Non-technical summary
A newly discovered trilobite fauna from the Cambrian Honey Creek Formation marks a distinct interval that follows an extinction event. Dominated by the genus Monocheilus in association with Ptychaspis, it resembles faunas from Alberta, Canada, and the Upper Mississippi Valley region of the United States. Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959 is a species that has been reported widely in North America. However, restudy of various museum collections shows that the various occurrences record a set of more narrowly distributed species. The pattern of distribution is similar to groups of modern “pseudocryptic species” identified by a combination of genetic and anatomical data.
Introduction
Cambrian trilobites of Oklahoma have been studied intensively for 70 years (e.g., Frederickson Reference Frederickson1948, Reference Frederickson1949; Stitt, Reference Stitt1971, Reference Stitt1977; Westrop et al., Reference Westrop, Waskiewicz Poole and Adrain2010), so it is surprising to discover an entirely new fauna in the succession. This fauna is of interest because it includes early representatives of two major clades (Ptychaspididae Raymond, Reference Raymond1924 and Eurekiidae Hupé, Reference Hupé1953) that radiated in Laurentia during the Sunwaptan Stage. The composition of the fauna, which is dominated by Monocheilus Resser, Reference Resser1937 and also contains Ptychaspis Hall, Reference Hall1863, resembles assemblages that occur in the same homotaxial position in Minnesota and Wisconsin (e.g., Nelson, Reference Nelson1951) and in the southern Canadian Rocky Mountains (Westrop, Reference Westrop1986). As such, these assemblages may mark a distinct biostratigraphic interval in a transition from low-diversity faunas in the aftermath of the end-Steptoean extinction to more-diverse Sunwaptan faunas (e.g., Westrop and Cuggy, Reference Westrop and Cuggy1999).
Monocheilus (considered here to be a senior synonym of Stigmacephalus Resser, Reference Resser1937; see Systematic paleontology, which follows) is the earliest representative of the family Eurekiidae, and the occurrence of Ptychaspis is likely the oldest record of the family Ptychaspididae. Early representatives of Ptychaspis have often been assigned to a single species, P. bullasa Lochman and Hu, Reference Lochman and Hu1959 (e.g., Bell and Ellinwood, Reference Bell and Ellinwood1962; Stitt, Reference Stitt1971, Reference Stitt1977). Restudy of type material shows that there is in fact a plexus of pseudocryptic species (e.g., Westrop and Adrain, Reference Westrop and Adrain2007; Westrop et al., Reference Westrop, Landing and Dengler2018) that are differentiated readily by cranidial and pygidial anatomy (see Systematic paleontology).
Stratigraphy and study area
The study area lies in the Slick Hills, immediately north of the Wichita Mountains. The Honey Creek Formation and the underlying Reagan Sandstone compose the Timbered Hills Group (Stitt, Reference Stitt1971). The Timbered Hills onlaps the Carlton Rhyolite, which was exposed in late Cambrian Oklahoma as a volcanic archipelago with at least 300 m of relief (Donovan, Reference Donovan1986; Donovan and Bucheit, Reference Donovan and Bucheit2000; Donovan et al., Reference Donovan, Ayan and Bucheit2000). The boundary between the Reagan and the Honey Creek is gradational through an interval of sandstone with bioclastic carbonate interbeds and lenses. Following Donovan and Ragland (Reference Donovan and Ragland1986), we place the base of the Honey Creek at the lowest occurrence of bioclastic carbonate, and this definition differs from the one offered by Stitt (Reference Stitt1977, p. 5), who placed the boundary above the highest sandstone interbed. Lithologically, the formation is a pelmatzoan-rich, glauconitic, bioclastic grainstone and rudstone with thin siliciclastic drapes. Cross-bedding and ripple marks are common, as are sandstone interbeds. Heterolithic intervals are composed of fine- to medium-grained sandstone with recessively weathering bioclastic carbonate lenses (Westrop et al., Reference Westrop, Waskiewicz Poole and Adrain2010, fig. 1). Bucheit and Donovan (Reference Bucheit and Donovan2000, fig. 16) interpreted the Honey Creek as a tidally influenced deposit that formed between rhylolite islands.
The Fort Sill Formation (lowest unit of the Arbuckle Group) succeeds the Honey Creek Formation and is composed of lime mudstone–wackestone (Stitt, Reference Stitt1971; Donovan and Ragland, Reference Donovan and Ragland1986). Intraclastic rudstone is also present, and microbial buildups occur in the upper part of the formation (Stitt, Reference Stitt1971). Quartz sand and glauconite are minor components, suggesting that the archipelago was largely flooded during deposition of the Fort Sill.
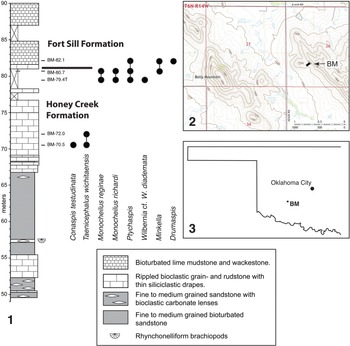
The trilobites were collected from the succession in the Bally Mountain region (section BM) of Kiowa County, which was deposited near a rhyolite island (Donovan and Bucheit, Reference Donovan and Bucheit2000). The section (Fig. 1.1) was measured and logged on the west-facing slope of an unnamed ridge about one kilometer to the east of Bally Mountain (Fig. 1.2, 1.3; 34°57′39″N, 98°39′03″W). Elvinia Zone faunas are well represented in a carbonate unit about 52.5 m above the base of the section. An overlying 12 m sandstone unit did not yield trilobites, but Taenicephalus wichitaensis Resser, Reference Resser1942 and Conaspis testudinata Ellinwood in Bell and Ellinwood, Reference Bell and Ellinwood1962 are present in the succeeding carbonate unit, between 70.5 m and 72 m above the base of the section (Fig. 1.1); the systematics of these species will be treated elsewhere. The newly discovered, low-diversity fauna dominated by new two species of Monocheilus, M. reginae and M. richardi (Figs. 2–6), occurs about 8 m higher in the section, in the Honey Creek–Fort Sill boundary interval (Fig. 1.1). It is referred to here as the Monocheilus reginae fauna.

Figure 1. (1) Stratigraphic column and species range chart for the Honey Creek–Fort Sill boundary interval, Bally Mountain section (BM), Kiowa County, Oklahoma. (2) Map showing the location of the section. (3) Map showing location of the study area in Oklahoma.

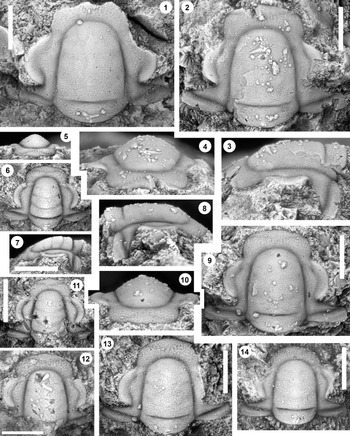
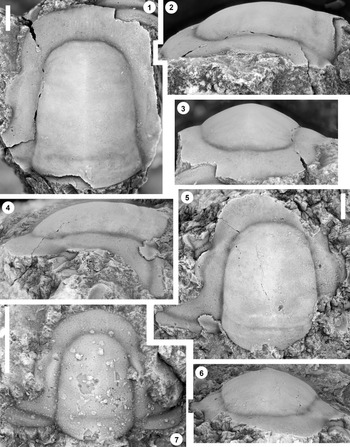
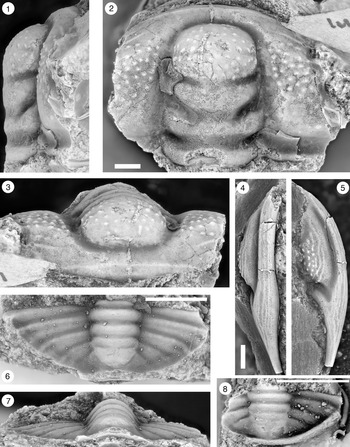
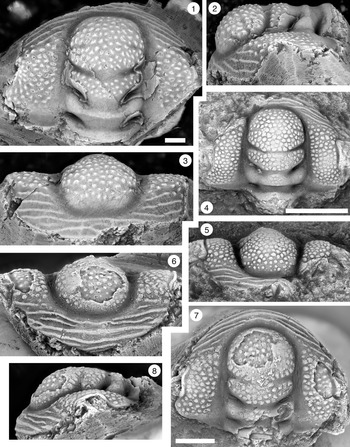
Figure 2. Monocheilus reginae n. sp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are cranidia from collection BM 79.4T. All are paratypes. (1) OU 238356, dorsal view. (2–4) OU 238357: (2) dorsal view; (3) lateral view; (4) anterior view. (5–7) OU 238358: (5) anterior view; (6) dorsal view; (7) lateral view. (8–10) OU 238359: (8) lateral view; (9) dorsal view; (10) anterior view. (11) OU 238360, dorsal view. (12) OU 238361, dorsal view. (13) OU 238362, dorsal view. (14) OU 238363, dorsal view. Scale bars = 1 mm.

