‘Empathy’ has been defined as the ability or competence to share the feelings of others (Decety & Lamm, Reference Decety and Lamm2006; Saudino et al., Reference Saudino, Carter, Purper-Ouakil and Gorwood2008). Baron-Cohen's (Reference Baron-Cohen2002) definition includes additional competencies: ‘the drive to identify another person's emotions and thoughts, and to respond to these with an appropriate emotion’ (p. 248). The complexity of empathy from both phylogenetic and ontogenetic perspectives is clear from different, intertwined scientific areas of investigation such as developmental and social psychology, cognitive neuroscience, clinical neuropsychology, and behavioral evolution (Baron-Cohen & Wheelwright, Reference Baron-Cohen and Wheelwright2004; Blair, Reference Blair2005; Davis et al., Reference Davis, Luce and Kraus1994; Decety & Jackson, Reference Decety and Jackson2004; Decety & Lamm, Reference Decety and Lamm2006; de Waal, Reference de Waal2008; Preston & de Waal, Reference Preston and de Waal2002).
In evolutionary terms, prosocial behaviors can be observed in different species of non-human primates; in particular, in those species in which individuals live in close social systems, such as the common marmoset (Callithrix jacchus; Burkhart et al., Reference Burkhart, Fehr, Efferson and van Schaik2007; see also Cronin, Reference Cronin2012). Empathy can find its evolutionary roots in different forms of observed prosocial behavior. In fact, de Waal (Reference de Waal2012) argues that empathy could be the main motivator of prosocial behaviors in different species of primates. These observations suggest that prosociality and empathy have, in fact, a phylogenetic history. However, the complexity of prosocial behavior and empathy manifestations in humans does not find correspondence in other species. Indeed, empathy likely plays a central role in the development and occurrence of prosocial behavior in humans. Investigators have frequently recognized the role of empathy-related responding in facilitating human cooperation (Eisenberg & Miller, Reference Eisenberg and Miller1987), and have suggested that empathy provides a major pathway to prosocial functioning (Batson, Reference Batson1991; Batson et al., Reference Batson, Ahmad, Stocks and Miller2004; de Waal, Reference de Waal2008). Individual differences in empathy-related responding levels have been recorded among individuals of all ages, in infancy (Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008), in childhood (Bryant, Reference Bryant1982), and in adulthood (Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004). However, few researchers have investigated the role of genetic and environmental factors in the expression of the trait in adulthood (Knafo & Uzefovsky, Reference Knafo, Uzefovsky, Legerstee, Haley and Bornstein2013).
Individuals who are higher in empathy during childhood, regardless of gender, tend to remain higher later in development, demonstrating the stability of this trait (Eisenberg et al., Reference Eisenberg, Guthrie, Murphy, Shepard, Cumberland and Carlo1999; Reference Eisenberg, Guthrie, Cumberland, Murphy, Shepard, Zhou and Carlo2002). Such evidence is consistent with the idea that empathy is partially heritable (e.g., Baron-Cohen, Reference Baron-Cohen2002; Chakrabarti & Baron-Cohen, Reference Chakrabarti, Baron-Cohen, Baron-Cohen, Tager-Flusberg and Lombardo2013; Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008; Rushton, Reference Rushton2004; Zahn-Waxler, Radke-Yarrow et al., Reference Zahn-Waxler, Radke-Yarrow, Wagner and Chapman1992; Zahn-Waxler, Robinson et al., Reference Zahn-Waxler, Robinson and Emde1992; Zahn-Waxler et al., Reference Zahn-Waxler, Schiro, Robinson, Emde, Schmitz, Emde and Hewitt2001) or at least that there are some evolutionary differences already present at birth. Furthermore, from twin studies comes the strongest evidence that empathy is heritable and that the heritability increases with age (Davis et al., Reference Davis, Luce and Kraus1994; Hatemi et al., Reference Hatemi, Smith, Alford, Martin and Hibbing2015; Hur, Reference Hur2007; Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008; Rushton, Reference Rushton2004; Rushton et al., Reference Rushton, Fulker, Neale, Blizard and Eysenck1984).
It is also hypothesized that the well-known gender differences in empathy levels, favoring females (Baron-Cohen, Reference Baron-Cohen2002; Eisenberg & Lennon, Reference Eisenberg and Lennon1983; Sucksmith et al., Reference Sucksmith, Allison, Baron-Cohen, Chakrabarti and Hoekstra2013; Volbrecht et al., Reference Volbrecht, Lemery-Chalfant, Aksan, Zahn-Waxler and Goldsmith2007), may have an evolutionary origin due to the emergence of animal species characterized by K-selection (i.e., those species characterized by prolonged and intensive parental care facilitated by the fact that mothers have relatively few children). With this strategy, mothers tend to be in tune with the expression of discomfort displayed by the infants, perhaps in part due to their ability to spend considerable time in close contact with their offspring. Given this framework, an important question might be whether gender differences in human empathy can be mirrored by the pattern of heritability for empathy traits. According to the empathizing-systemizing theory (Baron-Cohen, Reference Baron-Cohen2009), which defines five different ‘brain types’ with the ‘systemizing profile’ more common in males and the ‘empathizing profile’ more common in females, it appears crucial to generate more knowledge on possible gender-specific contributions of innate and environmental factors to empathy-related functioning in adult life, taking into account the potential influence of age.
Thus, our research was designed (1) to shed light on the possible contributions of innate and environmental factors in the empathy-related expression in adulthood, looking at the emotional, cognitive, and social components delineated within the trait (Berthoz et al., Reference Berthoz, Wessa, Kedia, Wicker and Grezes2008; Dimitrijevic et al., Reference Dimitrijevic, Hanak, Vukosavljevic-Gvozden and Opacic2012; Groen et al., Reference Groen, Fuermaier, Den Heijer, Tucha and Althaus2015; Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004; Muncer & Ling, Reference Muncer and Ling2006; Preti et al., Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011) and (2) to explore possible gender differences in these contributions.
