Introduction
Three challenges limit the conversion of biomedical research discoveries in the academic environment into clinically useful applications: inadequate funding sources for early stage proof-of-concept studies or validation assessment, limited expertise in product development, and lack of experience in commercialization strategies. To address these impediments, in 2013, the National Institutes of Health (NIH) established the National Centers for Accelerated Innovations (NCAI) [Reference Antman, DiCorleto and Freeman1] program through the National Heart, Lung, and Blood Institute. One of these centers, the Boston Biomedical Innovation Center (B-BIC), strove to identify technologies with clear clinical potential, and to provide the inventors with financial support, project management, mentorship, education, and other resources, which are designed to facilitate successful translation to clinical application and commercialization. As the time-limited program nears its completion, we review the history, structure, progress, and outcomes of B-BIC’s program, and share our view of those elements that accounted for success. In this way, we offer a blueprint for similar programs that can be developed at other academic medical centers.
Organization and Operations
B-BIC comprised a consortium of 16 member institutions (universities, research institutes, and academic medical centers) striving to accelerate the development of promising technologies (Table 1). B-BIC was designed to enable investigators to access the help they need when they need it; to assess commercialization opportunities; and to gain access to funding, industry expertise, and potential partners. A continuum of services was built providing multiple tools and the means by which commercial outcomes could be fostered reliably and consistently to further public investment in research.
The 16 B-BIC member institutions each supported their investigators in marketing and licensing specific technologies, but each of the institutions was unique, as were each investigator and technology. Coaching, project management, and skills development offerings were synthesized into a coherent program provided by the lead institutions [Brigham and Women’s Hospital (BWH), Massachusetts General Hospital (MGH), Mass General Brigham (formerly, Partners HealthCare System), and Harvard Medical School (HMS)], which are aimed at building a pipeline and providing the resources to support investigators regardless of where they were on the commercialization pathway. With funding, coaching, project management, and skills training, B-BIC provided one-on-one experiential learning and broad offerings to give investigators both an overall and specific understanding of the processes and risks involved in commercialization.
The central oversight and advisory structure of B-BIC comprised several committees: the Institutional Oversight Committee, with representatives from each of the core and affiliated member institutions; the Steering Committee, responsible for the scientific direction and operational oversight of the Center; and the Technology Assessment and Commercialization Group (TACG), which provided input into specific technologies as well as general advice for the Center (Fig. 1). The Center was led by three Program Directors (one each from BWH, MGH, and HMS), and was supported by the work of coaches (0.6 FTE), project managers (2 FTE), skills development personnel (3 FTE), and operations staff (1.2 FTE) (Fig. 2).
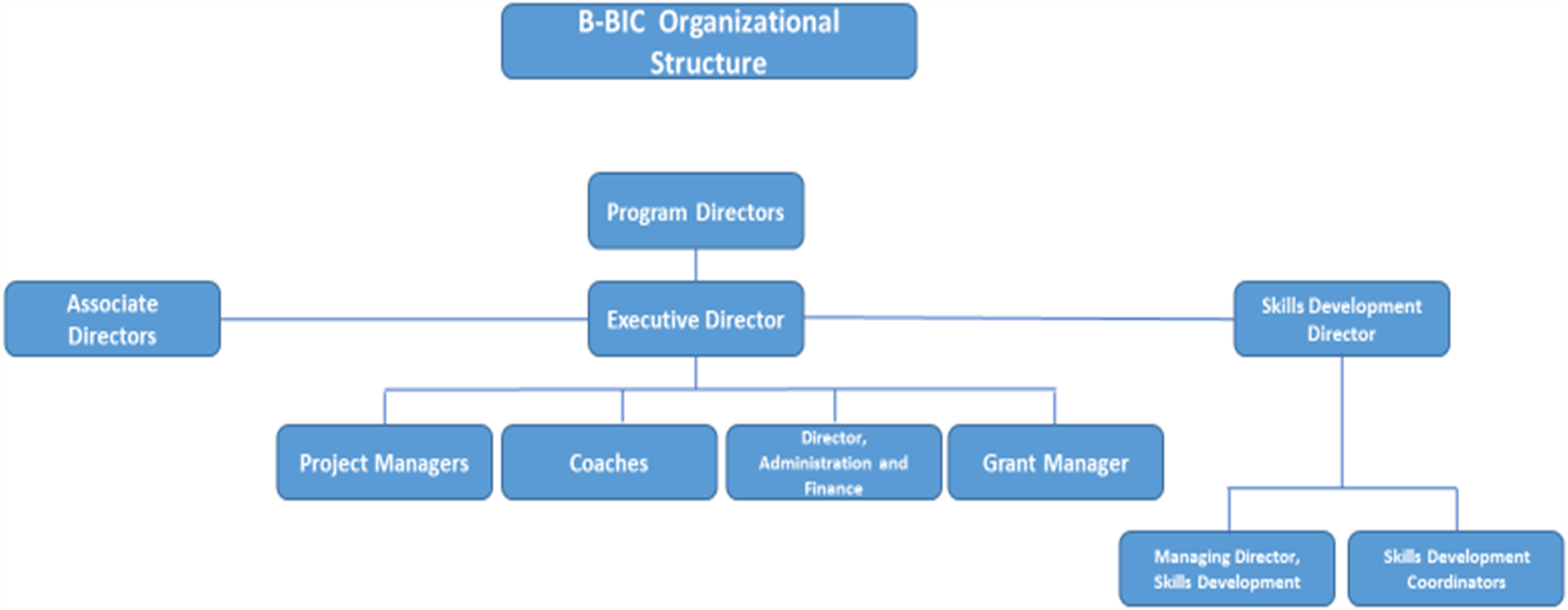
Fig. 1. B-BIC * Organizational structure.