Figure 3. Monocheilus reginae n. sp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are cranidia from collection BM 79.4T. (1–3) Paratype OU 238364: (1) dorsal view; (2) anterior view; (3) lateral view. (4) Paratype OU 238164, dorsal view. (5–7) Holotype OU 238158: (5) anterior view; (6) dorsal view; (7) lateral view. (8, 9) paratype OU 238365: (8) dorsal view; (9) anterior view. (1–7) Scale bars = 2 mm; (8, 9) scale bar = 1 mm.

Figure 4. Plot of palpebral lobe length against preoccipital glabellar length for Monocheilus reginae (black fill) and M. richardi (white fill). Reduced major axis regression lines were fitted in PAST v.4.09 (Hammer et al., Reference Hammer, Harper and Ryan2001). For M. reginae, y = 0.39x + 0.37; for M. richardi, y = 0.275x + 0.32. Comparisons of slopes in PAST showed that they were significantly different (p < .0002).

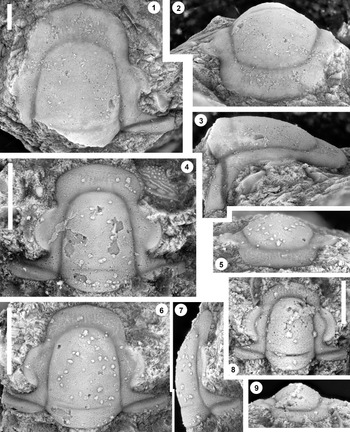
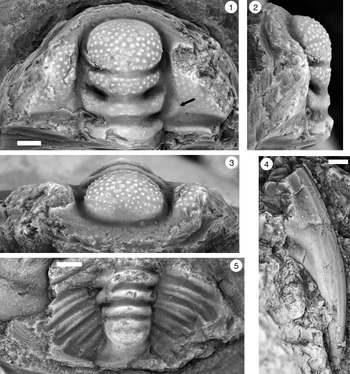
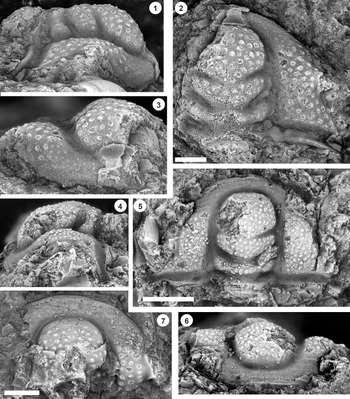
Figure 5. Monocheilus richardi n. sp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are cranidia from collection BM 79.4T. All are paratypes. (1–3) OU 238366: (1) dorsal view; (2) lateral view; (3) anterior view. (4–6) OU 238163: (4) lateral view; (5) anterior view; (6) dorsal view. (7–9) OU 238367: (7) anterior view; (8) lateral view; (9) dorsal view. (10–12) OU 238368a: (10) lateral view; (11) anterior view; (12) dorsal view (associated free cheek, OU 238368b, is visible). (13) OU 238369, dorsal view. (14) OU 238370, dorsal view. Scale bars = 1 mm.

Figure 6. Monocheilus richardi n. sp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are cranidia from collection BM 79.4T. (1–3) Paratype OU 238371: (1) dorsal view; (2) lateral view; (3) anterior view. (4–6) Holotype OU 238372: (4) lateral view; (5) dorsal view; (6) anterior view. (7) Paratype OU 238373, dorsal view. Scale bars = 2 mm.
Ptychaspis is rare at the Bally Mountain section, and our study includes archival material from elsewhere in Oklahoma as well as Texas and Idaho. The type material of P. matuszaki n. sp. was collected from exposures of the Fort Sill Formation, along a section-line road 4.4 km southeast of Hennepin (SE Sec. 4, T1S, R1W; 34°29′46″N, 97°18.0′4″W) separating sections 3 and 4, Murray County, Oklahoma (Matuszak, Reference Matuszak1957). This site is 450 m northeast of section DR of Westrop and Adrain (Reference Westrop and Adrain2007, p. 989, fig. 1c). Sclerites assigned to P. occulta n. sp. and Ptychaspis spp. are from the Morgan Creek Member, Wilberns Formation, central Texas, and were illustrated previously by Bell and Ellinwood (Reference Bell and Ellinwood1962) and Longacre (Reference Longacre1970) under the name Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959. Revision of P. bullasa is based on restudy of the type material from the Mink Creek region, near Preston, southern Idaho (Lochman and Hu, Reference Lochman and Hu1959).
Age and correlation of the Monocheilus reginae fauna
The Monocheilus reginae fauna occurs in two collections (Fig. 1.1). The lower of these (BM 79.4T) is a float sample of trilobite grain- to rudstone from a covered interval 1.6 m below the base of the Fort Sill Formation. It includes abundant sclerites of Monocheilus and rare specimens of Ptychaspis and Wilbernia Walcott, Reference Walcott1924. Collection BM 80.7 is lithologically similar to BM 79.4T but was recovered in place 1.3 m higher in the section and 30 cm below the base of the Fort Sill. Monocheilus and Ptychaspis are both minor components of this collection, with Minkella Lochman and Hu, Reference Lochman and Hu1959 dominating. The latter genus is under study by S.R.W. and will not be treated in this paper.
Both species of Monocheilus described in this paper are related to M. oweni (Hall, Reference Hall1863). However, that species is based on sandstone internal molds that provide no information on such features as sculpture of the exoskeleton and, as a result, is difficult to evaluate. It is best restricted to the types (see discussion of M. richardi that follows), and we use the shutter-mark convention (Wiley, Reference Wiley1979) in the following discussion to underscore uncertainty about reports of the species in various regions. Monocheilus “oweni” (under the name Stigmacephalus) has been reported from a collection (B57) from the Bison Creek Formation of Alberta (Westrop, Reference Westrop1986), which, as in Oklahoma, also includes Ptychaspis and a species of Wilbernia. Westrop (Reference Westrop1986, p. 17) placed this collection in his Stigmacephalus oweni fauna, which encompassed a poorly fossiliferous interval whose base was defined broadly by the first occurrences of either the name-bearing species, Idahoia cf. I. lirae (Frederickson, Reference Frederickson1949) or Taenicephalina sp. 1. Idahoia cf. I. lirae is distinct from Frederickson's types of I. lirae from Oklahoma (Westrop, Reference Westrop1986, pl. 16, figs. 5–7) by virtue of, among other characters, a long occipital spine (Westrop, Reference Westrop1986, p. 42). This species does not co-occur with M. “oweni” in any of Westrop's (Reference Westrop1986) sections: Idahoia cf. I. lirae occurs in his section D, whereas M. “oweni” is from his sections B and S. As a result, there is some uncertainty about the relative ranges of these two species, although M. “oweni” seems to extend into younger strata (see Westrop, Reference Westrop1986, p. 18 for discussion). In Oklahoma, the M. reginae fauna lies above the I. lirae Zone, which is present in the Honey Creek Formation about 16 km to the southeast at section KR1 (see Westrop et al., Reference Westrop, Waskiewicz Poole and Adrain2010 for locality information), 3.1 m above the highest occurrence of Taenicephalus wichitaensis Resser, Reference Resser1942 (unpublished data, Blackwell and Westrop, 2023). The evidence suggests that the ranges of M. reginae, M. richardi, and M. oweni likely overlap, and the M. oweni fauna and the M. reginae fauna may be broadly correlative.
In the Minnesota–Wisconsin border region of the St. Croix Valley (Nelson, Reference Nelson1951), M. “oweni” occurs above the Conaspis Zone (= Taenicephalus Zone; Grant, Reference Grant1965), confirming that this species is among the older representatives of the genus. Sections farther to the south, along the bluffs of the Mississippi (Grant, Reference Grant1962), apparently lie above the local range of M. “oweni” and instead contain M. anatinus (Hall, Reference Hall1863) in association with Ellipsocephaloides curtus (Whitfield, Reference Whitfield1878) and Idahoia wisconsinesis (Owen, Reference Owen1852) (e.g., Grant, Reference Grant1962, text-figs. 3, 6), among others. Both of the latter two species enter the succession in Alberta above the M. oweni fauna (Westrop, Reference Westrop1986).
Materials and methods
Unless indicated otherwise, treatment of each species is based on the figured specimens. Specimens were coated with a sublimate of ammonium chloride before photography. Depth of field was maximized by rendering digital images from stacks of images focused at 100 μm intervals using Helicon Focus 4.0 for the Macintosh <http://www.heliconsoft.com>. Proportions expressed in percentages in descriptions and diagnoses are means, with the following pair of numbers indicating the range of values. All measurements were made on digital images to the nearest tenth of a millimeter using the Measure Tool of Adobe Photoshop.
Repositories and institutional abbreviations
Illustrated specimens are housed at the Oklahoma Museum of Natural History, University of Oklahoma, Norman (OU), and at the National Museum of Natural History, Washington D.C. (USNM).
Systematic paleontology
Family Eurekiidae Hupé, Reference Hupé1953
Remarks
Adrain and Westrop (Reference Adrain and Westrop2004, p. 19) raised the possibility that Monocheilus Resser, Reference Resser1937 and Stigmacephalus Resser, Reference Resser1937 were related to younger Sunwaptan genera assigned to the family Eurekiidae. As discussed in the following, these genera are considered to be synonyms, and Resser (Reference Resser1937) published both names in the same publication. As first revisers, we choose Monocheilus as the senior synonym because the type material (Hall, Reference Hall1863) includes both cranidia and a pygidium (e.g., Westrop, Reference Westrop1986, pl. 14, figs. 1, 2).
Although pygidia of typical species of Eurekiidae, including those of Eurekia Walcott, Reference Walcott1916 and Corbinia Walcott, Reference Walcott1924, possess as many as six pairs of marginal spines and well-defined pleural furrows (e.g., Adrain and Westrop, Reference Adrain and Westrop2004, pl. 12, pl. 13, figs. 18–20; Westrop et al., Reference Westrop, Palmer and Runkel2005, figs. 2.10–2.12, 8.1–8.3, 8.6–8.10), Wisarcadiaspis Westrop and Palmer, Reference Westrop and Palmer2009 is much closer to Monocheilus. Although more convex than Monochelius, the pygidium has an aspinose margin and weak pleural furrows (Westrop et al., Reference Westrop, Palmer and Runkel2005, fig. 6). The axis terminates well short of the posterior margin, and like Monocheilus (e.g., Westrop, Reference Westrop1986, pl. 15, fig. 4), it is composed of at least three segments, with two typically incorporated into the terminal piece. Thus, the presence of only a single pair of marginal spines in some species of Monocheilus is not a barrier to a phylogenetic relationship with other species of Eurekiidae.
Unlike typical Eurekiidae, the anterior border of Monocheilus lacks independent convexity, but the backwardly bowed border furrow (e.g., Figs. 2.2, 2.6. 2.11, 2.12, 3.6. 3.9; Westrop, Reference Westrop1986, pl. 15, figs. 7, 8) is similar to those in some species of Corbinia (e.g., Adrain and Westrop, Reference Adrain and Westrop2004, pl. 13, figs. 2, 13; Westrop et al., Reference Westrop, Palmer and Runkel2005, fig. 7) and Wisarcadiaspis (e.g., Westrop et al., Reference Westrop, Palmer and Runkel2005, fig. 4). Effacement of the anterior border and border furrow, which occurs later in ontogeny (e.g., Figs. 3.1, 3.2, 6.1–6.3; Westrop, Reference Westrop1986, pl. 15, figs. 1, 2), is matched in “Bayfieldia” simata Winston and Nicholls, Reference Winston and Nicholls1967 (pl. 9. figs. 20, 25). Moreover, the long genal spine of Monocheilus (e.g., Westrop, Reference Westrop1986, pl. 15, fig. 9), which contrasts with the miniscule spines of typical eurekiid librigenae (e.g., Westrop et al., Reference Westrop, Palmer and Runkel2005, fig. 8.4, 8.5), is also a feature of Wisarcadiaspis.
There are enough similarities in characters to provisionally view Monocheilus as a basal member of Eurekiidae. Further evaluation of this hypothesis must await a computer-based phylogenetic analysis of Eurekiidae that will also need to include a revision of “Bayfieldia” simata Winston and Nicholls, Reference Winston and Nicholls1967 (including their “var. A,” which represents a distinct species), which combines a Monocheilus-like cranidium with a conventional eurekiid pygidium that includes five to six pairs of marginal spines and well-furrowed pleural fields (e.g., Winston and Nicholls, Reference Winston and Nicholls1967, pl. 9, figs. 20, 23–26).
Genus Monocheilus Resser, Reference Resser1937
Type species
Conocephalites anatinus Hall, Reference Hall1863 from the Lone Rock Formation, Wisconsin (by original designation).
Diagnosis
Eurekiidae with preglabellar field of cranidium separated from anterior border by weak, backwardly curved anterior border furrow in small cranidia that becomes effaced in larger holaspids, producing an undifferentiated frontal area. Glabella parallel-sided to gently tapered anteriorly with very weakly incised glabellar furrows barely perceptible in larger holaspids. Transversely subelliptical pygidium bearing one to four pairs of short, triangular marginal spines. Short, convex axis consists of one axial ring and terminal piece composed of at least two segments. Pygidial pleural field is nearly flat.
Remarks
Previous authors (e.g., Grant, Reference Grant1962; Westrop, Reference Westrop1986) have treated Monocheilus and Stigmacephalus as distinct genera. Monocheilus reginae n. sp. (Figs. 2, 3) and M. richardi n. sp. (Figs. 5, 6) from the uppermost Honey Creek Formation are clearly related to the type species of Stigmacephalus, S. oweni (Hall, Reference Hall1863), and provide new information on the librigena and pygidium. They also provide character support for synonymy of Monocheilus and Stigmacephalus. Cranidia of M. reginae and M. richardi differ in the size of the palpebral lobes, with the relatively long-eyed M. reginae resembling such species as M. micros (Walter, Reference Walter1924) (Westrop, Reference Westrop1986, pl. 15, figs. 1–3, 5, 6). By contrast, M. richardi has smaller palpebral lobes that are comparable to S. oweni (e.g., Nelson, Reference Nelson1951, pl. 109, figs. 1, 2; Westrop, Reference Westrop1986, pl. 15, figs. 10, 11). As such, M. reginae and M. richardi bridge the cranidial morphologies of Monocheilus and Stigmacephalus.
As will be discussed, there appear to be two pygidial morphotypes associated with cranidia of M. reginae and M. richardi in BM 79.4T that differ in outline (Fig. 7.1–7.5 and Fig. 7.6, 7.7, respectively; species assignments are uncertain), and both are very similar to those of M. anatinus (Hall, Reference Hall1863; Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 33, fig. 5a; Westrop, Reference Westrop1986, pl. 14, fig. 3) and M. micros (Walter, Reference Walter1924; Westrop, Reference Westrop1986, pl. 15, fig. 4). In particular, all of these pygidia share triangular spines on the posterior corners and a single pair of broad, shallow pleural furrows on a relatively flat pleural field. The axis is short, occupying about half of pygidial length, with one well-defined axial ring and a second ring that is partly fused with the terminal piece. They lack a border and border furrow. All are distinct from the specimen attributed to S. oweni by Nelson (Reference Nelson1951, pl. 109, fig. 9), which has a long axis that terminates close to the pygidial margin, a narrow border, and distinct border furrow and lacks marginal spines. It is almost certainly misassigned, and consequently, use of pygidial characters to separate Monocheilus and Stigmacephalus (e.g., Westrop, Reference Westrop1986, p. 87) can no longer be justified.