To pursue our objectives, we applied the twin study design to a large sample drawn from the population-based Italian Twin Registry (ITR) (Brescianini et al., Reference Brescianini, Fagnani, Toccaceli, Medda, Nisticò, D'Ippolito and Stazi2013). The twin design has shed light on the causal mechanisms underlying an extraordinary variety of complex phenotypes and diseases, including psychosocial traits (Gregory et al., Reference Gregory, Light-Häusermann, Rijsdijk and Eleym2009; Knafo et al., Reference Knafo, Zahn-Waxler, Van Hulle, Robinson and Rhee2008), and its potential has enormously increased since the worldwide spread of twin registries (Hur & Craig, Reference Hur and Craig2013). This design allows for the estimation of genetic and environmental effects on the expression of human complex traits by comparing the level of trait resemblance between monozygotic (MZ) twins (genetically identical) and dizygotic (DZ) twins (who share on average only 50% of their genetic background, like ordinary siblings). Briefly, assuming that MZ and DZ twins share to the same extent all environmental exposures that are relevant to the trait under study (Equal Environments Assumption), a higher resemblance in MZ than in DZ pairs would point to genetic influences on the trait; if, instead, resemblance is not dependent upon zygosity, then environmental factors (family-based or individual-specific) would be supported as primary influences on trait expression (Neale & Cardon, Reference Neale and Cardon1992).
It is important to point out that the twin studies that have previously assessed the heritability of empathy (variously measured) among adults and young adults (e.g., Davis et al., Reference Davis, Luce and Kraus1994; Hur, Reference Hur2007; Rushton, Reference Rushton2004; Rushton et al., Reference Rushton, Fulker, Neale, Blizard and Eysenck1984) have not focused on possible gender differences, except for one study conducted by Hatemi et al. (Reference Hatemi, Smith, Alford, Martin and Hibbing2015). In a sample of young-adult twins in Australia, they found that individual differences in men's empathy, measured through the EQ, were largely due to genetic influences, and those in women's empathy almost entirely explained by non-shared environmental factors or error; however, they did not analyze the empathy subdimensions of the EQ. It may be hypothesized that gender differences in a psychosocial trait such as empathy vary as a function of gender differences in a given culture; thus, it is useful to examine these differences in a country like Italy, where different socialization and social roles for men and women have been maintained, perhaps more so than in some other Western countries.
Materials and Methods
Sample, Procedures and Measures
Study participants were recruited from a large multipurpose survey (Toccaceli et al., Reference Toccaceli, Fagnani, Gigantesco, Brescianini, D'Ippolito and Stazi2014) on twins aged 18–65 years who had been enrolled in the population-based ITR over a period of nearly 12 years, from 2001 to 2012. It is important to point out that twins as individuals often have not differed from non-twins with respect to social and behavioral characteristics (e.g., Klemmensen et al., Reference Klemmensen, Hobolt, Dinesen, Skytthe and Nørgaard2012); therefore, it is reasonable to consider that our results might be generalized to the general population as well. The procedures that led to the establishment of the ITR are described in detail elsewhere (Brescianini et al., Reference Brescianini, Fagnani, Toccaceli, Medda, Nisticò, D'Ippolito and Stazi2013). Currently, the ITR contains information on approximately 28,000 twins, and is involved in both general population and clinical-based studies on various complex phenotypes, with behavioral and psychiatric genetics as major research areas.
Between June and October 2012, 4,894 adult twins aged 18–65 years were contacted by mail and were asked to participate in the survey. The mailed material included a brief letter explaining the aims of the survey, as well as an informed consent form that the respondents had to return separately in order to safeguard individuals’ privacy and confidentiality. In the same contact, the twins received the questionnaire for the assessment of empathy.
Empathy assessment
Empathy was assessed with the Italian version (Preti et al., Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011) of the Empathy Quotient (EQ; Baron-Cohen & Wheelwright, Reference Baron-Cohen and Wheelwright2004). The EQ is a well-known instrument comprised of 60 items (40 items tapping empathic behavior and 20 filler items not counted in the scoring). The EQ items are rated on a four-point Likert scale (1 = strongly agree to 4 = strongly disagree). A large number of studies have previously validated the EQ by demonstrating the typical gender differences in various European countries (Dimitrijevic et al., Reference Dimitrijevic, Hanak, Vukosavljevic-Gvozden and Opacic2012; Preti et al., Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011; Vellante et al., Reference Vellante, Baron-Cohen, Melis, Marrone, Petretto, Masala and Preti2013; Von Horn et al., Reference Von Horn, Backman, Davidsson and Hansen2010; Zeyer et al., Reference Zeyer, Boelsterli, Brovelli and Odermatt2012), in Canada and the United States (Berthoz et al., Reference Berthoz, Wessa, Kedia, Wicker and Grezes2008; Wright & Skagerberg, Reference Wright and Skagerberg2012) and, to a minor extent, in Asian countries (Kim & Lee, Reference Kim and Lee2010; Wakabayashi et al., Reference Wakabayashi, Baron-Cohen, Uchiyama, Yoshida, Kuroda and Wheelwright2007). Following Baron-Cohen and Wheelwright (Reference Baron-Cohen and Wheelwright2004), a three-point scoring system was adopted, in which the 21 forward items were scored 2 for strongly agree, 1 for slightly agree, and 0 for strongly disagree and slightly disagree, while the 19 reversed items were scored 2 for strongly disagree, 1 for slightly disagree, and 0 for strongly agree and slightly agree. According to this system, scores can range from 0 to 80, with a cut-off of 30 that best differentiates between mean levels of empathy in the general population and autism spectrum conditions. The original version of the EQ has shown adequate internal consistency, concurrent and convergent validity, and good test–retest reliability (Baron-Cohen & Wheelwright, Reference Baron-Cohen and Wheelwright2004; Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004). High validity of the instrument has been reported also in the Italian population (Preti et al., Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011). Moreover, previous studies using a factor-analytic approach, based either on 28 items or on 15 items (Berthoz et al., Reference Berthoz, Wessa, Kedia, Wicker and Grezes2008; Dimitrijevic et al., Reference Dimitrijevic, Hanak, Vukosavljevic-Gvozden and Opacic2012; Groen et al., Reference Groen, Fuermaier, Den Heijer, Tucha and Althaus2015; Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004; Muncer & Ling, Reference Muncer and Ling2006; Preti et al., Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011), but not the 40-item version, have identified three EQ subscales labeled ‘cognitive empathy’ (CE; i.e., items that tap the ability to effectively understand the emotions of another individual and assume his/her perspective; Volbrecht et al., Reference Volbrecht, Lemery-Chalfant, Aksan, Zahn-Waxler and Goldsmith2007), ‘emotional empathy’ (EE; i.e., items that tap the individual's emotional response to another's expressed emotion; Duan & Hill, Reference Duan and Hill1996), and ‘social skills’ (SS; i.e., items that tap the sensitivity to social situations through, for example, the presence of spontaneous use of SS and of intuitive social understanding; Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004; Muncer & Ling, Reference Muncer and Ling2006). Therefore, besides calculating a global score for the individual proneness to empathy based on the full (40-item) version, we also conducted subdimension analyses based on the 28-item EQ scale.