Note: *Boston Biomedical Innovation Center.
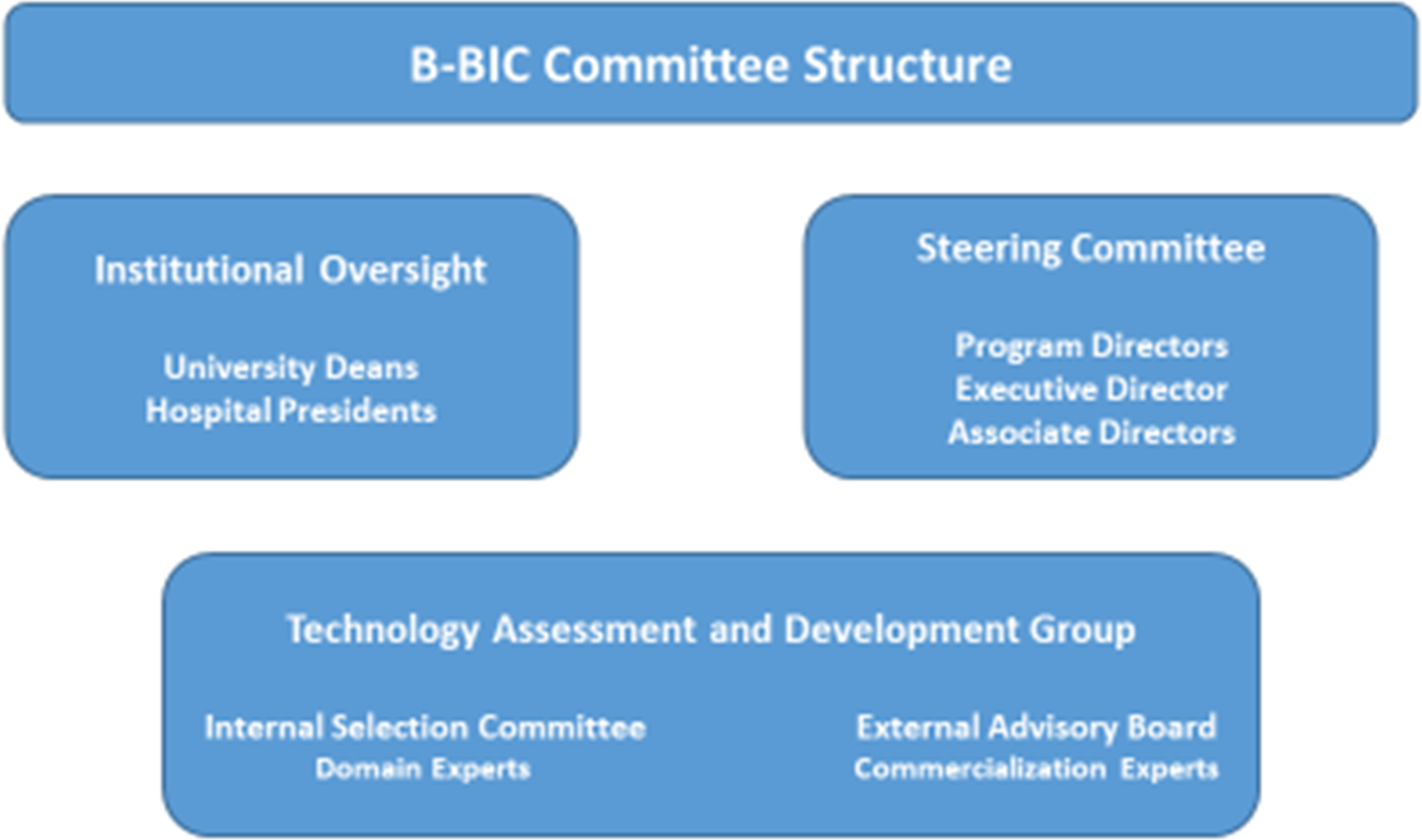
Fig. 2. B-BIC * Key committee structure.
Note: *Boston Biomedical Innovation Center.
The Institutional Oversight Committee met annually to ensure alignment of the program goals with the needs of these key stakeholders and member institutions. The TACG met three times a year to review individual projects in depth, and to provide project investigators with external feedback and direction from the private industry perspective. The Steering Committee met every 2–4 weeks to discuss proposals, opportunities, and outstanding issues. Staff met every 2 weeks to discuss the progress of individual projects and their needs for external resources, project hand-offs, sponsor requirements, and upcoming educational events.