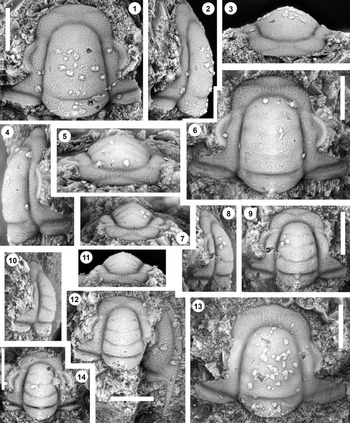
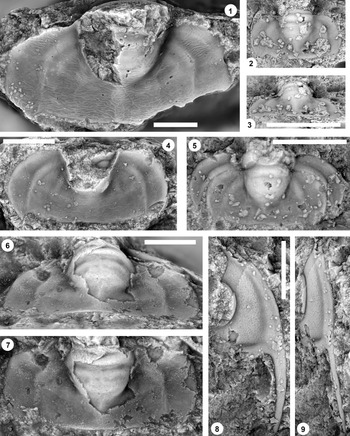
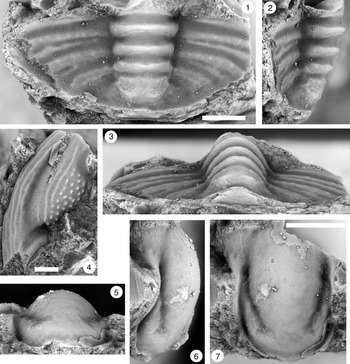
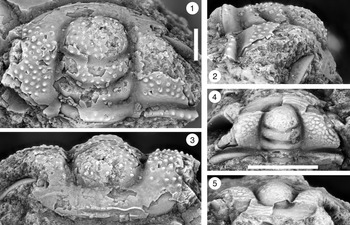
Figure 7. (1–7) Monocheilus spp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are pygidia from collection BM 79.4T. (1) OU 238374, dorsal view. (2, 3) OU 238375: (2) dorsal view; (3) posterior view. (4) OU 238162, dorsal view. (5) OU 238376, dorsal view. (6, 7) OU 238161: (6) posterior view; (7) dorsal view. (8, 9) Monocheilus reginae n. sp. from collection BM 79.4T, librigena, OU 238377: (8) dorsal view; (9) lateral view. Scale bars = 2 mm.
Librigenae of M. micros (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 33, fig. 4a; Westrop, Reference Westrop1986, pl. 15, fig. 9) and M. reginae (Fig. 7.8, 7.9) are very similar, with long genal spines, and are separable only on the basis of sculpture: the former has a smooth external surface, like the rest of the cephalon, whereas the latter is pitted. Aside from sculpture, cranidia of these two species are differentiated primarily on the position of the palpebral lobe. Monocheilus reginae resembles S. oweni (e.g., Westrop, Reference Westrop1986, pl. 15, figs. 11, 13) in possessing a palpebral lobe that is separated from the glabella both anteriorly and posteriorly by a distinct, continuous band of fixigena (Figs. 2, 3), as does M. richardi (Figs. 5, 6). By contrast, larger cranidia of M. micros (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 33, fig. 4b; Westrop, Reference Westrop1986, pl. 15, fig. 1) have palpebral lobes that are located closer to the glabella so that the palpebral furrows and axial furrows merge anteriorly, and only the posterior tip of the lobe is separated by a narrow strip of fixigena; similar palpebral lobe positions characterize M. anatinus (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 33, fig. 5c, pl. 34, fig. 2; Westrop, Reference Westrop1986, pl. 14, fig. 1), M. truncatus Ellinwood in Bell and Ellinwood, Reference Bell and Ellinwood1962 (e.g., Westrop, Reference Westrop1986, pl. 14, figs. 3–5), and M. orestes Westrop, Reference Westrop1986 (pl. 15, figs. 14–17). However, small cranidia of M. micros clearly have palpebral lobes that are farther from the glabella (Westrop, Reference Westrop1986, pl. 15, fig. 7), resembling the condition in M. reginae and M. richardi, and the palpebral lobe shifts toward the glabella during holaspid ontogeny. The polarity of ontogenetic change indicates that a palpebral lobe and, therefore, eyes that are located close to the glabella represent the apomorphic condition. This is also supported by potential outgroups such as Minkella (e.g., Westrop, Reference Westrop1986, pl. 14, figs. 7–16), in which the palpebral lobe is separated from the glabella by a broad, continuous band of fixigena. Stigmacephalus appears to rest only on the retention of this plesiomorphic character state, and recognition of Monocheilus on the basis of a palpebral lobe that abuts the glabella likely makes Stigmacephalus paraphyletic. Stigmacephalus is therefore treated as a junior synonym of Monocheilus. As revised here, Monocheilus includes, at minimum, M. anatinus, M. oweni, M. oweni var. A of Nelson, Reference Nelson1951 (which represents a distinct species; see Westrop, Reference Westrop1986, p. 89), M. micros, M. truncatus, M. orestes, M. reginae, and M. richardi. Pygidial characters, including the relatively flat pleural field, short axis, and triangular marginal spines (up to four pairs; Westrop, Reference Westrop1986, pl. 14, fig. 6; Stitt and Straatmann, Reference Stitt and Straatmann1997, fig. 8.14), are potential apomorphic characters supporting monophyly. Effacement of the anterior border and border furrow is shared with species such as “Bayfieldia” simata Winston and Nicholls, Reference Winston and Nicholls1967. The differences in pygidial anatomy between “B.” simata (e.g., Winston and Nicholls, Reference Winston and Nicholls1967, pl. 9, fig. 23) and Monocheilus (e.g., Fig. 7.1–7.7) raise the possibility that similarities in frontal area anatomy are homoplastic, in which case effacement would be another character supporting monophyly of the latter.
Holotype
A cranidium (Fig. 3.5–3.7; OU 238158) from the Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collection BM 79.4T.
Paratypes
Eleven cranidia (OU 238164, OU 238356–OU 238365) and one librigena (OU 238377), all from the Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collection BM 79.4T.
Diagnosis
Monocheilus with large palpebral lobe about 60% (59%; 42%–64%; lower values in larger specimens [e.g., Fig. 3.1]) of preoccipital glabellar length; both anterior and posterior ends separated from glabella by narrow strips of fixigenae. Faint anterior border furrow curved backward; anterior border roughly rhombic in outline. Pitted sculpture with pitted sculpture augmented by caecal network on frontal area that is best expressed on smaller specimens (e.g., Fig. 2.9, 2.10) and faint to absent on anterior border.
Occurrence
Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collections BM 79.4T and BM 80.7, Monocheilus reginae fauna.
Description
Cranidium exclusive of posterolateral projection subrectangular in outline with gently rounded anterior margin; width across palpebral lobes equal to length (101%; 98%–103%). Axial and preglabellar furrows finely etched grooves. Glabella conspicuous, accounting for about 81% (79%–83%) of cranidial length and about 65% (57%–70%; lower values in smaller cranidia) of cranidial width, and gently convex; weakly tapered, with anterior margin rounded. Occipital furrow (SO) shallow, curved gently backward, terminating short of axial furrow. Occipital ring (LO) occupies about one-fifth (21%; 19–23%) of glabellar length. Short anterior border expressed on some specimens but not elevated above adjacent part of preglabellar field; border furrow faint and curved backward. Palpebral lobe forms nearly flat, arcuate band centered in front of glabellar mid-length, length about 60% (59%; 42%–64%; lower values in larger specimens) of preoccipital glabellar length; palpebral furrow lightly impressed curved groove. Narrow palpebral area accounts for about 26% (21%–35%; higher values in smaller specimens) of glabellar width opposite midpoint of palpebral lobe. Anterior branches of facial sutures nearly parallel before curving inward along anterior cranidial margin; posterior branches diverge backward in faintly sigmoid curve. Posterior border convex, expands abaxially, with maximum length (exsagittal [exsag.]) equal to 14% (12%–15%) of glabellar length; posterior border furrow is clearly defined groove directed obliquely forward from axial furrow. Sculpture of irregular pits on external surface except for furrows and palpebral lobes; overprinted with caecal network on preglabellar field that becomes faint to absent on anterior border.
Librigena with long genal spine. Librigenal field well inflated; carries same pitted sculpture as the cranidium, which also extends to the anterior sections of the librigenal border. Librigenal border furrow weakly impressed; border gently convex. Faint terrace ridges on posterior librigenal border and genal spine.
Etymology
For Regina Blackwell, Sean Blackwell's mother.
Additional material
In addition to the types, six cranidia were complete enough to provide morphometric data.
Ontogeny
Small cranidia (e.g., Fig. 2.6, 2.12) have a roughly rhombic anterior border and backwardly curved border furrow (small cranidia of M. micros are similar in this respect [Westrop, Reference Westrop1986, pl. 15, figs. 7, 8]). The border furrow is lost in larger specimens, but the border may be identifiable by a change in surface sculpture on the frontal area from caecal markings to a more pitted surface (Figs. 2.9, 3.6). However, although the frontal area in smaller specimens (e.g., Fig. 2.12, 2.14) includes caecal ridges, they fade in larger specimens (e.g., Fig. 3.4) so that the border is more difficult to identify. The palpebral lobe becomes shorter, with length dropping from about 60% of preoccipital glabellar length in smallest specimens (e.g., Fig. 2.6, 2.11, 2.12, 2.13) to slightly more than 40% in the largest (e.g., Fig 3.1). Glabellar furrows also exhibit ontogenetic variation. Smaller specimens have finely etched, transglabellar S1 and S2 furrows and faint S3 lateral furrows, and SO extends across the entire width of the glabella (e.g., Fig. 2.6, 2.11). SO is expressed in larger specimens but becomes shallower and terminates short of the lateral glabellar margin (e.g., Figs. 2.1, 3.4, 3.6). Other glabellar furrows become faint (e.g., Fig. 2.13) and are eventually lost entirely (e.g., Fig. 3.4), even on internal molds (e.g., Fig. 3.1).
Remarks
A comparison between cranidia of M. reginae and M. richardi, co-occurring species that differ in the size of the palpebral lobe (Fig. 4), is presented in the description of the latter. There appear to be two distinct pygidial morphotypes associated with the cranidia, raising the possibility that other characters may separate these species. However, more material is needed to confirm this. The most common morphotype (e.g., Fig. 7.1–7.5) is relatively long and narrow, with length (measured to the intersection of the anterior margin and the axis) equal to 46% of width, but one specimen (Fig. 7.6, 7.7) is distinctly shorter and wider, with length equal to 40% of width.
Among other species, M. reginae is similar to sclerites from the Bison Creek Formation, southern Alberta, that were assigned to M. micros (Walter, Reference Walter1924) by Westrop (Reference Westrop1986, pl. 15, figs. 1–9). As noted earlier, free cheeks can be distinguished only on the basis of sculpture (smooth in M. micros and pits on M. reginae; a difference that extends to the cranidia). The position of the palpebral lobe is also diagnostic; it is much closer to the axial furrow in M. micros, particularly at the anterior tip (compare Figs. 2, 3 with Westrop, Reference Westrop1986, pl. 15, figs. 1, 2). A consequence of this position in M. micros is a relatively narrower (transverse [tr.]) frontal area that is also longer than in M. reginae. Monocheilus anatinus (Hall, Reference Hall1863) (e.g., Westrop, Reference Westrop1986, pl. 14, fig. 1), M. truncatus Ellinwood in Bell and Ellinwood, Reference Bell and Ellinwood1962 (e.g., Westrop, Reference Westrop1986, pl. 14, figs. 3, 4), and M. orestes Westrop, Reference Westrop1986 (pl. 15, figs. 14–17) also differ from M. reginae in having larger palpebral lobes that are much closer to the glabella.
Holotype
A cranidium (Fig. 3.5–3.7, OU 238158) from the Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collection BM 79.4T.
Paratypes
Nine cranidia (OU 238163, OU 238366, OU 238367, OU 238368a OU 238369–OU 238373) and a librigena (OU 238368b) from the Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collection BM 79.4T.
Diagnosis
Monocheilus with small palpebral lobe slightly more than 40% (42%; 30%–50%; lower values in larger specimens [e.g., Fig. 6.5]) of preoccipital glabellar length; both anterior and posterior ends separated from glabella by narrow strips of fixigenae. Faint anterior border furrow expressed on smaller specimens, curved backward; anterior border roughly rhombic in outline. Cephalon with pitted sculpture augmented by caecal network that is best expressed on smaller specimens (e.g., Fig. 5.1).
Occurrence
Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collections BM 79.4T and BM 80.7, Monocheilus reginae fauna.
Description
Monocheilus richardi is sufficiently similar to M. reginae that a comparison can be presented instead of a full description. Monocheilus reginae differs from co-occurring cranidia of M. richardi in the size of the palpebral lobe (Fig. 4). Compared with M. richardi (Figs. 5, 6), M. reginae is a relatively large-eyed species with a palpebral lobe that is equal to about 60% (59%; 42%–64%; lower values in larger specimens) of preoccipital glabellar length, whereas the palpebral lobe is noticeably smaller in similarly sized specimens of M. richardi, averaging slightly more than 40% (42%; 30%–50%; lower values in larger specimens). In other respects, the cranidia are similar.
Etymology
For Richard Blackwell, the name of both Sean Blackwell's father and Sean Blackwell's grandfather.
Additional material
In addition to the types, three cranidia were complete enough to provide morphometric data.
Remarks
As in Monocheilus reginae, the palpebral lobe of M. richardi becomes proportionately smaller during ontogeny. However, the palpebral lobe of M. richardi is relatively smaller throughout growth (Fig. 4). In smaller specimens of M. richardi (e.g., Fig. 5.1–5.13), the palpebral lobe is 44% (42%–50%) of preoccipital glabellar length, well below the values (61%; 56%–64%) of similarly sized M. reginae (e.g., Fig. 2) and in fact similar to the proportions of the largest cranidia of M. reginae (42%).
The pitted sculpture is a diagnostic feature of both M. reginae and M. richardi, whereas other species, including M. micros and M. orestes, are smooth (e.g., Westrop, Reference Westrop1986, pl. 15, figs. 5, 6, 14, 15). Cranidia of M. richardi are similar to the types and other specimens of M. oweni (Hall, Reference Hall1863) from the Upper Mississippi Valley region (e.g., Nelson, Reference Nelson1951, pl. 109, figs. 1, 2) in possessing a relatively small palpebral lobe, although M. richardi differs in having a wider palpebral area of the fixigena so that the palpebral lobe is farther from the glabella. Unfortunately, all of the specimens from the Upper Mississippi Valley are preserved as sandstone internal molds, and the nature of the sculpture cannot be determined. It is impossible to compare M. oweni completely with other species that preserve the skeleton. As noted earlier, we recommend that the name M. oweni be restricted to the types, and the shutter-mark convention (M. “oweni”) should be used for internal molds from other collections in the Upper Mississippi Valley, as well as for specimens attributed to this species from Alberta (Westrop, Reference Westrop1986, pl. 15, figs. 10–13).
Monocheilus spp.
Figures 7.1–7.7, 8

