a central roundish nucleus and an abundant cytoplasm containing numerous characteristic mitochondria and several small lipid droplets (Fig. 4). Mitochondria are spherical, large and packed with laminar cristae (Fig. 5). Other organelles are poorly developed. Brown adipocytes produce heat. Fatty acids are used here for thermogenesis. Their β-oxidation is not coupled to ATP formation because of the presence of an uncoupling protein (uncoupling protein1) in the inner membrane of mitochondria(Reference Ricquier12, Reference Cannon and Nedergaard13). Importantly, this protein is synthesized only by brown adipocytes and therefore is considered to be the unique marker of this cell type(Reference Frontini, Rousset and Cassard-Doulcier14).
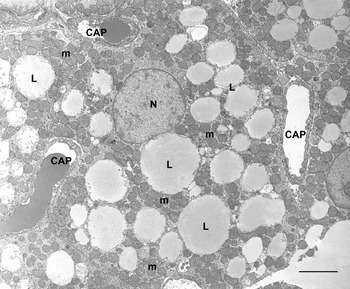
Fig. 4. Rat interscapular brown adipose tissue. Brown adipocytes show many classic ‘brown’ mitochondria (m) packed with transverse cristae. Many small lipid droplets (some indicated; L) are present. CAP, capillary; N, nucleus. Transmission electron microscopy; —, 2·5 μm.

Fig. 5. Interscapular brown adipose tissue showing numerous brown adipocyte mitochondria packed with transverse cristae. L, lipid droplet. Transmission electron microscopy; —, 1 μm.
Both types of adipocyte are surrounded by a distinct basal membrane that mainly consists of type IV collagen. Lipid droplets of both cell types are composed of TAG. The surface of lipid droplets contains the protein perilipin and no plasma membrane is visible by electron microscopy at this level. Both cell types have β3-adrenoceptors.
The two cell types are quite different in their morphology and physiology, but they share important characteristics such as the ability to store and release considerable amounts of lipids and the presence of specific adrenoceptors.
BAT and WAT are present together in different depots of the adipose organ(Reference Cinti3, Reference Cinti5, Reference Cinti6). It is visually evident that the interscapular, axillary and some of the cervical projections of the anterior subcutaneous depot are formed of BAT (visually brown) and the rest are WAT (visually white). The deep cervical depot and the mediastinic and perirenal depots are also brown. Thus, it is visually evident that the depots of the adipose organ are mixed, i.e. composed in part of WAT and in part of BAT. In adult female Sv129 mice it is calculated that approximately 60% of all adipocytes of the adipose organ are in the form of multilocular cells (brown adipocytes) and that all studied depots (subcutaneous anterior, subcutaneous posterior, mesenteric, retroperitoneal, perirenal, periovaric, parametrial and perivescical) are mixed, with the exception of the mediastinic depot, which is only brown(Reference Murano, Barbatelli and Giordano15, Reference Murano, Zingaretti and Cinti16).
Which cell is therefore the parenchymal leader cell of the adipose organ? Why are two different cells with such different physiologies located in the same organ? Is there any holistic view that explains a unitary role?
Apart from Sv129 mice, most adult mammals have a prevalence of WAT in the adipose organ, but WAT:BAT varies with strain, gender, age and nutritional and environmental conditions.
In the human adult areas containing metabolically-active BAT have been identified recently by positron emission tomography and it has been shown that they represent authentic uncoupling protein 1-expressing BAT(Reference Zingaretti, Crosta and Vitali17). The capillary network is more dense in BAT and adrenergic fibres can be found both in WAT and in BAT, but their density is higher in BAT(Reference De Matteis, Ricquier and Cinti18–Reference Giordano, Song and Bowers24).
Plasticity of the adipose organ
The plasticity of the adipose organ offers an explanation for WAT and BAT cooperation in partitioning the energy reserve between thermogenesis and other metabolic functions. The adipose organ has been shown to be plastic and its WAT:BAT composition variable. After 10 d of cold (6°C) exposure 80% of adipocytes in the adipose organ of adult mice (Sv129) are brown adipocytes(Reference Murano, Zingaretti and Cinti16). Notably, the total number of adipocytes does not differ from that of controls (maintained near thermoneutrality), in line with previous work showing the absence of any sign of DNA replication in WAT of cold-exposed animals(Reference Miller and Faust25, Reference Cousin, Bascands-Viguerie and Kassis26). The experiments with adult mice have shown that the reduction in the total number of white adipocytes corresponds with the increase in brown adipocytes(Reference Murano, Zingaretti and Cinti16). As there is no sign of white adipocyte degeneration or apoptosis it may therefore be concluded that most of the newly-formed brown adipocytes are derived from a direct transformation of white adipocytes into brown adipocytes, in line with previous experimental observations in vivo using β3-agonist (CL316243) administration(Reference Himms-Hagen, Melnyk and Zingaretti27) that have subsequently been confirmed by a different group(Reference Granneman, Li and Zhu28).