Moderating and control variables
It was hypothesized that gender differences in the levels of empathy may reflect gender-specific genetic and environmental effects on empathic behavior; therefore, model-fitting analyses were stratified by gender. Furthermore, given the wide age range (18–65 years) of the study sample, possible interactions of age with genetic and environmental influences were also tested. Because no age moderation emerged, inter-individual age differences were simply considered a potential confounding factor and were controlled for in model-fitting analyses (see Statistical Analyses).
Statistical Analyses
For sample descriptives, confirmatory factor analyses (CFA), reliability, gender differences, and correlation with the autism spectrum quotient (AQ), twins from both complete and unmatched pairs were used and were considered as individual subjects.
The socio-demographic background of the sample in terms of age, gender, education, and marital status was examined using Stata (version 13.0).
Confirmatory factor analyses (CFA)
In order to determine whether the three-factor structure of the EQ (i.e., CE, EE, and SS) could be replicated in this Italian sample, factor analysis was conducted in R (version 3.1.0) by using the ‘lavaan’ package. The following separate CFA were performed for the 40-item and the 28-item version (Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004): (1) one-factor model for the 40-item version, (2) one-factor model for the 28-item version, and (3) three-factor model (CE: 11 items; EE: 11 items; and SS: 6 items) for the 28-item version. The three-factor model was tested only for the short 28-item version because no factor structure had been proposed by previous studies for the EQ containing all original 40 items. The Diagonally Weighted Least Square method of estimation was applied in all CFAs, given the ordered categorical response format. All the models were evaluated using the following indices: normed chi-square (i.e., chi-square/df), the root mean squared error of approximation (RMSEA) with its 90% confidence interval (CI), and the comparative fit index (CFI). Recommended values for these measures are between 2 and 5 for the normed chi-square (Hooper et al., Reference Hooper, Coughlan and Mullen2008), below 0.08 for the RMSEA (Hooper et al., Reference Hooper, Coughlan and Mullen2008), and above 0.90 for the CFI (Hu & Bentler, Reference Hu and Bentler1999). The metric invariance of the three-factor model (i.e., CE, EE, and SS) by gender was assessed by first fitting a two-group, three-factor model to both genders simultaneously (configural model) and then evaluating the chi-square change when equating factor loadings between males and females. To check whether the non-independence of data within twin pairs affected the results, all CFAs were first conducted on the total sample of twins and were then replicated considering only twin-1 and only twin-2 in each pair.
Reliability
The internal consistencies of the EQ scale and its subscales (CE, EE, and SS) were estimated in R (version 3.1.0) by the Cronbach's alpha and the McDonald's omega in their ordinal versions (Gadermann et al., Reference Gadermann, Guhn and Zumbo2012; Zumbo et al., Reference Zumbo, Gadermann and Zeisser2007), considering as acceptable those values higher than 0.70 (DeVellis, Reference DeVellis2012). Possible effects of within-pair data clustering were inspected by performing separate reliability analyses on the twin-1 sample and the twin-2 sample.
Gender differences analyses and the correlation of EQ with the autism-spectrum quotient
Gender differences for the total EQ (both 40-item and 28-item version) were tested with an independent-samples t-test in its cluster-adjusted version to account for the non-independence of observations within twin pairs. Between-gender comparisons were also performed for the EQ subscales (CE, EE, and SS). The correlation between the EQ score and the AQ score (Baron-Cohen et al., Reference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley2001) was estimated using the Pearson's correlation coefficient; this latter analysis was performed on a subsample of twins who had been administered the AQ questionnaire in a previous study by our group (Picardi et al., Reference Picardi, Fagnani, Medda, Toccaceli, Brambilla and Stazi2015). Gender differences analyses and correlation with the AQ were carried out with Stata (version 13.0).
The following analyses (i.e., twin correlations and genetic model fitting) were based on complete twin pairs only, and were conducted with the Mx statistical software (version 1.7.03; Neale et al., Reference Neale, Boker, Xie and Maes2006) using raw data as input.