Several essential functions were also leveraged from the member institutions, including the negotiation of technology license and confidentiality agreements, conflict of interest review and management, education and compliance for the use of human subjects and live vertebrates, and materials management. Investigators, department administrators, project managers, and operations staff worked closely together to ensure integration with these essential institutional departments. Center staff also worked with synergistic programs within the member institutions to leverage their funding opportunities, direct investigators to the appropriate resources, and tie together elements of the ecosystem in order to provide cohesive information for the investigators. Project managers also served as project reviewers, while operations staff provided input and helped to manage similar funding opportunities, such as the HMS Q-FASTR and Partners Innovation Discovery Grants programs.
The Application Process
B-BIC offered two distinct funding programs. Pilot awards provided up to $50,000 to support direct costs, and were expected to be completed within 1 year for proof-of-concept work that is necessary to examine the proposed scientific opportunity and business development approaches. DRIVE awards supported validation studies that were necessary to mitigate risk and explicate the value proposition sufficiently to attract private follow-on investment. DRIVE awards funded up to $200,000 for direct costs over 2 years, and were released in accordance with tranche plans that were aligned with project milestones and a series of go/no-go decision points. Pilot and DRIVE grants were made available on a continuous basis; investigators did not need to wait for an artificially determined timeline to apply for assistance. Proposals were reviewed on a rolling basis, as presented to the Steering Committee by project managers with input from the coaches, member institution licensing managers, and at least three reviews from TACG external members. This rolling, on-demand model for program funding we found to be essential for program success as it does not create artificial deadlines, is responsive to the sense of urgency in competitive technology development, and offers prompt feedback on an ongoing basis as project planning evolves.
Investigators learned about B-BIC through a referral from their licensing manager or colleague(s), email notifications, or Pitch Day events where multiple investigators signed up for 20-minute sessions with staff members at a common location. Formal engagement with the Center began with a two-page pre-proposal that was submitted to one of the two funding programs. Investigators could apply to either program at any time during the year using the online application system available on our website B-BIC, April 29, 2021 (https://b-bic.org/). A fundamental program principle was that the full range of B-BIC infrastructure was available throughout the year. Investigators could receive assistance whenever their preliminary data were ready or whenever they were inspired to take the next step toward commercializing their technology.
As soon as an application was received, it was assigned for review by the appropriate coach, project manager, institutional licensing manager, and member of the TACG who was a clinical domain expert. The pre-proposal and its reviews were then presented by the Project Manager to the Steering Committee for discussion and determination at the first go/no-go decision point. A decision was made as to whether the technology was within program scope, at the right stage of development, and could benefit from B-BIC assistance beyond simple funding. Suggestions for further consideration by the investigator were often raised during the discussion, and this information, along with the reviews, was passed back to the investigator. The Steering Committee could decide to invite submission of a full proposal, request further information, invite resubmission after consideration of reviewers’ suggestions, direct the applicant to the other B-BIC funding program, or decline the pre-proposal.
If a full proposal was invited, the investigator was referred to the appropriate coach and project manager to help the investigator develop the project plan. All full proposals were required to incorporate several elements including consideration of the business opportunity, a description of the project plan and milestones, and authorization by the investigator’s institution. Institutional authorization of full proposal submission was key to ensuring integration with the member institutions’ compliance programs, conflict of interest procedures, and plans for matching funds (where offered), should an award be made. Formal review criteria were developed alongside the NCAI Common Proposal Application in collaboration with the other NCAI programs and staff, and can be downloaded from the B-BIC website at B-BIC (April 24, 2021) https://b-bic.org/funding/#funding. The review criteria address the unmet need, competitive landscape, differentiation from existing technologies, intellectual property, the regulatory pathway, and potential risks and mitigation strategies, among others. A full proposal submitted to the DRIVE program also needed to include a tranche plan developed in conjunction with the project manager. The tranche plan mapped the budget to the milestones and served as a key tool in implementing the go/no-go decision points described in the project plan. Once the full proposal was complete and submitted, it was then reviewed by three members of the External Selection Committee, as well as by the relevant domain expert for the second time to ensure that the proposed outcomes continued to offer a potentially significant impact on the field. The full proposal and reviews were then presented to the Steering Committee by the project manager, and discussion was focused on whether the proposal merited submission to the NIH Technology Review Committee (TRC). The TRC comprised several representatives from relevant federal or affiliated agencies including the Centers for Medicare and Medicaid, the US Patent and Trademark Office, three branches of the Food and Drug Administration, other members of the federal NCAI program including Entrepreneurs-in-Residence, and Kaiser Permanente representatives. If a proposal was submitted to the TRC, its review comments were then disseminated to the Steering Committee, and a funding decision was made by its nine voting members. All comments were anonymized and returned to the investigators with the funding decision.