These data suggest that the adipose organ has two parenchymal leader cells and the prevalent physiology reflects the need of the organism subsequent to the environmental conditions, thus implying a new physiological phenomenon, reversible transdifferentiation.
In human subjects BAT increases in cold-exposed subjects as well as in those suffering for pheocromocitoma (adrenaline- and noradrenaline-producing tumour) and other pathologies(Reference Huttunen, Hirvonen and Kinnula29, Reference Garruti and Ricquier30).
The reversible transdifferentiation phenomenon
Adult cells have a specific phenotype that defines the physiology of any cell type. The phenotype can be changed by pathological stimuli(Reference Tosh and Slack31). With reversible transdifferentiation a physiological phenomenon is intended in which a cell reversibly changes its phenotype under physiological stimuli without passing through a stage of de-differentiation. This concept implies that adult cells can spontaneously reprogramme their genes to sustain different physiological characteristics required by physiological variations in the environment in which they live. This phenomenon could have relevant implications because WAT–BAT transdifferentiation could be useful in the control of obesity and type 2 diabetes in human subjects. In this context it is well known that mice lacking BAT or without β-adrenoceptors (necessary for BAT stimulation) became massively obese and diabetic(Reference Lowell, S-Susulic and Hamann32, Reference Bachman, Dhillon and Zhang33).
Furthermore, it has been shown that:
ectopic uncoupling protein 1 expression in WAT induces obesity resistance in mice(Reference Kopecky, Clarke and Enerback34);
mice lacking insulin receptor only in BAT become diabetic(Reference Guerra, Navarro and Valverde35);
murine strains prone to develop obesity and diabetes have less BAT than obesity- and diabetes-resistant mice(Reference Guerra, Navarro and Valverde35, Reference Almind, Manieri and Sivitz37);
obese rats treated with β3-agonists control obesity and diabetes and develop BAT(Reference Champigny, Ricquier and Blondel38–Reference Ghorbani and Himms-Hagen41);
many different models of transgenic mice with a clear brown phenotype of adipose organ are lean and obesity resistant(Reference Cummings, Brandon and Planas42–Reference Cederberg, Gronning and Ahren44);
human subjects with more brown phenotype of abdominal subcutaneous fat have less insulin resistance then those showing a reduced brown phenotype(Reference Yang, Enerback and Smith45);
brown fat genes are reduced in visceral depots of obese individuals(Reference Oberkofler, Dallinger and Liu46).
The term transdifferentiation has been used to define both a direct transformation of one cell into another with different morphology and function and to define an unusual differentiation of a stem cell that usually differentiates into a different lineage(Reference Tosh and Slack31). It is considered that this last phenomenon is not a true differentiation because it is a differentiation process from a poorly-differentiated state into a differentiated state, even if it usually undergoes a different differentiation pathway. Furthermore, true transdifferentiation should not include a process of de-differentiation and should be a result of physiological stimuli of the organism.
In vivo experimental data suggest that white adipocytes of the murine adipose organ are able to undergo a true reversible transdifferentiation process in physiological conditions, i.e. white adipocytes can be converted directly and reversibly into brown adipocytes. This notion is derived from published(Reference Himms-Hagen, Melnyk and Zingaretti27) and unpublished results (G. Barbatelli, I Murano, L Madsen, Q Hao, M Jimenez, K Kristiansen, JP Giacobino, R De Matteis and S Cinti). The main data are morphological (light and electron microscopy), biochemical and immunohistochemical combined with data for genetic expression. Both in animals treated with the β3-agonist CL316243 and in mice exposed to cold conditions a series of images have been observed and molecular data obtained that suggest a progressive direct transformation of white adipocytes into brown adipocytes without any morphological or molecular sign of cell de-differentiation or proliferation. Importantly, the absence of β3-adrenoceptors dampen this transformation(Reference Jimenez, Barbatelli and Allevi47) in line with the transformation of brown adipocytes into white adipocytes in mice lacking all β-adrenoceptors(Reference Bachman, Dhillon and Zhang33). Thus, the sympathetic nervous system seems to play a fundamental role in the plasticity of the adipose organ and a positive correlation has recently been shown between the density of noradrenergic parenchymal fibres and the density of brown adipocytes in the adipose organ under different environmental temperatures(Reference Murano, Barbatelli and Giordano15).