Figure 8. Monocheilus spp. from the uppermost Honey Creek Formation, Bally Mountain section, Kiowa County, Oklahoma. All are thoracic segments except for (1) (pygidium), and all are from collection BM 79.4T. (1) OU 238378, dorsal view. (2, 3) OU 238379: (2) dorsal view; (3) lateral view. (4, 5) OU 238380: (4) dorsal view; (5) posterior view. Scale bars = 2 mm.
Occurrence
Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collections BM 79.4T and BM 80.7, Monocheilus reginae fauna.
Remarks
Monocheilus reginae and M. richardi are differentiated clearly by the sizes of their palpebral lobes. As noted in the preceding, there is sufficient variation in pygidia from collection BM 79.4T that we anticipate larger samples will demonstrate that these species are also differentiated by pygidial anatomy, although the correct sclerite associations cannot be made at present. Pygidial morphotypes are separated by their outlines. Most are relatively long and narrow (e.g., Fig. 7.1–7.5), with length (measured to the intersection of the anterior margin and the axis) equal to 46% of width. The other pygidial morphotype (Fig. 7.6, 7.7) is relatively shorter and wider, with length equal to 40% of width, and has less strongly curved lateral margins. In other respects, the morphotypes are similar, with a single pair of triangular marginal spines. The axis is short with one distinct axial ring bounded posteriorly by a complete ring furrow, and the terminal piece comprises at least two segments. The pleural field is nearly flat and crossed by a single pair of wide, shallow pleural furrows that curve sharply backward, separating narrow, convex anterior and posterior pleural bands.
Associated thoracic segments (Figs. 7.5, 8.2–8.5) have short (tr.) pleurae with well-defined, broad pleural furrows. The posteriormost segment is curved strongly backward (Fig. 7.5), but other specimens have transverse pleurae and were presumably positioned farther forward in the thorax (e.g., Fig. 8.4).
Family Ptychaspididae Raymond, Reference Raymond1924
Genus Ptychaspis Hall, Reference Hall1863
Type species
Dikelocephalus miniscaensis Owen, Reference Owen1852 from the Lone Rock Formation, Minnesota (subsequent designation by Miller, Reference Miller1889; see Bell et al., Reference Bell, Feniak and Kurtz1952).
Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959
Figures 9–12
- Reference Lochman and Hu1959
Ptychaspis bullasa Lochman and Hu, p. 422, pl. 58, figs. 21–42.
- non Reference Bell and Ellinwood1962
Ptychaspis bullasa; Bell and Ellinwood, p. 405, pl. 58, figs. 14–17 [= Ptychaspis occulta n. sp.].
- non Reference Longacre1970
Ptychaspis bullasa; Longacre, p. 44, pl. 2, figs. 4, 5 [= Ptychaspis spp.].
- Reference Hu1971
Ptychaspis bullasa; Hu, p. 97, pl. 17, figs. 1–34; text-fig. 46.
- non Reference Stitt1977
Ptychaspis bullasa; Stitt, p. 43, pl. 2, fig. 4.
- non Reference Westrop1986
Ptychaspis bullasa?; Westrop, pl. 8, figs. 9–12 [= Ptychaspis spp.].
- non Reference Stitt and Straatmann1997
Ptychaspis bullasa; Stitt and Straatmann, p. 90, fig. 7.16 (= Ptychaspis sp. indet).
Holotype
A cranidium (USNM 137099) from the St. Charles Formation, Idaho (Fig. 9.1–9.3).