All of the process and evaluation events occurred on a rolling basis, with an average time from full proposal receipt to award release of just over 4 months. Significant value was added through this process, regardless of whether an investigator received an award. At the very least, the specific advice and input provided to the investigators greatly increased their knowledge of the commercialization path.
The Steering Committee members often asked for further information or invited the Principal Investigator (PI) to give further consideration to and elaborate on specific aspects of the proposal. Because the project managers presented the proposals and reviews for consideration by the Steering Committee, they were intimately familiar with the substance of the deliberations and were, thus, able to discuss the information and concerns of the Steering Committee knowledgeably with the PI as soon as that information was shared with the PI following a decision. The PI was then able to respond to the issues raised by revising his or her proposal without having to wait for another application cycle.
A Continuum of Services
The continuum of assistance provided by B-BIC generally began with coaching to help an investigator develop a high-level, strategic understanding of the opportunity his or her technology presented. Academic investigators often do not have the experience needed to answer the questions that surround a business opportunity. Inviting them to complete a proposal or an application form containing questions about the market size, product differentiation, and regulatory requirements would be an exercise in futility if we were to provide no assistance in exploring these business development issues. Project plans for both Pilot and DRIVE awards were, therefore, developed with significant input from coaches, project managers, members of the TACG, and members of the Steering Committee, in addition to the input received from the NIH NCAI federal partners.
One of the most significant elements of the B-BIC program was the project management component that was interwoven with the investigator’s work plan and approach. Project management guided the investigator’s focus on critical experiments that were needed to achieve the next inflection point for commercial development. PIs consistently found this resource to be one of the most helpful aspects of the program as it taught them the importance of decision-based planning once a translational approach was selected. This approach, which emphasized the necessity of achieving sequential milestones in the execution of a plan with a clear commercial development goal, stood in stark contrast to the standard hypothesis-driven scientific method commonly used by academic investigators, which emphasizes the relative importance of pursuing scientific observations wherever they may lead.
Another important program element was the involvement of the external members of the TACG. These individuals came from industry and brought experience in venture capital, pharma research and development programs, manufacturing, and other relevant areas. Every proposal was reviewed by TACG members as part of the rolling development and online review process, and their comments were returned to the investigators. Awarded projects were presented by the investigators and discussed with the TACG at a regularly scheduled meeting. These members were also brought in on an as-needed basis to provide input at key decision points through smaller, more focused group meetings. These smaller meetings were used to determine the direction of a work plan in accordance with anticipated go/no-go decision points.