Figure 6 summarizes the morphological and immunohistochemistry stages of the physiological and reversible transdifferentiation of white into brown adipocytes, which is supported by data from a number of different research groups(Reference Himms-Hagen, Melnyk and Zingaretti48–Reference Mercader, Ribot and Murano54).

Fig. 6. Scheme showing stages of white to brown adipocyte transdifferentiation. UCP1, uncoupling protein 1.
Physiological reversible transdifferentiation in mammary glands
The search for new examples of physiological reversible transdifferentiation in the adipose organ has shown that the well-known tissue plasticity of the mammary glands during pregnancy, lactation and post-lactation stages could be explained as a transdifferentiation phenomenon(Reference Morroni, Giordano and Zingaretti55).
The mammary glands of female mice, which correspond to the anterior and posterior subcutaneous depots, include WAT and BAT components as described earlier. After weaning these depots are infiltrated by branched ducts collected at the nipple, and the presence of three symmetrical bilateral nipples in the anterior subcutaneous depot and two symmetric bilateral nipples in the posterior subcutaneous depot define the ten mammary glands of mice. During pregnancy a new anatomical structure develops, i.e. the lobulo-alveoli that are the milk-producing part of the gland. During lobulo-alveoli development adipocytes disappear. Immediately after the end of lactation adipocytes re-appear and the lobule-alveoli disappear. This plasticity has been explained as a result of slimming of adipocytes during pregnancy and re-filling of adipocytes post lactation together with degeneration as a result of apoptosis of the milk-secreting glands. The slimmed (de-lipidated) adipocytes are thin elements not visible among the rest of the gland(Reference Elias, Pitelka and Armstrong56, Reference Blanchette-Mackie, Dwyer and Barber57). A different interpretation of the phenomenon is that during pregnancy adipocytes undergo a progressive process of transdifferentiation and transform into the milk-secreting epithelial cells of the lobulo-alveoli. Post lactation most of the epithelial cells of the lobulo-alveoli transdifferentiate back into adipocytes. If this interpretation is correct it would be the second example of physiological reversible transdifferentiation in the adipose organ.
Data have been collected that support this theory, including electron microscopy evidence of several intermediate steps that suggest a gradual transition from the adipocyte phenotype to the epithelium phenotype and vice versa(Reference Morroni, Giordano and Zingaretti55). The Cre-lox fate mapping technique is considered to be the most appropriate technique to prove the fate of a specific cell type during development or transdifferentiation processes(Reference Tosh and Slack31, Reference Soriano58, Reference Sauer59). Briefly, a marker driven by specific gene promotors (e.g. β-galactosidase) is detectable in a specific cell type whatever the transformation destiny of the cell (as a result of development or transdifferentiation) because the process producing the expression of the marker is irreversible. Double transgenic mice (aP2-Cre_R26R and WAP-Cre_R26R) have been used in the investigation. In the mammary gland aP2-Cre_R26R mice express β-galactosidase only in adipocytes. During pregnancy β-galactosidase has been found in the milk-producing epithelial glands, suggesting that these glands derive from adipocytes. The WAP-Cre_R26R mice express β-galactosidase only in the epithelial cells during pregnancy and in the post-lactation stage also in adipocytes, suggesting that adipocytes derive from epithelial cells. Considering these data in combination with electron microscopy data showing all intermediate stages of adipo–epithelial–adipo transformations it is considered that the plasticity of the mammary gland is, at least in part, a result of the reversible transdifferentiation properties of adipocytes.
Recent unpublished results (R De Matteis, MC Zingaretti, I Murano, A Vitali, A Frontini, I Giannulis, G Barbatelli, F Marcucci, M Bordicchia, R Sarzani, E Raviola and S Cinti) suggest that the ability to undergo adipo–epithelial transdifferentiation is not exclusive to mammary fat, but can be induced also in visceral fat and in fat from male mice. In fact, it has been found in explants of visceral fat and fat from male mice.
