Non-alcoholic fatty liver diseases (NAFLD) are hepatic manifestations of the metabolic syndrome( Reference Marchesini, Brizi and Bianchi 1 ), and represent a major public health issue, affecting about 30 % of the general population in the USA. NAFLD encompass a wide range of diseases, all initiated by an abnormal accumulation of lipids in the liver – that is, hepatic steatosis. This accumulation can be worsened by an increase in free fatty acid and endotoxin levels in the plasma, which activate Toll-like receptors (TLR) and induce, via the NF-κB signalling pathway, the production of pro-inflammatory cytokines (IL-6 and TFNα), which in turn exacerbate inflammation( Reference Thuy, Ladurner and Volynets 2 – Reference Rivera, Adegboyega and van Rooijen 4 ). From a mechanistic point of view, in addition to the excess energy intake, dietary factors such as excessive consumption of fructose-enriched products( Reference Ouyang, Cirillo and Sautin 5 ) and high-fat diets( Reference Henriksen, Curtis and Fillmore 6 ) can act on the liver, with the induction of de novo lipogenesis( Reference Faeh, Minehira and Schwarz 7 , Reference Osei-Hyiaman, Liu and Zhou 8 ) and insulin resistance, and also on the intestine, with increased intestinal permeability and plasma endotoxin levels( Reference Thuy, Ladurner and Volynets 2 ). Endotoxin translocation, and the inflammatory process it induces, can play a major role in the progression from a simple reversible steatosis to NAFLD and non-alcoholic steatohepatitis (NASH)( Reference Buzzetti, Pinzani and Tsochatzis 9 , Reference Bergheim, Weber and Vos 10 ).
Although the role of massive endotoxin translocation from the intestinal lumen into the bloodstream is well known as a trigger of the inflammatory response in sepsis, an increasing number of studies highlight the existence of a low-grade endotoxin translocation in a number of metabolic disorders( Reference De Bandt, Waligora-Dupriet and Butel 11 ). This translocation can be induced or amplified by various factors such as diet (fructose, high-fat diet, etc.) or even by slight alterations in intestinal trophicity. At the whole-body level, this has several consequences, starting with a chronic inflammatory condition contributing to alterations in insulin sensitivity. The liver, as the first organ exposed to these endotoxins via the portal vein, can be the target of inflammatory and oxidative stress leading, for example, to endoplasmic reticulum stress and worsening hepatic damage, with ensuing hepatic dysfunction (NAFLD) and subsequent inflammation and fibrosis (NASH).
Given the high prevalence of NAFLD and its possible evolution, with a significant frequency, towards cirrhosis and hepatocellular carcinoma, the development of strategies to counter this process has become a public health issue. Protection of the liver against stress due to an endotoxin-induced inflammatory process could thus be a new therapeutic target. We set out to assess whether citrulline (Cit) supplementation in the diet could be a useful strategy for this purpose. Indeed, acute or chronic Cit administration can limit inflammation and oxidative stress in various organs including the liver( Reference Jegatheesan, Beutheu and Ventura 12 – Reference Moinard, Le Plenier and Noirez 14 ). We have shown that Cit administration prevents the development of fructose-induced NAFLD( Reference Jegatheesan, Beutheu and Ventura 12 ) and its deterioration into NASH, and also that it is associated with decreased hepatic endoplasmic reticulum stress( Reference Jegatheesan, Beutheu and Freese 15 ).
Our working hypothesis was that, during the initial stages of NAFLD development, hepatic steatosis sensitises the liver to endotoxin-induced injuries, and that this can be prevented by Cit supplementation, by virtue of the anti-inflammatory and antioxidative properties of this amino acid (AA).
The aim of the present study was to evaluate ex vivo, using an isolated perfused rat liver model, the consequences of acute in vivo exposure to endotoxin in a previously validated model of fructose-induced mild NAFLD( Reference Jegatheesan, Beutheu and Ventura 12 ), and the potential protective effect of Cit supplementation in this situation.
Methods
Animals
In all, thirty male Sprague–Dawley rats weighing 190–220 g (Charles River) were acclimatised at our animal facility for 1 week in temperature-controlled rooms with a 12 h light–12 h dark cycle. They had free access to water and standard rodent chow (UAR A04; SAFE).
For feeding experiments, animals received either standard rodent chow, or a 60 % fructose-enriched diet (U8960; SAFE). The compositions of the diets are given in Table 1.