In addition to offering robust general resources that were broadly available to all investigators, the B-BIC approach to skills development was unique, and in that it was interlaced with project management plans. Specific skills development activities were identified with the project PI and the project manager at the outset of all DRIVE projects. These areas of focus could include the development of a targeted product profile, creation of user feedback assessment tools, one-on-one coaching for investor pitches, presentation skills, etc. These skills development resources were made available to address needs anticipated in the specific project plans, as well as interests expressed by the investigators’ team members.
The Skills Development Center (SDC) activities received strong support and matured into an essential part of the B-BIC program. Original content was generated and offered to the expanding B-BIC consortium. Over 6 years, we organized 134 skills development sessions, and a total of 2,779 individuals registered for events ranging in attendee size from 2 (closed consultation for funded investigators) to 110 (pitch competitions open to the public). The summary data for SDC registrants is shown in Fig. 3, which are organized by institutional role (self-identified).
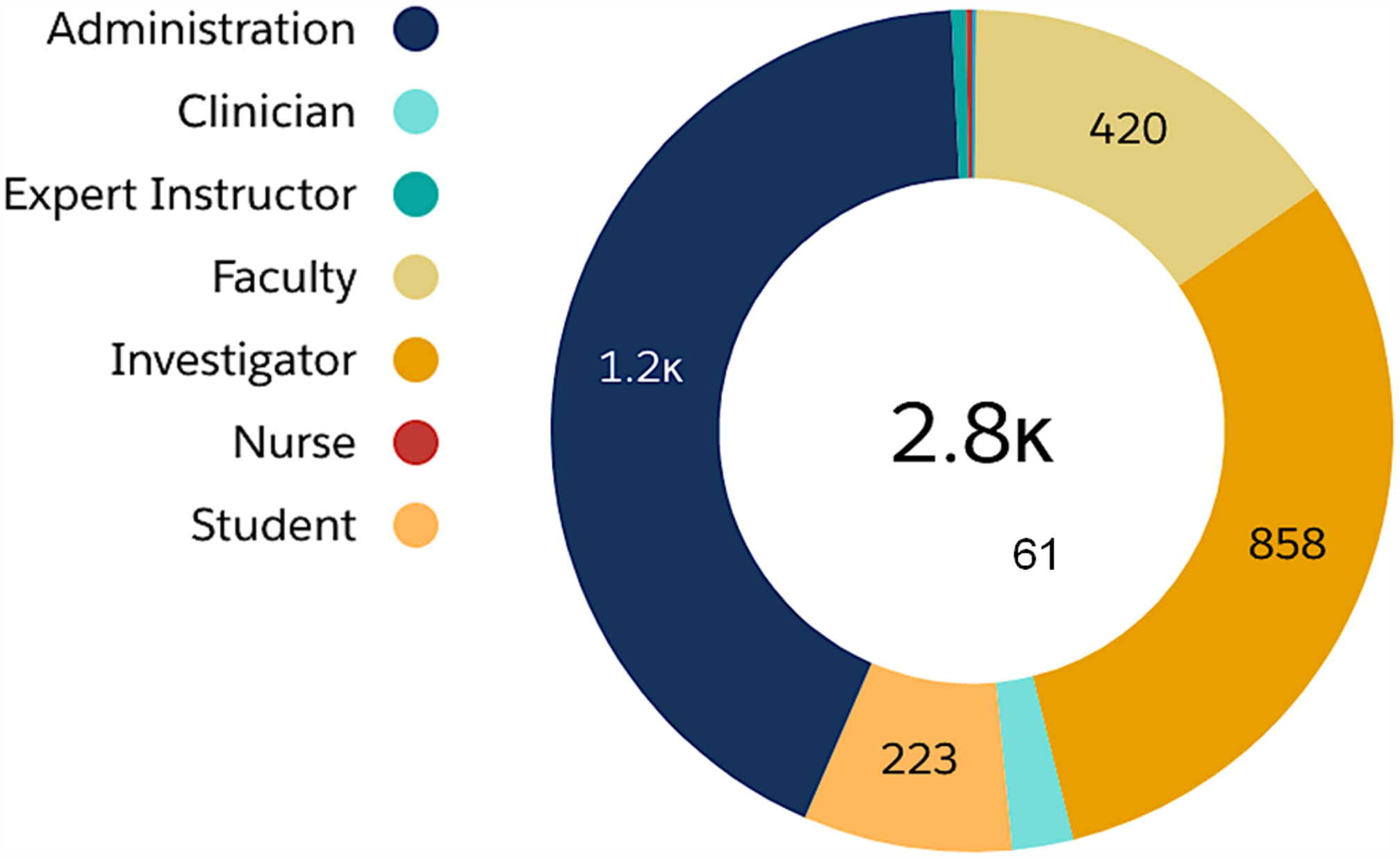
Fig. 3. Skills Development Center (SDC) events. Events attracted a diverse population of professionals interested in innovation and entrepreneurship including licensing managers, project managers, program directors, and business development professionals (all under “Administration”), as well as clinicians, instructors, faculty, investigators (in academics and industry), nurses, and students.
Prior experience in curriculum development for commercialization described phases (concept, development, market entry, market expansion) of commercialization of technology, which were viewed through the market, business, and technical lenses. These curricular models were not designed for the commercialization of new healthcare technologies, nor were they considered suitable to facilitate learning in the academic biomedical science community. We noted that innovators served by B-BIC were diverse with respect to interests, past experiences, and goals for translation of technology to the commercialization space. In order to provide the best possible skills development support for this heterogeneous community, we organized our efforts around principles of adult learning (self-directed, of immediate interest, application-oriented) and the general characteristics of our specific population (scientific, academic, and high-achieving). We also set a high priority on creating original content that could be shared with the broader research community on open-access platforms. The resulting support model can be described based on levels of customization (Fig. 4), designed with input from coaches and project managers. Our efforts were grounded in the development and delivery of content for Tier 2 offerings, which enabled expansion to open-access digital content for Tier 1, and the generation of worksheets for individualized consultations leveraged for Tier 3 support.