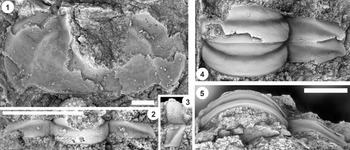
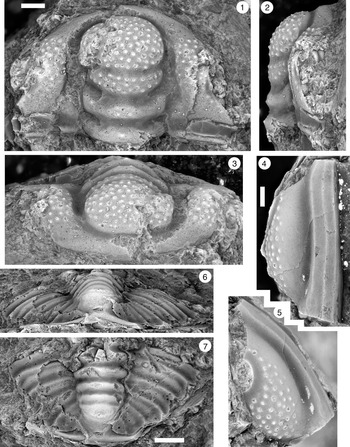
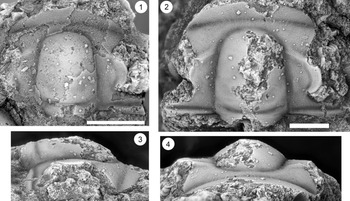
Figure 9. (1–6) Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959, St. Charles Formation, Bear River Range, Mink Creek, southern Idaho. All are cranidia. (1–3) Holotype USNM 137099: (1) lateral view; (2) dorsal view; (3) anterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, figs. 39, 40). (4–6) Paratype USNM 137100k: (4) lateral view; (5) anterior view; (6) dorsal view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 37). (7) USNM 137100, dorsal view (previously unfigured and not a designated paratype; see Lochman and Hu, Reference Lochman and Hu1959, p. 423 for the list of paratypes). Scale bars = 2 mm.
Diagnosis
Ptychaspis with palpebral lobes short and narrow, with midpoint situated roughly opposite S2 glabellar furrow. Anterior cranidial margin gently curved so that frontal area maintains nearly even width (sagittal [sag.], exsag.). Cranidial sculpture of tubercles extends forward on fixigena to point near level of S3 (where expressed), and in smaller cranidia extends back to posterior border furrow (e.g., Fig. 10.4, 10.10); tubercles are lost on posterior fixigenae on larger specimens (e.g., Figs. 9.6, 10.1). Frontal area lacks tubercles and has up to two striate ridges running parallel to anterior cranidial margin. Anteriorly, pygidial pleural and interpleural furrows extend nearly transversely (10°–15° from transverse plane) from axis before curving backward near pygidial margin.