Twin correlations
For the total EQ and subscales (CE, EE, and SS) scores, the within-pair correlations between the twin-1 and twin-2 samples were estimated according to zygosity (MZ vs. DZ pairs) and gender, and were interpreted under the assumptions of the twin design (Neale & Cardon, Reference Neale and Cardon1992). This was achieved by fitting multigroup saturated models that allowed different parameters (means, variances, and covariances) for MZ and DZ pairs, and for males and females, so containing five zygosity-by-gender groups (MZ male, DZ male, MZ female, DZ female, and DZ unlike-gender pairs).
In order to gain insights into the complex interplay of empathy facets and to test whether this interplay could affect gender differences in the genetic and environmental components, the within-individual phenotypic correlations between the EQ subscales (CE, EE, and SS) were also estimated by multivariate multigroup saturated models.
Genetic model fitting
Univariate gender-limitation structural equation models (Neale & Cardon, Reference Neale and Cardon1992) with five zygosity-by-gender groups (see above, ‘Twin correlations’) were fitted to estimate genetic and environmental effects on the total EQ and subscales (CE, EE, and SS) scores. In particular, total variance in the scores was decomposed into the following components: (1) additive genetic variance (A), resulting from the additive effects of all loci relevant to the trait; (2) shared environmental variance (C), representing the influence of environmental factors that are shared by the twins, especially within the family during early childhood (e.g., socio-economic status of the family, cultural background and practice, parents’ job, and parents’ child-rearing style), but possibly also during prenatal life; and (3) unshared environmental variance (E), due to acquired experiences that are unique to an individual (e.g., traumatic events, health status, social engagement, education level, chance friendships, and job experience), including measurement error effects. Gender-specific proportions of total variance explained by each of the above components were calculated, with the additive genetic proportion known as the trait ‘heritability’; these proportions were used to summarize the genetic-environmental influences on empathic behavior (i.e., total EQ and subscales) in men and women, and to perform gender comparisons. Given that statistical power issues may seriously affect model selection, we considered it prudent to avoid submodel fitting and to report the estimates under the full (ACE) model for both genders. Possible age-related modification of genetic and environmental effects on the EQ scales was also tested by interaction models (Purcell, Reference Purcell2002) that incorporated age as a continuous moderator to avoid arbitrary categorizations.
Results
Data from 1,687 individual twins (corresponding to 34% of the survey population) were analyzed; of these, 445 were unmatched twins because the co-twin did not respond to the survey. The demographic background (i.e., age, gender, education, and marital status) of the study sample did not differ from that of non-respondents and is shown in Table 1. Individuals were aged 18–65 years (M age = 39 years, SD = 14 years), with men representing 39% of the sample. A proportion of 12% of the sample completed only secondary school, 40% high school, and 38% college or university (3-year and 5-year degree); 53% of the twins were unmarried.
TABLE 1 Socio-Demographic Characteristics of the Study Sample

Confirmatory Factor Analyses (CFA)
Table 2 presents the (standardized) item loadings and the goodness-of-fit statistics of the CFAs applied to the 40-item version (one-factor model) and to the 28-item version (one-factor and three-factor model) of the EQ. The CFA supported the previously proposed three-factor structure of the EQ in the 28-item version. The item loadings of the three-factor model ranged from 0.53 to 0.79 for the CE factor, from 0.17 to 0.87 for the EE factor, and from 0.47 to 0.73 for the SS factor. All items of the three-factor model, except three, had loadings ≥0.30. Item loadings of the one-factor model ranged from 0.01 to 0.76 (40-item version) and from 0.13 to 0.77 (28-item version). Whereas the one-factor model of the 28-item version contained six items with loadings <0.30, the one-factor model of the 40-item version contained 13 such items. The three-factor model on the 28-item version clearly fit the data better than the one-factor models (40-item and 28-item version).
TABLE 2 Standardized Item Loadings and Goodness-of-Fit Indices of the Confirmatory Factor Analysis (CFA) of the EQ
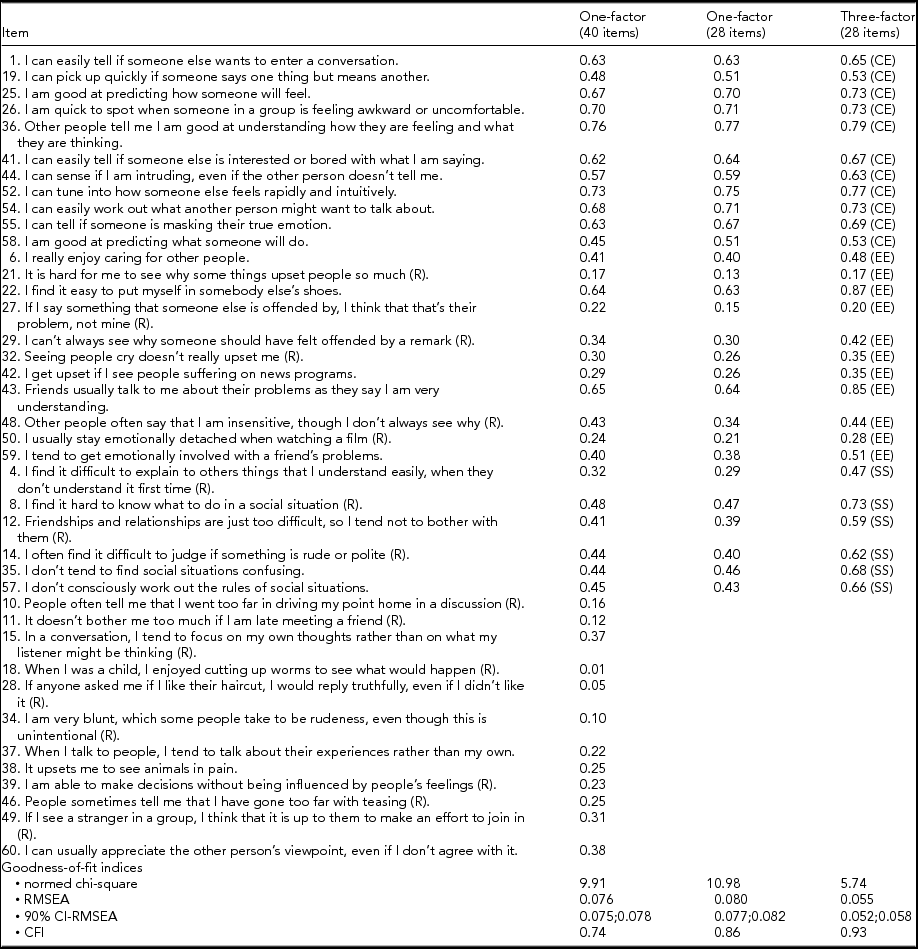
R = reversed item; CE = cognitive empathy subscale; EE = emotional empathy subscale; SS = social skills subscale; RMSEA = root mean squared error of approximation; 90% CI-RMSEA = 90% confidence interval of RMSEA; CFI = comparative fit index.