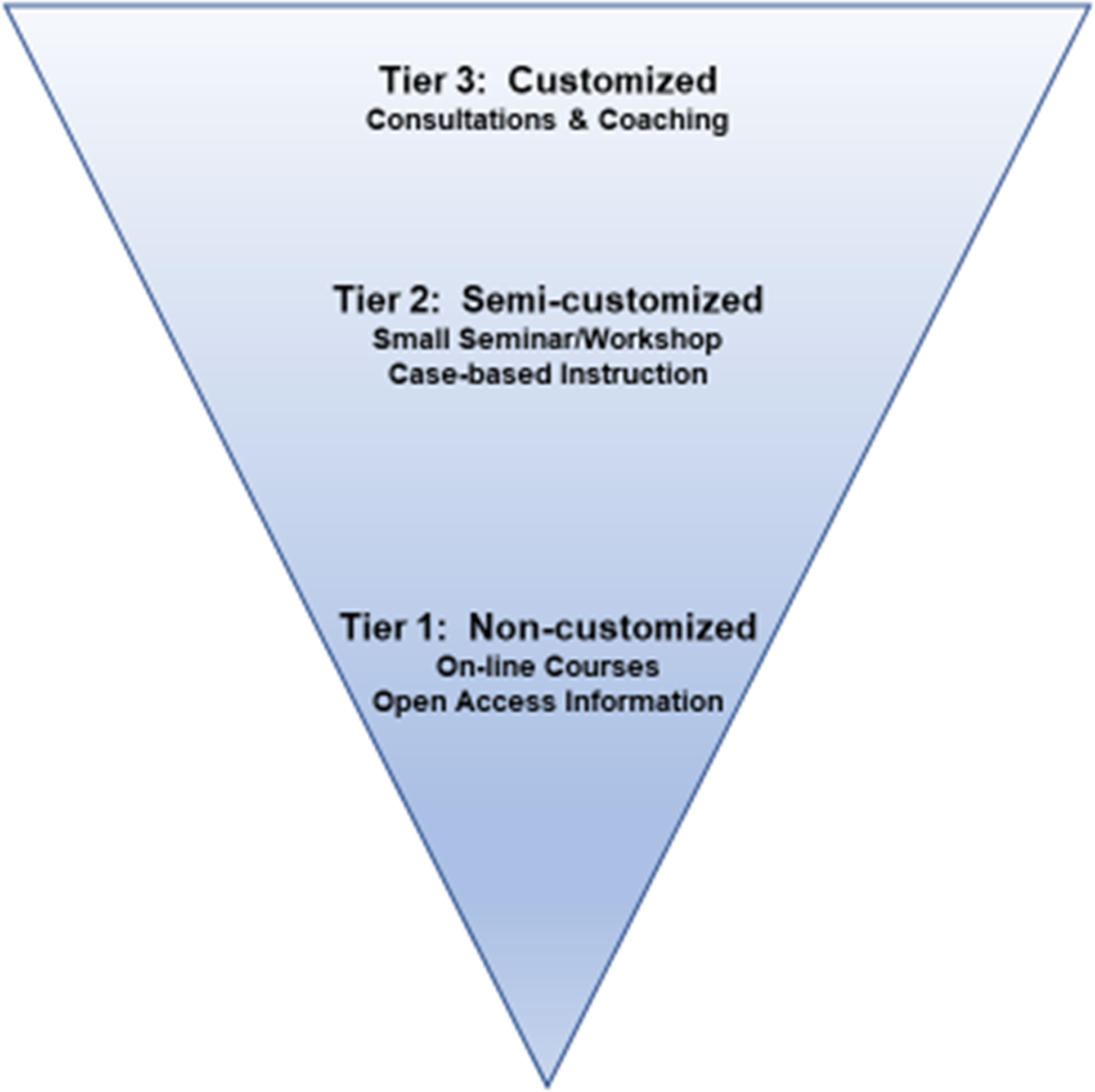
Fig. 4. Three tiers of learning. Tier 1 – resources produced from Tier 2 activities are repurposed and made broadly available for self-directed learning; Tier 2 – small-group, interactive, face-to-face learning opportunities led by expert instructors; Tier 3 – high-impact learning opportunities created specifically for a research team.
Tier 3 skills development activities were highly customized, and learning needs were identified with the project PI and the Project Manager at the beginning of each DRIVE project (as discussed above). They were identified at the start of the project, in conjunction with the PI and project manager, to further the technology development goals as well as the investigators’ and team members’ knowledge about the commercialization process. Tier 2 content development produced a broad range of resources from workshops and panel discussions to small-group presentation coaching. Overarching topics for these broad-based offerings included technology transfer, communications, entrepreneurship, management, and leadership development. We hosted a total of 123 of these small and mid-sized workshops (5–25 participants). Tier 1 digital resources were created in collaboration with Harvard Catalyst, the CTSA within HMS. The seminar portions of all workshops hosted between 2014 and 2016 were recorded, edited, and assembled as content playlists on our YouTube channel [2]. There were a total of 94 videos, organized as part of 20 different playlists, and these were distributed to a broad global community in all 50 states and 156 countries. The SDC YouTube channel was viewed more than 18,000 times for more than 2,300 h in the first 6 years. The playlist content was organized into short learning segments. The material from our five playlists on the Technology Transfer series was republished as chapters in a free, downloadable book available in the iBooks bookstore [Reference Basu, Prakash, Vaughan and Antman3]. This book was created with the intention of helping the absolute beginner understand some basic elements of the process by which technologies are developed and moved toward commercial exits. We included information to help other institutions run the workshops that were integrated into the seminar content. Workshop descriptions, worksheets, and discussion questions were embedded in this resource.
The five program elements – targeted funding, coaching, project management, the involvement of external advisory members, and skills development – comprised a continuum of support available to investigators that were flexible and could be designed to meet individual needs. These elements were held together by the leadership of the Steering Committee, which regularly met to discuss the opportunities described in investigators’ proposals, the specific needs of and any significant changes to the awarded projects, and the Center as a whole. As a result of this remarkable group effort, the whole was truly greater than the sum of its parts.