Table 1 Composition of the dietsFootnote *
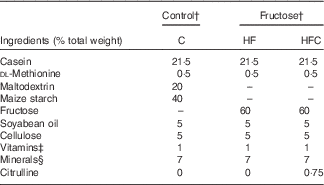
C, control; HF, high fructose; HFC, high fructose + citrulline.
* Control and fructose diets were supplied by SAFE. These diets were prepared from the same sources, except for maltodextrin and starch for the control diet, which were replaced with fructose in the fructose diet.
† Energy content of the diet is 3481 kcal/kg (14565 kJ/kg).
‡ SAFE premix vitamin 200.
§ SAFE premix mineral 205B.
Animal care and experimentation techniques complied with both French and European Community regulations. All procedures were conducted in accordance with the regional ethics committee’s (Comité régional d’éthique pour l’expérimentation animale d’Ile-de-France) guidelines for animal care, and the study protocol was approved by the same committee (registration no. 00737.02).
Experimental design (Fig. 1)
The rats (Fig. 1) were randomly assigned to three groups (n 10 rats/group) to receive, for 4 weeks, a standard rodent chow (control (C)) or a 60 % fructose diet alone (high fructose (HF)) or supplemented with Cit: 1 g/kg per d (high fructose+citrulline (HFC)). The dose of Cit was chosen on the basis of our previous studies( Reference Osowska, Moinard and Neveux 16 – Reference Elwafi, Curis and Zerrouk 18 ). Body weight and food intake were monitored throughout the experiments.

Fig. 1 Citrulline and endotoxin-induced injury in fructose-induced non-alcoholic liver disease: experimental design and main results. P, perfusate; L, liver; LPS, lipopolysaccharide; ALT, alanine aminotransferase; TLR4, Toll-like receptor 4.
Blood samples were taken 2 days before the end of the 4-week feeding period from the tail vein of fasting animals for metabolic evaluation.
On the last day, after an overnight fast, rats were anaesthetised by isoflurane inhalation (4·5 %, Vetflurane; Virbac Schweiz AG) and received a single SC injection of buprenorphine (100 mg/kg, Temgesic; Reckitt Benckiser). The animals then received an intraperitoneal injection of a non-lethal dose of endotoxin (2·5 mg/kg lipopolysaccharide (LPS) from Escherichia coli 0127B:8 serotype; Sigma). After 1 h, the rats were anaesthetised by isoflurane inhalation, and the livers were prepared for ex vivo perfusion and isolated.
All experiments were carried out between 08.00 and 13.00 hours.
Isolated liver perfusion experiments
The livers were prepared according to our standard procedure as described previously( Reference Jourdan, Cynober and Moinard 19 – Reference Schuster, Blanc and Bonnefont-Rousselot 21 ). In brief, immediately after laparotomy, liver ligaments were cut and the bile duct was cannulated. After heparin injection (1000 IU in saline) into the inferior vena cava, a catheter was placed in the portal vein, and the liver was directly perfused with a warm oxygenated perfusion medium at a flow rate of 10 ml/min, isolated and transferred to the perfusion apparatus.
The livers were perfused in a recirculating system via the portal vein at a constant temperature of 37°C and at constant physiological pressure (12 cm H2O). The perfusion medium (150 ml) was a Krebs–Henseleit buffer supplemented with 30 g/l albumin, 8·5 mm glucose and 2 mm Ca. The perfusate was maintained at pH 7·4 and oxygenated with an O2/CO2 mixture (95 %/5 %). At the beginning of the perfusion (t 0), the perfusate was supplemented with an antiproteolytic AA mixture. After a 30-min equilibration period (t 30), a complete AA mixture was added, and the perfusion was continued for 30 min.
Perfusate pH, portal flow and bile flow were continuously monitored. Bile was collected in Eppendorf tubes, and bile flow was estimated by gravimetry, assuming a specific mass of 1 g/ml.
During the perfusion, perfusate samples were taken at t 35, t 45 and t 60 to measure aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) activities, as well as AA, glucose, urea, ammonia and nitrite/nitrate concentrations.
At the end of the perfusion, the livers were weighed on an electronic scale. Samples of the left lobe were immediately frozen in liquid N2.