As a reassurance that our results on the 28-item version provided a faithful picture of the full version, an exploratory factor analysis on the 40-item EQ revealed three factors explaining 93% of the variance in the data; these empirical factors overlapped considerably with the CE, EE, and SE components previously identified (data not shown).
The three-factor model showed metric invariance by gender; indeed, constraining factor loadings of the two-group configural model (goodness of fit: chi-square/df = 2.43, RMSEA = 0.043, CFI = 0.92) to be equal between males and females yielded a non-significant (p = .75) increase of the chi-square. The clustering of observations within the twin pairs did not seem to affect the results of CFAs because these results remained unchanged when the analyses were replicated using twin-1 and twin-2 samples separately (data not shown).
Reliability
The internal consistencies of the 40-item and 28-item EQ (Cronbach's alpha = 0.88 and 0.90, respectively; McDonald's omega = 0.87 and 0.90, respectively) and of the subscales of the 28-item EQ (Cronbach's alphas = 0.91 [CE], 0.79 [EE], and 0.79 [SS]; McDonald's omegas = 0.91 [CE], 0.79 [EE], and 0.80 [SE]) were good. The higher internal consistency of the CE subscale compared to the EE and SS subscales was in line with previous reports showing that reversed items (0 out of 11 for CE, 6 out of 11 for EE, 4 out of 6 for SS) were less reliable than forward items (Groen et al., Reference Groen, Fuermaier, Den Heijer, Tucha and Althaus2015). Results of reliability analyses on twin-1 and twin-2 samples were almost identical (data not shown).
Gender Differences Analyses and the Correlation of EQ with the AQ
Gender-specific means and standard deviations of the 40-item EQ and the 28-item EQ with its subscales are reported in Table 3. The expected gender differences in mean level were detected, with women showing significantly higher scores than men on the 40-item and 28-item EQ. With regard to the EQ subscales, the largest gender difference was found for EE followed by CE, with significance reached for both subscales; for SS, men's and women's scores were similar and were no longer significantly different. The same gender pattern held true when stratifying the sample by age; more precisely, in each of the three age-groups 18–25, 26–50, and 51–65 years the levels of the 40-item and 28-item EQ, as well as of the CE and EE subscales, remained significantly higher for women, whereas similar levels of the SS subscale were observed across genders. The mean values for men and women for the 40-item EQ in the 18–25 age group were in line with those reported by Preti et al. (Reference Preti, Vellante, Baron-Cohen, Zucca, Petretto and Masala2011) for an Italian sample of students aged 18 to 30 years.
TABLE 3 Summary Statistics of the EQ Scales by Gender

CE = cognitive empathy subscale; EE = emotional empathy subscale; SS = social skills subscale.
For each EQ scale, missing items’ scores were replaced with the mean scores over available items when the number of missings did not exceed 10% of the total number of items (i.e., four missings for the 40-item EQ, three missings for the 28-item EQ, and one missing for each of the CE, EE, and EE subscales).
*p value for t-test on means (the test was performed with robust regression analysis — as implemented in Stata [version 13.0] — adjusted for age and accounting for within-pair clustering of observations).
For a subsample of 99 twins with available data on the AQ, the AQ score was negatively correlated with both the 40-item EQ (r = -0.44) and the 28-item EQ score (r = -0.46). These data may provide slight support for the divergent validity of the EQ.
Twin Correlations
The responders were 1,242 twins from complete pairs and 445 unmatched twins, for a total 1,687 subjects. Correlation analyses (and genetic model fitting, below) were based on 606 complete pairs with available information on zygosity, after excluding 15 pairs of unknown zygosity. Of the 606 pairs, 117 were MZ male, 53 DZ male, 193 MZ female, 113 DZ female, and 130 DZ unlike gender. Table 4 shows the within-pair correlations between twin-1 and twin-2 samples in the five zygosity-by-gender groups. For the 40-item and 28-item EQ, the difference between the correlations in MZ and same-gender DZ pairs was less for men than for women; furthermore, DZ unlike-gender pairs were substantially less correlated than were DZ same-gender pairs. This suggested possible gender differences in the underlying genetic-environmental architecture of empathic behavior as measured by the total EQ (both 40-item and 28-item version). In particular, the correlational pattern seemed consistent with a weaker or absent role of the shared environment in women. Inspection of the correlations for the two zygosity groups for each of the EQ subscales suggests that the gender differences might be driven by the cognitive and emotional components, whereas the correlations for the social component seemed to indicate similar genetic and environmental effects for men and women.