Technology Highlights
The most successful B-BIC-funded projects exited the program in a stronger position for future funding than at entry. Jonathan Thon and his team formed Platelet Biogenesis (now, PlateletBio) prior to their receiving a B-BIC award for the proposal, “A Biomimetic Human Platelet Bioreactor.” During the project, they worked with their assigned project manager to identify an external product development company, Fikst Product Development, to help them continue to scale the design of their bioreactor technology. In addition, they worked with the project manager to conduct more structured market research with leaders of blood banking organizations, and were able to tap into resources within the Centers for Medicare and Medicaid Services (CMS) to learn more about blood product pricing and reimbursement. This information was used to refine the business case for presentation to investors and for application to development-focused programs, such as Small Business Innovation Research (SBIR) grants, and to secure additional investment from private sources. After exiting from B-BIC, PlateletBio has gone on to secure over $40 million in private investment, as well as several government-sponsored development awards, including a contract from the Biomedical Advanced Research and Development Authority (BARDA) worth up to $56 million. As a result of this corporate and technological journey, PlateletBio has refined their understanding of their “platelet-like products (PLCs)” into an evolving pipeline addressing a range of indications, with IND-enabling work predicted to begin as early as this year.
Bruce Bean and colleagues received an award for the proposal, “Silencing Airway Nociceptors for Treatment of Cough and Airway Inflammation,” and collaborated with the supporting project manager and a B-BIC domain expert prior to and during the award to develop a project plan for evaluating their novel small molecule for treating cough and airway inflammation in a clinically relevant animal model. After generating compelling proof-of-concept data, a start-up company, Nocion Therapeutics, was launched to advance the development of the novel therapeutic. Nocion raised $27 million from investors, and was named a “Fierce 15” Biotech Company of 2019 by FierceBiotech [4]. More recently, Nocion expanded its efforts into the COVID-19 arena, evaluating their therapeutic agent for efficacy in reducing the spread of SARS-CoV-2 through effective cough suppression.
Other start-up companies formed to support the development of technologies initially funded through B-BIC also experienced enhanced investor interest as a result of program participation. A few months after Warren Zapol and colleagues received a B-BIC award to advance the development of a novel iridium electrode-based nitric oxide generator, the start-up company, Third Pole Therapeutics, formed through attracting seed investment. Collaboration with B-BIC project managers and external advisors helped Third Pole negotiate the challenges of transitioning the research and development efforts from an academic setting into the newly funded start-up, and helped identify other support opportunities through the NCAI central program. After winning a Johnson & Johnson Innovation Award, Third Pole has since entered into a collaboration with Actelion Pharmaceuticals, Ltd., a Janssen pharmaceutical company subsidiary of Johnson & Johnson, to develop their technology as a commercializable platform for the delivery of inhaled nitric oxide therapy for a range of indications.
An important determinant of our most successful programs was a strong collaboration between the project team and the B-BIC support team, one that often extended beyond the funded project period. Sylvie Breton, who received funding to develop a therapeutic and diagnostic (theranostic) agent for acute kidney injury, worked closely with a B-BIC commercialization coach during her project award period. That initial collaboration helped the investigators secure seed funding for a new start-up company, Kantum Pharma, from private investors.
These examples illustrate the key elements of a successful technology exit from B-BIC. Project management, networking with potential investors, well-timed financing and resource allocation, and a collaborative commitment to successful commercialization were all essential requirements for optimal success.
Outcomes
More than 270 proposals were received over a 7-year period from 213 unique investigators. Just over 40% of the awards were granted at the Pilot level. To date overall, B-BIC has awarded approximately $11.7 MM to 63 projects, and the funded technologies have raised approximately $242 MM in follow-on funding, an increase (multiplier) of 21X (Table 2). Participating institutions provided support from program operations and key personnel. Approximately 59% of the follow-on funding has come from private investment, including venture capital; 40% from public awards, including SBIR grants; and 1% from licensing deals. Fifteen new companies formed around the B-BIC supported technologies are active as of this writing, and among those with investments, the median amount of funding raised from venture capital is $2.75MM.
Table 2. B-BIC * Outcomes
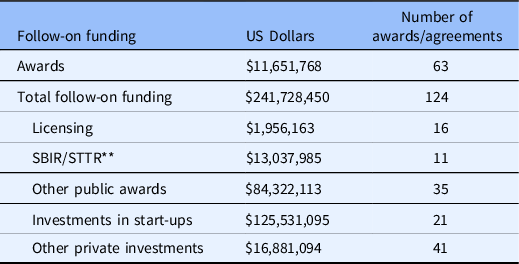
* Boston Biomedical Innovation Center.
** Small Business Innovation Research/Small Business Technology Transfer.
None of this success would have been possible without the commitment and support of the member institutions and their technology transfer offices. Technology transfer officials served as helpful referral sources alerting investigators with technologies at the appropriate stage of development to submit applications to the program, and also participated in technology development at every stage. Commercial revenue-sharing was not pursued as an option for institutional support of the B-BIC program, and licensing revenues were not a significant portion of the follow-on funding received. It is worth noting that dilutive funding was viewed as counterproductive in the early stages of technology development. Early valuation requirements set up potential conflict at the beginning of the relationship with investigators, and create distrust at a time when we are trying to educate the investigators about the different aspects of commercialization of their specific technologies. It was essential to align to the interests of the member institutions and recognize their needs to recover reasonable fees and licensing costs. Because the future value of their assets was neither diminished nor encumbered by dilutive funding, institutional technology transfer offices sought B-BIC input and were not restrained in seeking technology referrals. Consequently, the B-BIC pipeline was not constricted by any lack of commercialization knowledge on the part of the investigators, or by any reticence on the part of the participating institutions. In this way, B-BIC was viewed as a neutral party and “honest broker” that catalyzed the path toward successful commercialization of biomedical inventions and technologies.