Figure 10. Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959, St. Charles Formation, Bear River Range, Mink Creek, southern Idaho. All are cranidia and all paratypes. (1–3) USNM 135100r: (1) dorsal view; (2) lateral view; (3) anterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 35). (4–6) USNM 137100e: (4) dorsal view; (5) lateral view; (6) anterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 26). (7–9) USNM 137100d: (7) lateral view; (8) anterior view; (9) dorsal view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 24). (10–12) USNM 137100c: (10) dorsal view; (11) lateral view; (12) anterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 23). Scale bars = 2 mm.
Occurrence
St. Charles Formation, ridge north of Mink Creek, near Preston, southern Idaho (Lochman and Hu, Reference Lochman and Hu1959).
Description
Cranidium with length equal to 95% (90%–99%) of width. Frontal area is short and lacks border furrow. Anterior cranidial margin gently curved medially so that frontal area maintains nearly even length (sag., exsag.) across most of width. Glabella convex, long, and broad, occupying 89% (87%–91%) of cranidial length and half (50%; 47%–53%) of cranidial width between palpebral furrows; glabellar width at S2 equal to 60% (56%–63%) of glabellar length. SO firmly impressed, nearly transverse. S1 and S2 deeply incised and connected across glabella. S1 transverse medially but deflected forward abaxially; S2 curved backward. LO occupies 13% (12%–14%) of glabellar length, transverse over most of width but curved forward near axial furrow. L1 accounts for 13% (12%–14%) of glabellar length (sag.), transverse medially, but curved sharply forward abaxially. L2 roughly trapezoidal in outline, with nearly transverse anterior margin; occupies about one-sixth (16%; 12%–20%; highest values in smallest cranidia) of glabellar length. S3 faint at best on larger cranidia (e.g., Fig. 11.1, 11.2), nearly transverse; not expressed on smaller individuals (e.g., Fig. 10). Consequently, L3 and L4 essentially undifferentiated, forming a suboval region equal to about 40% (42%; 40%–44%) of glabellar length. Palpebral lobe short, narrow band centered opposite S2; length equal to roughly one-quarter (23%; 19%–27%; lowest value in largest cranidium; Fig. 11.2) of glabellar length. Palpebral furrow firmly impressed, narrow groove. Palpebral area of fixigena very gently inflated, width equal to 41% (37%–48%; higher values in larger cranidia) of glabellar width at S2. Posterior branches of facial suture strongly divergent; anterior branches nearly parallel in front of palpebral lobe before swinging inward along anterior cranidial margin. Posterior border furrow well incised; posterior border strongly convex. Cranidial sculpture of tubercles extends forward to point just in front of anterior tip of palpebral lobe. On smaller cranidia, tubercles extend back to posterior border furrow (e.g., Fig. 10.4, 10.9, 10.10) but are lost on posterior fixigenae on larger specimens (e.g., Figs. 10.6, 11.2). Frontal area lacks tubercles and has up to two striate ridges running parallel to anterior cranidial margin. Tubercles absent from the posterior border throughout holaspid ontogeny. Internal mold includes scattered fine pits on glabella and fixigenae.

Figure 11. Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959, St. Charles Formation, Bear River Range, Mink Creek, southern Idaho. All are paratypes. (1–3) Cranidium, USNM 137100n: (1) lateral view; (2) dorsal view; (3) anterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 42). (4, 5) Librigena, USNM 137100j: (4) lateral view; (5) dorsal view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 36). (6, 7) Pygidium, USNM 137100s: (6) dorsal view; (7) posterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 30). (8) Pygidium, USNM 137100p, dorsal view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 31). Scale bars = 2 mm.
Librigena with stout genal spine. Lateral border furrow is well defined and merges posteriorly with posterior border. Lateral border is narrow and descends steeply at cephalic margin. Librigenal field strongly inflated with shallow eye socle furrow and low eye socle. Borders, spine, and abaxial parts of librigenal field carry coarse striate ridges; adaxially, librigenal field with tubercles.
Hypostome with convex median body divided into short posterior lobe and much longer anterior lobe by oblique middle furrow that is effaced medially. Macula indistinct oval region on internal mold. Lateral and posterior border furrows form deep grooves; narrow, rim-like borders. Broad, triangular anterior wing. Patches of exoskeleton preserve terrace ridges on lateral border and smooth surface on anterior lobe of median body; internal mold smooth.
Pygidium semielliptical in outline, wider than long. Axis convex, extending almost entire pygidial length and narrow, occupying less than a third of pygidial width; composed of three axial rings and terminal piece on largest specimen (Fig. 12.1–12.3). Articulating and axial ring furrows transverse and deep; posteriormost shallower than others on smaller specimens, so that third ring poorly differentiated from terminal piece. Articulating half ring very short, less than half length of first axial ring. Deep pleural and shallower interpleural furrows extend nearly transversely from axis at no more than 10°–15° from transverse plane before curving backward more strongly near pygidial margin; posteriormost furrows more strongly deflected. Anterior pleural bands roughly equal to posterior bands. Posterior pleural bands carry row of faint tubercles. Pygidial border narrow, rising vertically from weakly convex pleural field.

Figure 12. Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959, St. Charles Formation, Bear River Range, Mink Creek, southern Idaho. All are paratypes. (1–3) Pygidium, USNM 137100L: (1) dorsal view; (2) lateral view; (3) posterior view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 27). (4) Librigena, USNM 137100f, dorsal view (previously unfigured). (5–7) Hypostome, USNM 137100m: (5) posterior view; (6) lateral view; (7) ventral view (illustrated previously by Lochman and Hu, Reference Lochman and Hu1959, pl. 58, fig. 25). Scale bars = 2 mm.
Ontogeny
Through the size range available for study (cranidial lengths [sag.] from 1.5 to 15 mm), general cranidial proportions show little change. For example, the glabella occupies between 87% and 91% of cranidial length, and cranidial length/width falls modestly from 96%–99% in cranidia less than 3 mm (sag.) to 91%–95% in cranidia between 10 and 11 mm (sag.). SO, S1, and S2 furrows become broader (sag., exsag.) through ontogeny, even allowing for differing expression between the external surface and internal molds. The palpebral lobe is noticeably smaller in the largest cranidium (Fig. 11.2; equal to 19% of glabellar length) and is proportionally larger in smaller specimens (e.g., Fig. 10.1, 10.4; 26%–27% of glabellar length). This change is accompanied by a widening of the palpebral area of the fixigena (equal to 37%–39% of glabellar width in Fig. 10.1, 10.4 but 48% of glabellar width in Fig. 11.2).
Pygidia are incomplete, but the smallest (Fig. 11.8) is relatively narrower (tr.) than in the other specimens (Figs. 11.6, 12.1), and the third axial ring is poorly defined. In all three specimens, the axis remains long and terminates close to the posterior border.
Remarks
Restudy of the type material of Ptychaspis bullasa Lochman and Hu (Reference Lochman and Hu1959; Figs. 9–12) shows that this species is characterized by an anteriorly positioned palpebral lobe that is centered opposite the S2 glabellar furrow, and pygidial pleural and interpleural furrows that are almost transverse over most of their widths. This allows several records of P. bullasa from outside the type area in southern Idaho (Figs. 13–17) to be evaluated critically.

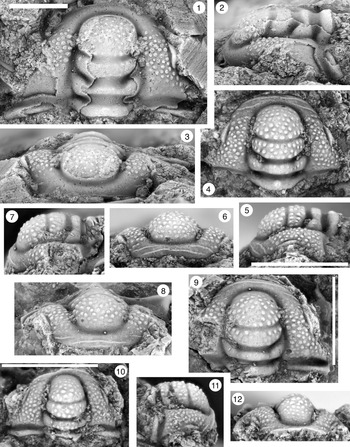
Figure 13. Ptychaspis matuszaki n. sp., Fort Sill Formation, about 4.4 km southeast of Hennepin, Murray County, Oklahoma. (1–3) Holotype cranidium, OU 4268: (1) dorsal view (note baccula, marked by arrow); (2) lateral view; (3) anterior view. (4) Paratype librigena, OU 238167, dorsal view. (5) Paratype pygidium, OU 4270, dorsal view. Scale bars = 2 mm.

Figure 14. Ptychaspis matuszaki n. sp., Fort Sill Formation, about 4.4 km southeast of Hennepin (SE Sec. 4, T1S, R1W), Murray County, Oklahoma. All are paratypes. (1–3) Cranidium, OU 238168: (1) dorsal view; (2) lateral view; (3) anterior view. (4, 5) Librigena, OU 4269: (4) lateral view; (5) dorsal view. (6, 7) Pygidium, OU 238169: (6) posterior view; (7) dorsal view. Scale bars = 2 mm.

Figure 15. Ptychaspis occulta n. sp., Morgan Creek Member, Wilberns Formation, central Texas. All are cranidia. (1–3) Paratype USNM 185437, Sudduth section, Burnet County, collection SU 104, about 6.7 m below the top of the Morgan Creek Member (illustrated previously by Bell and Ellinwood, Reference Bell and Ellinwood1962, pl. 58, fig. 17): (1) dorsal view; (2) lateral view; (3) anterior view. (4, 5) Paratype USNM 185470, White Creek section, Blanco County, collection WC 950, about 5.5 m below the top of the Morgan Creek Member (illustrated previously by Bell and Ellinwood, Reference Bell and Ellinwood1962, pl. 58, fig. 14): (4) dorsal view; (5) anterior view. (6–8) Holotype USNM 185472, Little Llano River section, San Saba County, collection LL 725, about 6.4 m below the top of the Morgan Creek Member (illustrated previously by Bell and Ellinwood, Reference Bell and Ellinwood1962, pl. 58, fig. 16): (6) anterior view; (7) dorsal view; (8) lateral view. Scale bars = 2 mm.

Figure 16. Ptychaspis sp. 1, Honey Creek–Fort Sill boundary interval, Bally Mountain section, Kiowa County, Oklahoma. All are cranidia. (1–3) OU 238381, collection BM 80.7: (1) lateral view; (2) anterior view; (3) dorsal view. (4–6) OU 238171, collection BM 82.1: (4) lateral view; (5) dorsal view; (6) anterior view. (7) OU 238172, dorsal view, collection BM 79.4T. Scale bars = 2 mm.