TABLE 4 Twin Correlations for the EQ Scales by Zygosity and Gender
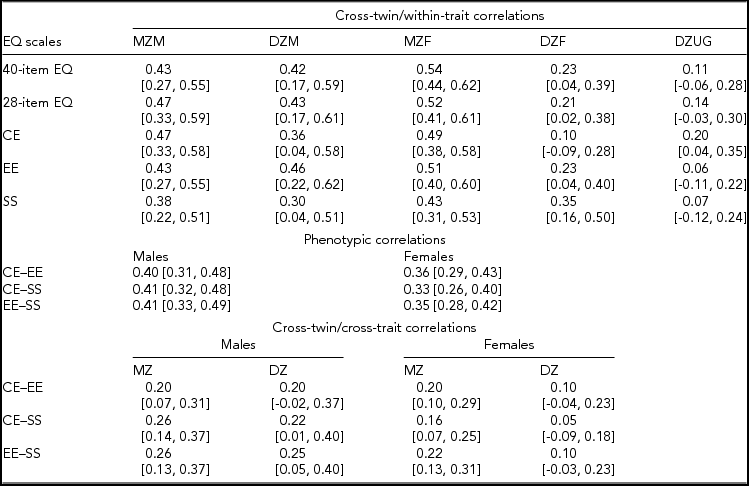
CE = cognitive empathy subscale; EE = emotional empathy subscale; SS = social skills subscale; MZM = monozygotic male; DZM = dizygotic male; MZF = monozygotic female; DZF = dizygotic female; DZUG = dizygotic unlike gender; MZ = monozygotic; DZ = dizygotic.
Cross-twin/within-trait correlations are between twins-1 and twins-2 samples for the same scale. Phenotypic correlations are between different subscales of the 28-item EQ in twins as individuals. Cross-twin/cross-trait correlations are between different subscales of the 28-item EQ in different twins within pairs (i.e., one subscale in twins-1 sample and another subscale in twins-2 sample, and vice versa).
Numbers in square brackets are 95% confidence intervals.
Table 4 also shows that the correlations among the 28-item EQ subscales by gender were of similar magnitude for men (about 0.40) and women (range: 0.33–0.36). Furthermore, based on the twin design, higher cross-twin/cross-trait correlations (i.e., between one component in twin 1 and another component in twin 2) in MZ female compared to DZ female pairs support a possible genetic correlation between the empathy subdimensions in women.
Genetic Model-Fitting
Interaction analyses showed no moderation by age of the genetic and environmental proportions of variance for any of the EQ scales for either gender, confirming that age simply acted as a potential confounding factor for empathy heritability. With regard to age differences in level of empathy, a slight but non-significant inverse-U-shaped age pattern was observed in both genders, as already found in a previous study (O'Brien et al., Reference O'Brien, Konrath, Grühn and Hagen2013). Moreover, the quadratic effect (i.e., the square of age) played no role in the models for the estimation of twin correlations and heritability, and thus linear age was retained as the only covariate in these models. Genetic and environmental proportions of variance for all the empathy subscales, as obtained from the gender-limitation structural equation models, are reported in Table 5. Consistent with the pattern in the correlation analyses, for the 40-item and 28-item EQ, additive genetic factors explained marginal portions of variance (about 5% and 10%, respectively) in men's empathy, whereas they explained a substantial amount of variance (about 50%) in women's empathy. Accordingly, the environmental load was higher for men's than for women's empathy; specifically, 40% of variance in men's empathy was due to the shared environment, whereas this factor accounted for no variance in women's empathy. The unshared environmental proportions of variance in men's and women's empathy were similar for the 40-item EQ (56% and 47%, respectively) and identical for the 28-item EQ (50%).
TABLE 5 Genetic and Environmental Proportions of Variance for the EQ Scales by Gender
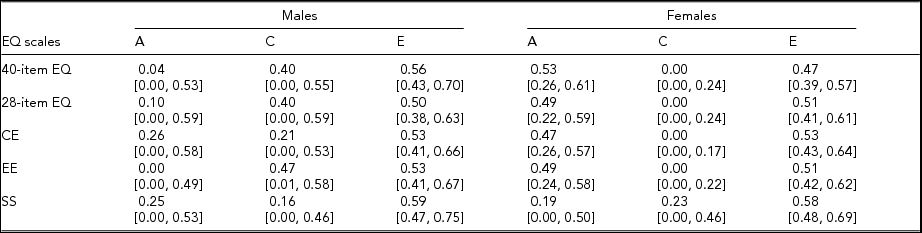
CE = cognitive empathy subscale; EE = emotional empathy subscale; SS = social skills subscale; A = additive genetic variance; C = shared environmental variance; E = unshared environmental variance.
Numbers in square brackets are 95% confidence intervals.
When looking at the EQ subscales, the cognitive and emotional components behaved similarly to the 40-item and 28-item EQ. Indeed, for CE, genetic effects explained almost half of the variance in women's empathy, whereas heritability was modest for men. Furthermore, the shared environmental contribution was sizeable for men and absent for women. Similarly, for EE, the estimates showed no genetic influence for males and around 50% heritability for females; prediction by the shared environment was approximately 50% for males and 0% for females. No gender differences were observed in the role of the unshared environment for either CE or EE. As expected from the wide and overlapping CI of both the genetic and environmental proportions of variance across genders, the formal chi-square test of gender heterogeneity for the 40-item and 28-item EQ, as well as for CE and EE, did not reach significance. However, for all these scales, heritability was significant only for women; furthermore, for EE, the estimate of the shared environmental contribution was significant only for men. For SS, genetic and environmental proportions of variance did not differ by gender; the additive genetic and shared environmental components each contributed about 20% of total variance in both males’ and females’ scores, with the remaining 60% of variance in both genders due to unshared environmental factors.