Molecular mechanisms that underlie the WAT–BAT transdifferentiation phenomenon seem to be of an adrenergic nature. β3-Adrenoceptors seem to be of pivotal importance. Administration of β3-agonists induce WAT–BAT transdifferentiation(Reference Giordano, Song and Bowers24, Reference Miller and Faust25). Furthermore, removal of β3-adrenoceptors strongly blunts the phenomenon(Reference Jimenez, Barbatelli and Allevi47). The PPARγ co-activator 1 seems to be an important key in the molecular post-receptor signalling, because its transfection into human white subcutaneous adipocytes in vitro induces the expression of the molecular BAT marker uncoupling protein 1(Reference Tiraby, Tavernier and Lefort60).
The molecular mechanisms of reversible adipo–epithelial transdifferentiation in the mammary gland are probably linked to the hormonal secretion of the pituitary gland responsible for milk production, but it cannot be excluded that local factors play an important role because only adipocytes of the mammary gland undergo the adipo–epithelial transformation during pregnancy in physiological conditions in vivo.
The adipose organ renews its cells
In 2003 it was shown by two independent groups that WAT of obese animals or human subjects is infiltrated by macrophages(Reference Xu, Barnes and Yang61, Reference Weisberg, McCann and Desai62). The grade of infiltration is correlated with BMI of patients as well as with the size of the adipocytes. This macrophage infiltration of fat seems to be relevant for health because the cytokines considered responsible for the insulin resistance that precedes type 2 diabetes seem to be mainly produced by these inflammatory cells rather than mature adipocytes, as previously thought. In line with this hypothesis is the coincidence between macrophage infiltration and the appearance of insulin resistance in both mice with diet-induced obesity and mice with genetic obesity. The data show that the majority (>90%) of macrophages are arranged in the adipose tissue to form characteristic structures that have been termed crown-like structures (CLS; Fig. 7). They are formed by macrophages reabsorbing residual fat and debris of dead adipocytes(Reference Cinti, Mitchell and Barbatelli63). The histopathology is therefore similar to the classic ‘foreign body reaction’ and frequent multinucleated giant cells are visible. CLS are visible also in the fat of lean animals but their frequency is about thirty times lower, which suggests that it could be the physiological means by which the adipose organ renews its cells. Of note, it has recently been elegantly shown that adipocytes of human adipose organ renew with a frequency that has been calculated at 10%/year(Reference Spalding, Arner and Westermark64). The hypertrophic adipose tissue of lean HSL−/− mice has the same type of macrophage infiltration of their fat, with classic CLS at a frequency comparable with that of genetically-obese mice, suggesting that adipocyte hypertrophy and not obesity is responsible for the death of fat cells(Reference Cinti, Mitchell and Barbatelli63). Mice and human subjects with hyperplastic obesity do not show CLS and are insulin sensitive(Reference Cinti, Mitchell and Barbatelli63, Reference Valet, Grujic and Wade65). The adipocytes in the newborn are small and progressively increase in size, because of a progressive increase in their lipid content, until they reach the characteristic size of mature adult cells(Reference Mercader, Ribot and Murano54). It has been calculated in rats that fat cell size increases progressively during the first weeks of life until puberty when they reach the adult cell size, which remains constant for the rest of the rat's life(Reference Greenwood and Hirsch66). In human subjects there seems to be a similar pattern(Reference Hager, Sjostrm and Arvidsson67). Thus, adipocytes seem to have a maximum size that is maintained with aging. It is therefore possible to consider that the adipocyte cycle is in some way linked to a maximum size they can reach. In physiological conditions the exposure of mice to different environmental temperatures leads to adipocytes with different sizes; smaller in cold conditions and larger in warm conditions. CLS (and therefore dead adipocytes) have been found almost exclusively in fat of warm-acclimatised mice showing the larger fat cells (I Murano, A Vitali, G Barbatelli and S Cinti, unpublished results).