Discussion
B-BIC has had remarkable success in its 7 years of operation. This success has been due not to one overriding factor but, rather, to a systematic and intentional paradigm shift in every facet of B-BIC’s operations. Compared to the typical academic research and education enterprise, B-BIC has not operated according to a “business as usual” paradigm.
B-BIC’s success starts with robust investigator engagement throughout its large and multi-institutional ecosystem. Solicitation of new, innovative translational research projects is accomplished using multiple modalities, including pitch competitions, requests for proposals, advertisements, the B-BIC website, and others. Critically important is B-BIC’s iterative engagement with PIs and their research team members throughout the application process. Many good ideas have been transformed into great ideas in the proposal stage through the intensive engagement of researchers with B-BIC’s outstanding cadre of project managers and coaches. While these efforts require some real-time commitment (which depends on the complexity of the project), the support structures within the program render the committed time highly efficient. The evaluation of proposals through B-BIC’s effective application process also gives researchers assurance that the organization cares about their success; biweekly meetings of the Steering Committee, extensive use of electronic reviews, and robust software support are all used to streamline the process so that proposals can be reviewed in a matter of weeks instead of months.
Robust proposal solicitation is followed by rigorous and efficient proposal selection. B-BIC utilizes multiple layers of reviews to evaluate the translational potential of research proposals from general experts with broad perspective (Steering Committee) to domain-specific experts from academia and industry (TACG) (as previously executed by the Center for Integration of Medicine and Innovative Technology in our ecosystem), all supported by project management experts and coaches. As in the proposal solicitation process, iterative engagement with PIs is critically important to ensure that projects selected for funding are initiated with the proper milestones, timeline, and internal and external supports (e.g., contract research organizations) in place.
Key to the success of B-BIC is its intensive support of funded projects, both in terms of scientific project management support and skills development support. Indeed, the partnership provided to B-BIC researchers by its project managers and skills development specialists is widely cited by PIs as the most valuable feature of B-BIC’s support, in many cases even more important than research project funding. These types of support are almost unheard of in an academic environment, especially at this intensive level, and it has taken much work, innovation, and iterative adaptation on the part of B-BIC leadership and staff to find the optimal approach. Now, after 7 years of experience, B-BIC and its awardees could not imagine operating in any other way.
B-BIC project managers and skills development specialists work with researchers collaboratively during every phase of project planning and execution. Although these interactions certainly include the more traditional development of project milestones and tracking, the input of the project managers is especially valuable in considering alternative translational approaches and using external resources such as CROs. Skills development in B-BIC has evolved as a tiered approach, offering all interested faculty and trainees education in relevant general topics such as translational research, entrepreneurship, market analysis, business development, and commercialization; and providing B-BIC-funded PIs and research teams with additional, intensive, targeted training that is specific to the needs of their translational research projects. The B-BIC skills development team has also published an open-access handbook on these topics that are helping hundreds to thousands of translational researchers worldwide [Reference Basu, Prakash, Vaughan and Antman3].
B-BIC has worked conscientiously over the past 7 years to build and support the translational research community in Boston and, in so doing, to effect culture change throughout our multiple institutions. The community has been nurtured with the view that teams of PIs, researchers, technology transfer professionals, domain experts, coaches, project managers, skills development specialists, technology experts, and commercialization experts are absolutely required to move forward with innovative and important biomedical technologies. Collaborations and partnerships among academic, biopharma, and the venture communities are also essential to the success of such technologies. B-BIC’s successful outcomes are well documented herein, and the future is bright, as well, as we move to a post-B-BIC era in which the B-BIC experience provides optimal guidance in a sustainable environment. Building on B-BIC’s example, many of our institutions have initiated institution-specific translational research funding mechanisms involving philanthropy, industry partnerships, and government grants – in many cases, borrowing some of the ingredients of B-BIC’s success such as scientific project management and skills development (among others).
In closing, we wish to acknowledge our enormous debt of gratitude to the NIH NCAI program. B-BIC simply would not have happened without the vision, support, and funding provided by the NCAI mechanism. Especially important was NIH’s foresight in envisioning that 7 years of operation would be required to stabilize the infrastructure and change the culture to the point that these innovations could be institutionalized and sustained, as our experience has clearly demonstrated.
Acknowledgments
The authors also wish to thank R. Cannistraro and S. Tribuna for expert technical and administrative support.
This work was supported in part by NIH grant U54HL119145 (B-BIC) and the NHLBI’s NIH Centers for Accelerated Innovatios program.
Disclosures
The authors have no conflicts of interest to declare.