Discussion
Gender differences in empathy, with women scoring higher than men on different assessment tools, are well documented in a multitude of developmental investigations (Christov-More et al., Reference Christov-Moore, Simpson, Coudé, Grigaityte, Iacoboni and Ferrari2014), and have proven to be persistent across the lifespan (Michalska et al., Reference Michalska, Kinzler and Decety2013; O'Brien et al., Reference O'Brien, Konrath, Grühn and Hagen2013). Moreover, these gender differences appear to be larger with age (Eisenberg et al., Reference Eisenberg, Fabes, Schaller and Miller1989; Michalska et al., Reference Michalska, Kinzler and Decety2013; Van Tilburg et al., Reference Van Tilburg, Unterberg and Vingerhoets2002).
Our results confirmed gender differences in adults’ empathy levels across a range of ages, showing an apparent stability of these differences across the adult lifespan, with females scoring significantly higher on the overall scale as well as on the cognitive and emotional subdimensions, but not on the SS scale, as already found in other studies (Gouveia et al., Reference Gouveia, Milfont, Gouveia, Neto and Galvão2012; Lawrence et al., Reference Lawrence, Shaw, Baker, Baron-Cohen and David2004). Moreover, the absence of a gender difference in SS suggests that social factors might not be real constituents of empathy (Gouveia et al., Reference Gouveia, Milfont, Gouveia, Neto and Galvão2012).
Such findings motivated further examination of factors involved in gender differences in adult empathy, taking advantage of the unmatched value of the twin method. Only a few investigations concern empathy subdimensions in adulthood and, more specifically, gender differences in the heritability of the trait. Our study was designed to address this gap in knowledge regarding adult empathic behavior in the Italian population, a non-North American and non-North European cultural and social setting with a history of relatively strong gender roles.
Although a number of investigators have found evidence of the role of heredity in empathy, in a more in-depth examination, we found possible completely different patterns of heritability of the total EQ score and of the 28-item scale, for men and women. Specifically, for males, no genetic contribution and substantial influences of both shared and individual environmental factors are likely to emerge even if these estimates are not statistically significant. Conversely, women's empathy might be predominantly driven by genetic factors and individual (non-shared) experiences. We tested for a moderating role of age to explain, at least in part, the gender differences in EQ heritability patterns; however, no moderation by age of genetic effects was found in our adult sample.
Moreover, because empathy on the EQ is a complex trait, we investigated whether any of its subdimensions had a primary role in this potential pattern. The heritability analyses of the EQ subscales indicated that the possible gender differences in the heritability of empathy might be driven by the cognitive and emotional components, whereas SS might have a similar gene-environment architecture across men and women; this latter finding, if confirmed in future studies, might strengthen the hypothesis that SS are not a core component of empathy; indeed, SS are not part of the many scholars’ definitions of empathy (e.g., Batson et al., Reference Batson, Ahmad, Stocks and Miller2004; Eisenberg & Fabes, Reference Eisenberg, Fabes, Damon and Eisenberg1998).
The gender differences in heritability patterns that seem to emerge in this study might mirror the gender differences in level of empathy and might be consistent with theoretical propositions from a phylogenetic perspective that we would like to suggest in purely speculative terms. From this perspective, the bond between mother and offspring is one of the key factors for survival. The quality of this bond is essential, and its adaptive value is important across different species. We can assume that behavioral traits with a highly adaptive value can be preserved by natural selection. A strong bond between mother and offspring might therefore become rather widespread across species, and empathy, at least in its cognitive and affective facets, could be a means to assure a potentially higher quality of such bond. Women might be then influenced by genetics (phylogenetic memory) and by individual experiences (necessarily involving the ‘quality’ of the relationship with another individual that might be originating from the relationship with offspring), whereas our data seem to show a different scenario for men.
The phylogenetic approach would provide an interesting framework in the case of significant results, but it would be even more complex to provide another likely explanation for a completely different causal pathway for men and women. Indeed, we think that there could be other possible reasons for this potential pattern of results. In particular, there are findings regarding the theme of a different approach by men and women to the ‘context’ experienced, which might ultimately lead to diverging patterns in the ‘response’ provided. There is, in fact, speculation regarding socio-biological differences between males and females, with biological hereditary differences sometimes manifesting themselves to a greater degree in social behavior rather than in biological indicators (Cullen et al., Reference Cullen, Baiocchi, Eggleston, Loftus and Fuchs2016). Developmental analyses have also shown that heritability is only meaningful in the specific context in which the trait is investigated, and therefore that heritability estimates might be influenced by the tool and procedures of the investigations (Knafo & Uzefovsky, Reference Knafo, Uzefovsky, Legerstee, Haley and Bornstein2013). Consequently, the measure of empathy and the type of recruitment (voluntary) might have affected our findings, although these influences cannot give an exhaustive explanation of the gender differences detected. In particular, we cannot exclude the possibility that, because empathy is a prosocial trait in which the tendency to volunteer might play a key role, the method of recruitment might have affected the sample composition and thus the heritability patterns found in this study. In other words, it is possible that the heritability architecture of empathy might be different in the sample that was not obtained through a request for volunteers. Moreover, social motivation — which appears to differ between males and females — is considered as one major driving force behind developmental gender differences in social capabilities such as empathy (e.g., Chevallier et al., Reference Chevallier, Kohls, Troiani, Brodkin and Schultz2012). Thus, perhaps environmental influences related to gender, and in particular adults’ social motives (e.g., in relation to work choice, career, and family planning), continue to be intertwined with genetics and to reinforce differences between adult men and women. Based on the aforementioned theories and empirical findings, it is important to consider not only the study context itself (e.g., the use of a questionnaire survey, the way of administering it, and the recruitment procedure), but also (and perhaps of more importance) how the cultural setting and gender-typed culturally mediated social motivations interact with genetics in adulthood.