Figure 17. Ptychaspis spp., Morgan Creek Member, Wilberns Formation, central Texas. All are cranidia. (1–3) USNM 192202, White Creek section, Blanco County, collection WC 968, at the top of the Morgan Creek Member (illustrated previously by Longacre, Reference Longacre1970, pl. 2, fig. 5): (1) dorsal view; (2) lateral view; (3) anterior view. (4, 5) USNM 192201, Gipson Ranch section, Blanco County collection GR 195, about 0.6 m below the top of the Morgan Creek Member (illustrated previously by Longacre, Reference Longacre1970, pl. 2, fig. 4): (4) dorsal view; (5) anterior view. Scale bars = 2 mm.
Cranidia that Bell and Ellinwood (Reference Bell and Ellinwood1962) identified as P. bullasa from the Morgan Creek Member of the Wilberns Formation, Texas (Fig. 15), have palpebral lobes that are larger and more posteriorly located (see also Longacre, Reference Longacre1970, p. 44) than those of the types (e.g., Figs. 9, 10). There are other differences in the cranidia from Texas, including a longer frontal area with subtriangular anterior cranidial margin, more densely packed tuberculate sculpture, and striate ridges that are more closely spaced on the anterior border (Fig. 15). The Texas material is assigned to a new species, P. occulta (see the following). However, the two cranidia from the Morgan Creek Member that Longacre (Reference Longacre1970) assigned to P. bullasa (Fig. 17) are distinct from both P. bullasa and P. occulta (see discussion of Ptychaspis spp. that follows).
The cranidium attributed to P. bullasa by Stitt (Reference Stitt1977, pl. 2, fig. 4) from the Fort Sill Formation of Oklahoma has been damaged since it was photographed and can no longer be evaluated fully. However, sclerites from the same stratigraphic interval in the lower Fort Sill Formation (Figs. 13, 14) represent a distinct species characterized by curved pleural and interpleural furrows on the pygidium, among other characters (see following discussion of P. matuszaki n. sp.).
The record of P. bullasa from the Deadwood Formation of South Dakota (Stitt and Straatmann (Reference Stitt and Straatmann1997, fig. 7.16) is supported by a single illustrated librigena and is difficult to evaluate. This specimen has far greater development of striate ridges and a smaller area of tuberculate sculpture than either of the librigenae in the type lot of P. bullasa (Figs. 11.4, 11.5, 12.4), and the identification cannot be corroborated.
Finally, Westrop (Reference Westrop1986, pl. 8, figs. 9–12) assigned sclerites from the Bison Creek Formation in the southern Canadian Rockies questionably to P. bullasa. The cranidia are from different localities and differ in details of the sculpture, with the larger specimen possessing highly irregular tubercles, some of which are connected by low ridges (Westrop, Reference Westrop1986, pl. 8, figs. 9, 10), whereas the smaller has rounded, isolated tubercles (Westrop, Reference Westrop1986, pl. 8, fig. 11). Tubercle shape is uniform through the holaspid ontogeny of P. bullasa (e.g., compare Fig. 10.4, 10.10 with Figs. 9.2, 9.6, 11.2), suggesting that the Bison Creek material records more than one species.
Rather than a single, widespread species, detailed comparisons of putative occurrences of P. bullasa indicate that there is in fact a geographically structured plexus of related species. Westrop et al. (Reference Westrop, Landing and Dengler2018) recently documented a similar pattern in the middle Cambrian trilobite Eodiscus, noting that such sets of pseudocryptic species are comparable to groups of modern species that are now recognized routinely in studies that integrate morphometric and genomic data.
Ptychaspis matuszaki new species
Figures 13, 14
- Reference Matuszak1957
Ptychaspis granulosa exsculpta Frederickson and Matuszak in Matuszak [nomen nudum], p. 29, pl. 2, figs. 1–3.
Holotype
A cranidium (OU 4268; Fig. 13.1–13.3) from the Fort Sill Formation, about 4.4 km southeast of Hennepin (SE Sec. 4, T1S, R1W), Murray County, Oklahoma.
Paratypes
A cranidium (OU 238168; Fig. 14.1–14.3), two incomplete free cheeks (OU 238167, OU 4269; Figs. 13.4, 14.4, 14.5), and two pygidia (OU 4270, OU 238169; Figs. 13.5, 14.6, 14.7).
Diagnosis
Ptychaspis with closely spaced tuberculate cephalic sculpture yielding to sparse striate ridges along cranidial and librigenal margins. In front of transglabellar S2 furrow, glabella subcircular in outline, divided by barely perceptible S3 and S4 lateral furrows (most clearly visible in lateral view; Fig. 13.2). Palpebral lobe small and located opposite S2. Gently convex baccula present on fixigena opposite L1 (arrow on Fig. 13.1). Pygidium possesses gently inflated pleural field and axis composed of three axial rings and terminal piece. Anteriorly, pleural and interpleural furrows deflected obliquely backward at angle of at least 20°–30° from transverse plane. Pygidial border forms narrow upturned rim that thickens toward axis.
Occurrence
Fort Sill Formation, Oklahoma, 4.4 km southeast of Hennepin (SE Sec. 4, T1S, R1W), Murray County, Oklahoma. Matuszak (Reference Matuszak1957, p. 30) did not provide detailed stratigraphic occurrence data for Ptychaspis in the Fort Sill Formation, but he indicated that sclerites now assigned to P. matuszaki (Figs.13.1–13.3, 13.5, 14.4, 14.5) occurred in the “basal Fort Sill Formation.”
Description
Cranidium subtrapezoidal in outline with rounded anterior margin; short (tr.) posterolateral projections. Frontal area short, accounting for about 10% of cranidial length, and slopes almost vertically forward. Anterior border furrow absent. Axial and preglabellar furrows firmly impressed grooves. Glabella well rounded anteriorly and inflated, comprising about 89% of cranidial length and about 49% of cranidial width. SO firmly impressed and of even incision across glabella; curved gently backward. LO occupies about 12% of glabellar length and curved forward abaxially; carries small median node on internal mold. S1 and S2 glabellar furrows connected across glabella. S1 nearly transverse medially but curves forward and deepens abaxially. L1 also curved forward and occupies about 10% of medial glabellar length. S2 shorter (sag., exsag.), shallower than S1, maintaining nearly even depth; less strongly curved. L2 accounts for about 17% of glabellar length medially but narrows slightly abaxially. S3 gently impressed, defined in part by break in sculpture, and nearly transverse. L3 and L4 similar in length (exsag.) to L2. Gently convex baccula present on fixigena opposite L1 (arrow on Fig. 13.1). Palpebral lobes incomplete, but palpebral furrows indicate that they are situated opposite S2. Palpebral area of fixigena equal to about 35% of glabellar width at S2. Anterior branches of facial suture converge forward in even curve. Posterior branches diverge gradually backward along a nearly straight path. Posterior border furrow deeply incised and broad with a narrow rim-like border. Surface sculpture uniformly tuberculate on L2, L3, and L4 but present only medially on L1; tubercles present on palpebral area and extend forward on preocular field to level of S3. On posterior area, tubercles confined largely to sutural margin. Internal mold includes scattered fine pits.
Free cheek with long, stout genal spine. Inflated librigenal field occupies 68% of width opposite eye; eye socle not preserved but eye socle furrow broad, shallow groove. Lateral border furrow well incised anteriorly but shallows near genal spine; lateral border narrow in dorsal view, descends nearly vertically from border furrow. Librigenal field with conspicuous tubercles that are lost near border furrow. Border with elongate, widely spaced striate ridges running parallel to lateral cranidial margin.
Pygidium subelliptical in outline, width greater than length; gently arched in posterior view. Axis narrow, convex, occupies 67% of pygidial length. Three axial rings and terminal piece of at least two segments separated by well-defined, transverse ring furrows; shallower furrow present on terminal piece. Articulating half-ring short; articulating furrow nearly transverse. Pleural field crossed by at least four pairs of broad (exsag.), oblique pleural furrows deflected backward at angle of at least 20°–30° from transverse plane; interpleural furrows narrower but well-defined grooves. Anterior and posterior pleural bands subequal, convex. Lateral and posterior borders form short (sag., exsag.), weakly upturned rim. External surface smooth; internal molds with pits; closely spaced on pleural field and border but widely spaced on crest of axis.
Etymology
For David R. Matuszak, who recognized that this species was new in an unpublished thesis.
Remarks
Cranidia of Ptychaspis matuszaki are similar to those of Ptychaspis bullasa Lochman and Hu, Reference Lochman and Hu1959 (Figs. 9–12). Both have tuberculate sculpture, but there are fewer, more widely spaced tubercles in P. bullasa (compare P. matuszaki [Figs. 13.1–13.3, 14.1–14.3] with P. bullasa [Figs. 9.1–9.3, 11.1–11.3]), which also has well-defined striate ridges on the frontal area. Both species have relatively small, anteriorly placed palpebral lobes that are centered near S2, and the overall proportions of the glabellar lobes are comparable. Ptychaspis matuszaki differs in having a distinct baccula next to L1 (seen most clearly in the best-preserved specimen; Fig. 13.1). In addition, cranidia of P. bullasa possess less rapidly diverging posterior suture branches, resulting in narrower (tr.) posterolateral projections. Pygidia share a raised, rim-like border, with those of P. matuszaki (e.g., Figs. 13.5, 14.6, 14.7) differing from P. bullasa (Figs. 11.6–11.8, 12.1–12.3) most clearly in having shorter axes and pleural and interpleural furrows that are deflected more strongly backward rather than being more transverse.