Sample treatment
Blood samples were collected in heparinised tubes. After centrifugation at 5000 g for 10 min at 4°C, plasma was separated and stored at −20°C until analysis. For the measurement of plasma AA, samples were immediately deproteinised with 30 % sulfosalicylic acid (1:10, v/v) and centrifuged. The supernatants were stored at −80°C.
Perfusate samples were frozen immediately, or after sulfosalicylic acid deproteinisation, and stored at −80°C.
Metabolic evaluation of animals
Plasma concentrations of cholesterol, TAG, urea and glucose, as well as plasma activity of ALT and AST were determined using standard techniques on a multiparameter analyser (Cobas C 6000; Roche).
Plasma insulin concentration was measured with a rat-specific ELISA kit (Rat Ultrasensitive Insulin ELISA; ALPCO Diagnostic) using the manufacturer’s protocol. Insulin sensitivity was evaluated using the homoeostasis model assessment of insulin resistance: (fasting insulin (mU/l)×fasting glucose (mm)/22·5).
Plasma AA were separated and quantified using ion-exchange chromatography with postcolumn ninhydrin detection using a JLC-500/V AminoTac™ amino acid analyser (Jeol Ltd).
Liver metabolism
Perfusate concentrations of urea, ammonia, glucose and AA, as well as plasma activity of ALT, AST and LDH were measured as described above.
Hepatic lipids
For the determination of hepatic TAG content, frozen liver samples (100 mg) were homogenised in 5 % NP-40 to extract lipids. The TAG content was evaluated using a commercially available TAG Quantification Kit (Abcam).
Liver inflammatory and oxidative status
Total RNA from liver samples was isolated using the TRIzol reagent (Invitrogen), and complementary DNA was synthesised using the QuantiTect Rev.Transcription kit (Qiagen). Real-time PCR was performed using the QuantiTect SYBER Green PCR kit (Qiagen) according to the manufacturer’s instructions. To control for variations in the reactions, all PCR data were normalised to β-actin expression. PCR primers for Tlr4 were F: 5'-ATTCCTGGTGTAGCCATTGCT-3', R: 5'-ACCACCACAATAACTTTCCGG-3'. The comparative cycle threshold method was used to quantify the target gene expression, normalised to an endogenous reference gene and relative to a calibrator (2
![]() $$^{{ {^{{{\minus}\Delta \Delta {\rm Ct} }} } }} $$
).
$$^{{ {^{{{\minus}\Delta \Delta {\rm Ct} }} } }} $$
).
Nitric oxide (NO) production was evaluated by the measurement of nitrites+nitrates in the perfusate using the Total Nitric Oxide and Nitrate/Nitrite Assay kit (R&D Systems) after centrifugation of the perfusate samples on a microfilter (Microcon-10kDa Centrifugal Filter Unit; Millipore).
For the measurement of hepatic antioxidative defenses, the liver samples were homogenised in ten volumes of 10 % TCA–0·5 mm EDTA buffer, with an Ultra Turrax homogeniser (Ika Labortechnik). GSH and GSSG in the surpernatant were assayed using reverse-phase HPLC coupled to MS. For vitamin A and vitamin E assays, liver samples were ground in liquid N2. The vitamins were assayed, after hexane extraction, using reverse-phase HPLC and spectrophotometric detection.
Liver oxidative status was evaluated by measuring 4-hydroxynonenal (4HNE) protein adducts in the liver homogenate using an OxiSelect HNE Adduct Competitive ELISA kit (Cell Biolabs Inc.) according to the manufacturer’s protocol.
Hepatic protein content was measured in liver homogenates using a Biorad DC protein assay.
Calculation and statistics
Substrate exchanges (F) during perfusion were calculated as follows:
where C 1 and C 2 are the concentrations of a given substrate at times t 1 and t 2 in two successive samples of perfusate, and V 1 and V 2 the perfusate volumes at the same times, t 1 and t 2. This formula takes into account variations in perfusate volume throughout the experiment due to evaporation (0·2 ml/min) and sampling. W is the liver wet weight. The different exchanges over the whole perfusion period were calculated as the mean of successive values corrected for time. Positive values indicate release of the substrate by the liver, negative values indicate uptake.
Data are presented as means with their standard errors. Prism 6.0 (GraphPad® Software) was used for data analysis. Comparison between the groups was performed using a one-way ANOVA with a Fisher post hoc test. The level of statistical significance was set at P≤0·05.