As previously noted, from childhood, males and females are confronted with different social stimuli and cultural expectations, and these experiences might be reflected in social experiences in adult life. In the Italian cultural context, specifically young girls have long been pressured by parents and family to do their best to help family members (Confalonieri et al., Reference Confalonieri, Bacchini, Olivari, Affuso, Tagliabue and Miranda2010; Olivari et al., Reference Olivari, Hertfelt Wahn, Maridaki-Kassotaki, Antonopoulou and Confalonieri2015), this could have contributed to lessen variability across families in women's social motivation compared to men and, consequently, shared environmental variance in empathy among adult females. This, in turn, could have made the genetic component of empathy more easily detectable for women. On the other hand, given that males are not expected to be as empathic as females, socialization toward empathy may vary more across families for them.
If the gender differences in empathy heritability found in this study are replicated and results are significant in future larger studies, it would be of interest to test whether and which environmental experiences and social gender-specific motivations moderate the genetic component of empathy. We are aware that this same heritability patterns might not apply to other populations in different social and cultural environments; however, robust inter-cultural comparisons are not possible at the moment, given the scarcity of available evidences from twin studies on adult empathy in other countries. Nonetheless, we cannot exclude the existence of specific psychosocial confounding or moderating factors that were not considered in our analyses and that could, once taken into account in the modeling, mitigate the observed differences.
Previous twin studies on adults’ and young adults’ empathy (e.g., Davis et al., Reference Davis, Luce and Kraus1994; Hatemi et al., Reference Hatemi, Smith, Alford, Martin and Hibbing2015; Hur, Reference Hur2007; Rushton, Reference Rushton2004; Rushton et al., Reference Rushton, Fulker, Neale, Blizard and Eysenck1984), as well as a recent genome-wide meta-analysis by Warrier and colleagues (Reference Warrier, Grasby, Uzefovsky, Toro, Smith, Chakrabarti and Baron-Cohen2017), found significant heritability estimates for the trait or for specific empathy components, with the remaining variance explained by individual environment only. However, to our knowledge, the only twin study that investigated the gene-environment architecture of adult empathy using a gender-stratified approach is the study by Hatemi and colleagues (Reference Hatemi, Smith, Alford, Martin and Hibbing2015). Their results, defined as ‘preliminary’ by the authors, are inconsistent with ours, with individual differences in men's empathy largely due to genetic influences, and differences in women's empathy almost entirely explained by non-shared environmental factors or error. Possible reasons for such conflicting results might be found in the different cultural and social background (Italy vs. Australia) and recruitment strategy of the Hatemi and colleagues’ study. In particular, as already highlighted above, the recruitment strategy (ours was completely voluntary, that of Hatemi's was based on incentives) draws attention to possible influences on study involvement that may reflect gender differences in participation choice, and may ultimately have a different impact on the estimates of genetic and environmental effects in the two genders for the trait under study. In addition, Hatemi et al. (Reference Hatemi, Smith, Alford, Martin and Hibbing2015) used a shortened version of the EQ and did not examine gender differences for the three subscales. The aforementioned divergent results indicate that further cross-cultural comparisons with similar measures of empathy (and the same subscales) are needed to identify factors contributing to gender differences in the genetic and environmental bases of empathy.
Finally, there is evidence from non-twin studies, as in the model proposed by Decety and Moriguchi (Reference Decety and Moriguchi2007), that cognitive and affective constituents of empathy are intertwined and jointly produce the experience of human empathy. Our results are in line with and extend this evidence in several ways. Indeed, we found a substantial correlation between not only the cognitive and the emotional components of empathy, but also between these components and the SS component. Furthermore, the observed intercorrelations among the subdimensions were similar for males and females, suggesting that there may be no gender difference in the co-manifestation of these different empathic (or empathy-related) skills.
Finally, taking advantage of the twin nature of our study sample, we also estimated cross-twin/cross-trait correlations in MZ versus DZ pairs for all three empathy facets, and derived evidence suggesting a possible genetic correlation between these facets only in females. This issue could be further investigated and better estimated in future studies on larger samples by using a multivariate twin model.
Limitations and Strengths
This is one of the few twin studies investigating empathy in adulthood and, to our knowledge, the second twin study addressing gender differences in the heritability of empathy. The sample size of this study is higher compared to previous investigations that made use of the ITR; nevertheless, we failed to reach statistical significance when formally testing gender differences in the genetic and environmental components of the targeted empathic traits, even if the point estimates of these components varied remarkably between men and women. Therefore, it would be desirable to replicate the investigation on a larger sample — and using as well a different assessment tool not based on self-report — possibly taking into account additional confounders or specific potential moderators of the effects (e.g., social status, family composition, and job experience) that we were not able to consider in the present work. Furthermore, to better analyze EE, it would be appropriate in a future study to administer a measure of personal distress (a self-oriented, aversive reaction to another's emotion or perceived need or emotional state; see Batson, Reference Batson1991) in order to address self-oriented, empathy-related emotion.
Conclusions
There is a need to better understand the bases of individual differences in empathy. Our findings suggest possible gender-specific innate and environmental contributions to the trait. These analytic differences, if replicated, might be exploited in the planning of empathy promotion interventions. In this respect, it would be worth thinking of different approaches to promote empathy as a strong social catalyst, sustaining empathic behavior in different social and work settings where it produces positive results for individuals’ and societal health and wellbeing (Mathews & Collin-Vézina, Reference Mathews and Collin-Vézina2016; Saffran, Reference Saffran2014; Shen, Reference Shen2015). Should the results of our work be confirmed in future studies, they would provide valuable evidence for the design of educational programs for use with young children; in particular for boys (who tend to be lower in empathy than girls; Eisenberg & Fabes, Reference Eisenberg, Fabes, Damon and Eisenberg1998), to shape and enhance their empathic behavior later in life.
Acknowledgments
We are grateful to Cristina D'Ippolito for her skillful technical assistance in manuscript formatting and database management. We also thank all the twins who participated in this survey. This research did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sector.
Conflict of Interest
None.