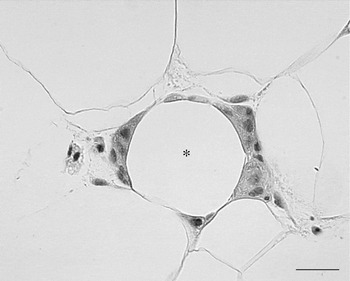
Fig. 7. Human subcutaneous white adipose tissue from an obese patient. Many macrophages surround the lipid droplet remnant of a dead adipocyte and form a crown-like structure CLS (*). Light microscopy; stain, haematoxylin and eosin; —, 25 μm.
Subsequent studies have shown that mice exposed to a high-fat diet have a progressive infiltration of the fat by macrophages forming CLS and the infiltration of visceral fat (epididymal) by macrophages is higher than that of subcutaneous fat(Reference Strissel, Stancheva and Miyoshi68). The infiltration of omental fat of obese human subjects is also higher than that of subcutaneous fat(Reference Cancello, Tordjman and Poitou69, Reference Harman-Boehm, Bluher and Redel70). This finding is in line with the well-known risk of visceral fat accumulation for metabolic disorders associated with obesity(Reference Bjorntorp and Rosmond71). Recent data show that the critical size triggering fat cell death is smaller in visceral fat than in subcutaneous fat. Thus, the smaller critical size could be responsible for the high grade of inflammation of visceral fat in obesity and this factor could be the link between visceral obesity and metabolic syndrome(Reference Murano, Barbatelli and Parisani72).
The reason for a smaller critical size in visceral fat has not been explained. It is hypothesized that visceral adipocytes could have a different origin from subcutaneous adipocytes. They may derive, at least in part, from a precocious transdifferentiation of brown adipocytes into white adipocytes (Fig. 8).

Fig. 8. Scheme showing a hypothesis explaining why visceral adipocytes have a smaller critical size triggering death than subcutaneous adipocytes. WAT, white adipose tissue.
This notion is supported by the following findings:
white adipocytes originating from brown adipocytes are smaller than white subcutaneous adipocytes(Reference Cinti, Frederich and Zingaretti73);
epicardial visceral fat is strongly correlated with abdominal visceral fat(Reference Iacobellis, Assael and Ribaudo74);
in some murine strains all mediastinic fat is BAT and epicardial fat is a component of mediastinic fat(Reference Murano, Barbatelli and Giordano15). UCP1 protein and its mRNA have recently been demonstrated in human epicardial fat(Reference Sacks, Fain and Holman75);
mediastinic fat of genetically-obese mice is composed of white adipocytes that are smaller than white adipocytes of subcutaneous fat (A Dover, A Frontini, S Cinti and B Walker, unpublished results).
Thus, it is proposed that visceral fat is composed of smaller adipocytes because they derive from transdifferentiation of brown adipocytes. These cells would have a smaller critical size triggering cell death because they have a different origin from those of subcutaneous fat originating in an anatomical site where insulation is one of the properties of that specific depot, allowing fat cells to reach an appropriate size for this specific function. This factor could be one of the reasons for the different metabolic risk of visceral fat in comparison with subcutaneous fat.
Conclusion
The data support the concept of a true organ with two types of parenchymal cells with a different anatomy and physiology, but both involved in energy partitioning and cooperating with each other because of their specific property, transdifferentiation.
This property would imply that adipocytes are able to undergo a process of physiological reversible reprogramming of their genome, allowing differentiated cells to change their phenotype and function. In this context, it is of great importance that the mammary glands of female animals show adipo–epithelial–adipo transdifferentiation phenomena during pregnancy, lactation and post-lactation stages. The β3-adrenoceptor-mediated transdifferentiation of white adipocytes into brown adipocytes could open up new therapeutic perspectives for obesity and type 2 diabetes, particularly in the light of data showing morphological and molecular evidence of BAT in areas corresponding to anatomical sites suspected of containing metabolically-active BAT by positron emission tomography in adult human subjects.
The presence of many fat-rich organs in mammals such as bone marrow, parotid glands, parathyroid glands, thymus, lymph nodes and pancreas implies that transdifferentiation of adipocytes is not a phenomenon limited to the examples reported here, but could also involve many other organs.
Acknowledgements
The research was supported by grants FIRB 2005RBN047PZY_000, Cofin 2007BRR57M_005 and COST BM0602 to S. C. The author declares no conflict of interest. aP2-Cre_R26R double transgenic mice were created by Dr Barbara Kahn, Boston, MA, USA and WAP-Cre_R26R by Dr Henninghausen, Bethesda, MD, USA.