Results
Nutritional and metabolic status
Plasma TAG and cholesterol concentrations were significantly higher only in the animals fed the HF diet alone, compared with C group animals. Cit tended to decrease fructose-induced hypertriacylglycerolaemia (−31 %), and significantly decreased the plasma cholesterol concentration (P<0·05), compared with the HF group (Table 2).
Table 2 Effect of fructose diet and citrulline on nutritional and metabolic status investigated at the end of the feeding period 2 d before euthanasia (Mean values with their standard errors; n 10 per group)
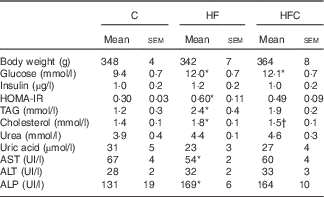
C, control; HF, high fructose; HFC, high fructose+citrulline; HOMA-IR, homoeostatic model assessment – insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase.
* Mean value was significantly different from the C group (P<0·05).
† Mean value was significantly different from the HF group (P<0·05).
Plasma glucose was significantly higher in the HF and HFC groups, but differences in plasma insulin were not statistically significant, owing to large inter-individual variations. However, the HF diet, when given alone, significantly decreased insulin sensitivity; this was attenuated by Cit (Table 2).
Plasma urea and uric acid levels did not differ between the three groups (Table 2). The HF diet alone resulted in limited changes in plasma AA levels. Cit supplementation was associated with significantly higher plasma Cit, ornithine (Orn) and arginine (Arg) levels in the HFC group than in the C and HF groups. The Arg:lysine (Lys) ratio, an important factor for Arg entry into the cells( Reference Wu, Bazer and Davis 22 ), was significantly increased in the HFC group compared with the C and HF groups. Cit also induced a significant decrease in plasma threonine in the HFC group compared with the C group. Global Arg bioavailability (i.e. the ratio of Arg:(Orn+Cit)( Reference Tang, Wang and Cho 23 )) was decreased by the HF diet and restored by Cit, but this decrease did not reach statistical significance (Table 3).
Table 3 Effects of citrulline on plasma amino acids related to arginine availability in fructose-fed rats‡ (Mean values with their standard errors; n 10 per group)
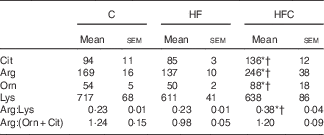
C, control; HF, high fructose; HFC, high fructose+citrulline.
* Mean value was significantly different from the C group (P<0·05).
† Mean value was significantly different from the HF group (P<0·05).
‡ Amino acid concentrations (µmol/l) in plasma were measured at the end of the feeding period 2 d before euthanasia.
Liver-enzyme activities remained within the normal range in all groups (Table 2).
Hepatic function and metabolism during endotoxaemia
After LPS injection, all animals exhibited classic signs of a non-lethal endotoxaemia, with piloerection, prostration and dacryorrhea.
Gross liver appearance was similar in all three groups. Compared with a reference value of about 4 ml/min per g at a physiological portal pressure of 12 cm H2O, portal flow was dramatically increased in all endotoxaemic rats in this study, but was lower in the HFC group than in the C group (C: 10·7 (sem 0·4) ml/min per g, HF: 10·0 (sem 0·6) ml/min per g, HFC: 8·7 (sem 0·5) ml/min per g; HFC v. C, P<0·05). Bile flow was also higher than the reference value, with no difference between groups (data not shown). At the end of the 60-min perfusion, ALT activity in the perfusate was significantly higher in the HF group than in the C group (C: 19 (sem 5) UI/l, HF: 34 (sem 6) UI/l, HFC: 26 (sem 4) UI/l; HF v. C, P=0·05). Final liver wet weight was similar between the three groups (C: 12·3 (sem 0·4) g, HF: 13·0 (sem 1·0) g, HFC: 14·5 (sem 0·9) g; NS).
In terms of global metabolism, only slight changes were observed because of large inter-individual variations (Table 4), with slightly lower urea production and higher ammonia and glucose release in the HF group compared with glucose uptake in the other two groups. The increase in hepatic TAG content in the HF group did not reach statistical significance.
Table 4 Viability and function of the isolated liver in endotoxaemic fructose-fed ratsFootnote * (Mean values with their standard errors; n 6–7 per group)
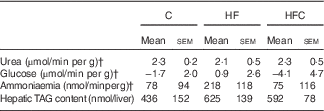
C, control; HF, high fructose; HFC, high fructose+citrulline.
* At the end of the feeding period, livers were isolated from endotoxaemic rats and perfused for 60 min. Hepatic fluxes were calculated from the variations in substrate concentrations in the perfusate between t 35 and t 60. Statistical analysis did not show any differences between groups.
† Negative values indicate uptake, positive ones indicate release.
AA fluxes (Table 5) were similar between the three groups.
Table 5 Hepatic amino acid (AA) fluxes (nmol/min per g) in endotoxaemic fructose-fed ratsFootnote * (Mean values with their standard errors; n 6–7 per group)
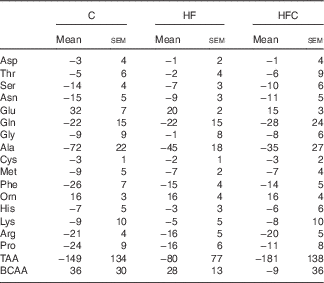
C, control; HF, high fructose; HFC, high fructose+citrulline; TAA, total amino acid flux; BCAA, branched-chain amino acid (Val+Leu+Ile) flux.
* At the end of the feeding period, livers were isolated from endotoxaemic rats and perfused for 60 min. AA fluxes were calculated from the variations in AA concentrations in the perfusate between t 35 and t 60. Negative values indicate uptake, positive ones indicate release. Statistical analysis did not show any differences between groups.
Hepatic inflammation and oxidative stress (Table 6)
As regards LPS-induced (Table 6) hepatic inflammation, fructose feeding tended to increase Tlr4 gene expression (P=0·084), but did not affect NO production. In the Cit-supplemented group, NO production and Tlr4 expression were similar to those in the C group (Fig. 2).

Fig. 2 Hepatic inflammatory status in endotoxaemic fructose-fed rats. At the end of the feeding period with a control or a fructose diet, livers were isolated from endotoxaemic rats and perfused for 60 min. Hepatic inflammatory status was evaluated by liver Tlr4 gene expression (presented as its ratio to β-actin) at the end of the 60-min perfusion (a) and by nitrites+nitrates concentration in the perfusate (b). C, control; HF, high fructose; HFC, high fructose+citrulline; Tlr4, Toll-like receptor 4. Values are means (n 6–7 per group), with standard errors represented by vertical bars. * HF v. C and HFC (P=0·084).
Table 6 Hepatic oxidative status in endotoxaemic fructose-fed ratsFootnote * (Mean values with their standard errors; n 6–7 per group)
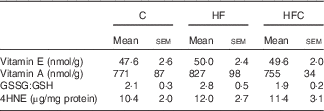
C, control; HF, high fructose; HFC, high fructose+citrulline; 4HNE, 4-hydroxynonenal.
* At the end of the feeding period, livers were isolated from endotoxaemic rats and perfused for 60 min. Hepatic oxidative status was measured at the end of the 60-min perfusion.
As regards oxidative status, the hepatic content of vitamins A and E, and GSSG:GSH ratio were similar in the three groups. LPS-induced oxidative stress, as evaluated by the hepatic content of 4HNE-protein adducts, was also similar between the three groups.
Discussion
In agreement with our previous studies( Reference Jegatheesan, Beutheu and Ventura 12 , Reference Jegatheesan, Beutheu and Ventura 24 ), our results confirm, in a model of mild NAFLD, that Cit supplementation in the diet improves the metabolic status of fructose-fed rats. Our data further show that in these conditions, hepatic steatosis is associated with increased sensitivity to LPS-induced alterations, compared with conventionally fed rats, and suggest that the preventive effects of Cit on the progression of NAFLD occur via a reduction in LPS-induced inflammation without modifications in oxidant/antioxidant status (Fig. 1).
In line with literature data, feeding a 60 % fructose diet to rats is associated with dyslipidaemia and decreased insulin sensitivity, as observed in other fructose-fed rat models( Reference Sievenpiper, de Souza and Cozma 25 ). In another set of experiments( Reference Jegatheesan, Beutheu and Ventura 12 ), we showed that 4-week fructose feeding, as used in the present experiments, led to dyslipidaemia and hepatic steatosis with increased hepatic TAG content and microvesicular steatosis. At this stage, hepatic aminotransferases remain in the normal range, making it a model of mild NAFLD. As previously described, the present data also show that Cit supplementation has a positive effect on these parameters. Besides specific effects on the liver, as discussed below, the protective effects of Cit may occur via several mechanisms( Reference Jegatheesan and De Bandt 13 ). In vivo studies in various conditions have shown that Cit supplementation is associated with improved muscle protein metabolism, decreased total and visceral fat mass, improved gut-barrier function and prevention of hepatic steatosis. Some of these studies showed a direct effect of Cit on adipocytes via a NO-dependent process, and on muscle cells through PI3K/MAPK/4E-BP1 signalling( Reference Jegatheesan and De Bandt 13 , Reference Le Plénier, Goron and Sotiropoulos 26 ). Cit supplementation is also associated with improved insulin sensitivity( Reference Capel, Chabrier and Pitois 27 ) and endothelial function( Reference Figueroa, Wong and Jaime 28 ). Interestingly, in the experiments of the present study, Cit supplementation was found to be associated with higher plasma Arg, a trend toward normalisation of the Arg:Orn+Cit ratio – a marker of global Arg availability for NO synthesis( Reference Tang, Wang and Cho 23 ) – and with a higher Arg:Lys ratio – a marker of Arg entry into the cells( Reference Wu, Bazer and Davis 22 ). Taking into account the role of NO in the peripheral effects of insulin( Reference Barrett, Wang and Upchurch 29 , Reference de Castro Barbosa, Jiang and Zierath 30 ), the role of NO synthesis from Arg in Cit-related effects needs to be investigated.
We note that the model used in our experiment is one of mild steatosis, compared with other studies from our laboratory( Reference Jegatheesan, Beutheu and Ventura 12 , Reference Jegatheesan, Beutheu and Ventura 24 ). This condition was chosen because hepatic alterations that favour the development and progression of NAFLD are cumulative and may occur before NAFLD is fully established. Given our experimental conditions, it was not possible to determine liver lipid content before perfusion experiments; hepatic steatosis was thus difficult to evaluate in our experiments. Only a slight increase in hepatic lipid content was noted in the HF group at the end of isolated liver perfusion. As hepatic TAG content was measured only at the end of the perfusion experiments, this was probably a consequence of our experimental conditions and the combination of the mildness of our model of steatosis and the ability of endotoxin to induce hepatic TAG release( Reference Arisqueta, Nuñez-Garcia and Ogando 31 ). Unfortunately, we were unable to determine TAG release precisely during our ex vivo experiments, owing to the too-low TAG concentration in the perfusion medium.
In this context of mild steatosis, fructose feeding tended to increase the susceptibility of the liver to LPS-induced inflammation, and this effect was dampened by Cit supplementation. Moreover, the response to an acute non-lethal endotoxaemia does not seem to be associated with higher hepatic oxidative stress, at least in the short term.
Our experimental conditions were chosen in order to induce transient non-lethal inflammation( Reference Chambon-Savanovitch, Felgines and Farges 32 ). Although this dose of LPS is not considered very high, it is eight times higher than that used by Cani et al.( Reference Cani, Amar and Iglesias 33 ) to obtain LPS levels similar to those found in metabolic endotoxaemia. However, comparison is difficult because both animal species (rats v. mice) and modes of administration (intraperitoneal bolus v. subcutaneous continuous perfusion) differ. We note that in Cani’s study LPS concentration was measured in the peripheral blood; given the role of the liver in endotoxin clearance( Reference Smedsrød, De Bleser and Braet 34 ), it is reasonable to assume that in the postprandial period, LPS level in the portal vein reached notably higher values, but probably not the level used in our experiments. We chose to isolate and perfuse the liver of animals used in our study 1 h after the LPS challenge, taking into account the kinetics of hepatic inflammatory response to LPS( Reference Hamburger, Broecker-Preuss and Hartmann 35 , Reference Nishiokada, Miyoshi and Fujiwara 36 ); this enabled us to evaluate direct effects of LPS on the liver, largely independent of the systemic response to LPS. Insofar as our aim was to detect possible sensitisation of the liver to endotoxin as a consequence of steatosis, and given the fact that Cit may not suppress – but simply mitigate – the response to LPS, only endotoxaemic rats were evaluated.
As regards liver function, the HF group presented portal and bile flows similar to those in the C group, but showed increased ALT activity in the perfusate. Interestingly, all groups presented a high portal flow relative to the applied physiological portal pressure (12 cm H2O). This may be related to the well-known stimulatory effect of LPS on inducible NO synthase and the vasodilatory effects of NO( Reference Ebisawa, Kono and Yoneda 37 ). Increased ALT release in the HF group suggests that fructose feeding is associated with a higher susceptibility to LPS-induced liver injury, consistent with the role of fructose in the development of cellular stress and a hepatic pro-inflammatory state( Reference Dekker, Su and Baker 38 ). This pro-inflammatory state is further confirmed by the trend towards higher Tlr4 expression in the same group. This probably explains the few perturbations observed in hepatic metabolism, with lower ureagenesis and higher ammonia release observed in the same group. Changes in AA exchange between groups were not significant, owing to large inter-individual variations. Surprisingly, fructose feeding was not associated with a higher LPS-induced oxidative stress, as demonstrated by similar levels of antioxidative vitamins and 4HNE, a marker of lipid peroxidation. Our data thus suggest that, at least in the initial stages of hepatic steatosis, the development of NAFLD is not a consequence of oxidative stress, but rather of fructose metabolism itself and of endoplasmic reticulum stress. However, although some alterations in hepatic metabolism – for example, in bromosulfophthalein clearance and excretion( Reference Utili, Abernathy and Zimmerman 39 ) – have been shown to occur very rapidly after exposure to LPS, we cannot rule out the possibility that other alterations may need more time to develop, a point that deserves further research.
In this setting, Cit prevents the LPS-induced increase in ALT release observed in the HF group and the associated increase in Tlr4 expression, suggesting decreased inflammation. It would have been of interest to measure pro-inflammatory cytokine production to make a fuller investigation of the inflammatory process; NO production was similar between the three groups, but we had previously observed( Reference Breuillard, Bonhomme and Couderc 40 ) in isolated peritoneal macrophages from Zucker diabetic fatty rats that Cit did not affect NO synthesis, but decreased TNF release. Surprisingly, oxidative stress was not affected by Cit supplementation. This contrasts with previous studies from our group( Reference Jegatheesan, Beutheu and Ventura 12 , Reference Jegatheesan and De Bandt 13 , Reference Jegatheesan, Beutheu and Freese 15 ); however, in these studies, oxidative stress was evaluated under conditions of chronic metabolic stress. Moreover, in the experiments of the present study, animals were studied in the fasted state – that is, at least 18 h after the Cit-supplemented diet had been withdrawn. It would be of interest to evaluate the effect of Cit on oxidative status in the postprandial phase.
As regards the mechanisms involved, besides Cit peripheral effects, our in vivo experiments( Reference Jegatheesan, Beutheu and Freese 15 ) and recent experiments in a hepatocyte cell line( Reference Tennoune, Bouslah and Le Plénier 41 ) indicate that Cit acts directly on the liver. In vivo, we showed that Cit prevents fructose-induced expression of the transcription factors SREBP1c and CHREBP, key inducers of hepatic de novo lipogenesis, and that it increases the expression of PPARα, favouring fat oxidation. This was associated with decreased endoplasmic reticulum stress and decreased expression of the pro-apoptotic CCAAT/enhancer-binding protein homologous protein (CHOP) protein( Reference Jegatheesan, Beutheu and Freese 15 , Reference Jegatheesan, Beutheu and Ventura 24 ). Our in vitro experiments on a model of fructose-induced steatosis in the hepatic H4IIE cell line( Reference Tennoune, Bouslah and Le Plénier 41 ) confirmed these observations, supporting the hypothesis of a direct effect of Cit on hepatic endoplasmic reticulum stress.
All these metabolic and liver function data suggest that 4-week fructose feeding is associated with increased liver sensitivity to the inflammatory effect of LPS, but not to LPS-induced oxidative stress. These data do not therefore support the idea of oxidative stress as a major factor contributing to the initiation of NAFLD. In this context, our data show that Cit supplementation reduces LPS-induced liver inflammation, independently of oxidative stress.
Acknowledgements
The authors thank the staff of the animal facility of the Faculty of Pharmacy, Paris Descartes University.
This project was supported by a French National Research Agency (ANR) grant (ANR-11-BSV1-0015, NAFLD-citrulline).
J.-P. D. B. and P. J. designed the study; W. O., P. J., J. M.-B., C. V., S. N., E. N. carried out the experiments and/or the biological assays; J.-P. D. B. and P. J. wrote the manuscript; all authors have read and approved the final manuscript.
Professor J.-P. D. B. is a shareholder of Citrage Company.