Cranidia of P. striata (Whitfield, Reference Whitfield1878) and Ptychaspis cf. P. miniscaensis (Owen, Reference Owen1852) from the Bison Creek Formation of Alberta (e.g., Westrop, Reference Westrop1986, pl. 8, figs. 1–3, 6, and pl. 7, figs. 1–4, respectively) are differentiated easily from P. matuszaki in having sculpture of coarse striate ridges that is expressed on both the external surface and the internal mold; tubercles are absent. In addition, the glabella of Ptychaspis cf. P. miniscaensis in front of S2 is tapered and subtrapezoidal, whereas the equivalent part of P. matuszaki is more rounded and subcircular in outline. S2 lateral furrows are connected across the glabella by a firmly impressed furrow in P. matuszaki, whereas S2 furrows are isolated to barely connected in Ptychaspis cf. P. miniscaensis. The raised, rim-like pygidial borders of both P. matuszaki and Ptychaspis cf. P. miniscaensis (e.g., Westrop, Reference Westrop1986, pl. 7, figs. 6, 11, 14) set them apart from P. striata, which has a flat pygidial border. Ptychaspis cf. P. miniscaensis has four axial rings in front of the terminal piece (e.g., Westrop, Reference Westrop1986, pl. 7, fig. 6), whereas P. matuszaki has only three (e.g., Fig. 13.5).
Ptychaspis miniscaensis is known only from sandstone internal molds from Lone Rock of Minnesota and Wisconsin (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 36, fig. 1a; Westrop, Reference Westrop1986, pl. 8, fig. 15) that are smooth or at best very weakly ridged even when well preserved. Ptychaspis tuberosa Feniak in Bell et al., Reference Bell, Feniak and Kurtz1952 (e.g., Westrop, Reference Westrop1986, pl. 8, fig. 14) also has a smooth internal mold.
Ptychaspis granulosa (Owen, Reference Owen1852) is known only from poorly preserved sandstone internal molds from the Upper Mississippi Valley. Superficially, it is quite similar to P. matuszaki and P. bullasa in terms of tuberculate sculpture of the cranidium (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 34, fig. 6b, pl. 35, fig. 1a). However, the pygidial border in P. granulosa is broad and flat (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 35, figs. 1c, d) whereas the border of P. matuszaki is narrow and forms an upturned rim.
Ptychaspis occulta new species
Figure 15
- Reference Bell and Ellinwood1962
Ptychaspis bullasa Lochman and Hu; Bell and Ellinwood, p. 405, pl. 58, figs. 14–17.
Holotype
A cranidium (USNM 185472; Fig. 15.6–15.8) from the Morgan Creek Member, Wilberns Formation, Little Llano River section, collection LL 725, San Saba County (illustrated previously by Bell and Ellinwood, Reference Bell and Ellinwood1962, pl. 58, fig. 16).
Paratypes
Two cranidia (USNM 185437, 185470; Fig. 15.1–15.5) from the Morgan Creek Member, Wilberns Formation, central Texas.
Diagnosis
A species of Ptychaspis with densely packed tuberculate cephalic sculpture on the postocular and palpebral areas and glabella yielding anteriorly to robust striate ridges on the frontal area. Rounded glabella with subspheroidal L4 and well-incised S1 and S2 glabellar furrows. S3 faintly impressed and oriented inward and slightly forward from axial furrow. Palpebral lobe narrow, ridge-like, located opposite L2 and extending from S1 to just in front of S2. Anterior branches of facial sutures converge forward, producing subtriangular anterior cranidial margin in anterior view (e.g., Fig. 15.6).
Occurrence
Morgan Creek Member (figured specimens collected between 5.5 m and 6.7 m below the top), Wilberns Formation, central Texas (see caption for Fig. 15 for detailed locality information).
Description
Ptychaspis occulta n. sp. is sufficiently similar to P. matuszaki n. sp. and P. bullasa Lochman and Hu, Reference Lochman and Hu1959 that comparisons can be presented in lieu of a full description. Ptychaspis occulta and P. matuszaki are superficially similar yet differ in detail, and although sample sizes are small, there are clear apomorphic character states that separate them. Diagnostic states for P. occulta include more robust sculpture than P. matuszaki in both the size and packing density of tubercules, as well as the striate ridges on the frontal area (compare Fig. 15 with Fig. 13.1–13.3); note that the ridges are well defined on internal molds of P. occulta (Fig. 15.1–15.3) but are barely perceptible on molds of P. matuszaki. Ptychaspis occulta also lacks a baccula, and the anterior branches of the facial suture are more rapidly convergent so that the frontal area is subtriangular in anterior view (e.g., Fig. 15.6). By contrast, P. matuszaki is bacculate (Fig. 13.1), and the reduced divergence of the facial sutures produces a frontal area that is gently rounded and nearly transverse in anterior view (Figs. 13.3, 14.3).
Ptychaspis occulta differs from P. bullasa in having larger, more posteriorly positioned palpebral lobes that are centered opposite L2 (e.g., Fig. 15.4, 15.7) rather than opposite S2 (e.g., Figs. 9.2, 9.6, 9.7, 10.1, 10.4, 10.9). In addition, P. bullasa has less-convergent anterior branches of the facial sutures, which yield a more rounded anterior cranidial margin (Fig. 9.3, 9.5), whereas P. occulta is differentiated further by having larger, more closely spaced tubercular sculpture on the glabella and fixigenae and numerous coarse, striate ridges on the frontal area that are also expressed on the internal mold (Fig. 15.1, 15.4, 15.7).
Etymology
From occulta (L), “having been hidden,” in reference to the species having gone unrecognized within the literature since 1962.
Remarks
Ptychaspis occulta differs clearly from P. miniscaensis (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 36, fig. 1a, d, e; Westrop, Reference Westrop1986, pl. 8, fig. 15), Ptychaspis cf. P. miniscaensis (e.g., Westrop, Reference Westrop1986, pl. 7, figs. 1–4), P. striata (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 35, figs. 2b, d, f; Westrop, Reference Westrop1986, pl. 8, figs. 1, 2), and P. tuberosa (e.g., Westrop, Reference Westrop1986, pl. 8, fig. 14) in the type of cranidial sculpture. All four of these species lack tubercles; external surfaces and internal molds of P. striata and Ptychaspis cf. P. miniscaensis have coarse, striate ridges over the frontal area, glabella, and fixed cheeks, whereas internal molds of both P. minscaensis and P. tuberosa are mostly smooth. In addition, P. tuberosa (e.g., Westrop, Reference Westrop1986, pl. 8, fig. 1), P. striata (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 35, figs. 2b, d, f), P. miniscaensis (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, fig. 36, fig. 1a), and Ptychaspis cf. P. miniscaensis (e.g., Westrop, Reference Westrop1986, pl. 7, figs. 1, 4) have smaller, more anteriorly positioned palpebral lobes (centered opposite or just in front of S2 rather than opposite L2) than P. occulta. Ptychaspis granulosa (e.g., Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 35, figs. 1a, e) has a granulose surface but lacks striate ridges, but it has smaller and more anteriorly positioned palpebral lobes (opposite S2) than P. occulta.
Ptychaspis sp. 1
Figure 16
Occurrence
Uppermost Honey Creek and basal Fort Sill formations, section BM, Bally Mountain, Kiowa County, Oklahoma, collections BM 79.4T, BM 80.7, and BM 82.1, Monocheilus reginae fauna.
Remarks
Ptychaspis cranidia from section BM are incomplete and cannot be identified to the species level. However, they record what is likely the oldest occurrence of the genus, at the top of the Honey Creek Formation.
Ptychaspis spp.
Figure 17
- Reference Longacre1970
Ptychaspis bullasa; Longacre, p. 44, pl. 2, figs. 4, 5.
Occurrence
Uppermost Morgan Creek Member (figured specimens collected within a meter of the top), Wilberns Formation, central Texas (see caption for Fig. 17 for detailed locality information).
Remarks
Longacre (Reference Longacre1970) identified two cranidia from the uppermost Morgan Creek Member as Ptychaspis bullasa. Not only are they different from the types of P. bullasa from Idaho, but they are also clearly distinct from cranidia from lower in the Morgan Creek that we assign to P. occulta n. sp. However, the two cranidia differ considerably in size, and contrasts in the anatomy make it difficult to be sure that they record a single species. The smaller of them (Fig. 17.4, 17.5) has large, closely spaced, irregular tubercles confined to the fixigenae between the posterior border furrow and palpebral ridge. The glabella is smooth, and the long frontal area carries several terrace ridge. The sculpture of small cranidia of P. bullasa (Fig. 10.4–10.12) consists of more-numerous, subcircular tubercles that extend over the glabella. Small P. bullasa are also characterized by a shorter frontal area and a narrower fixigena that includes a much shorter (tr.) posterolateral projection (e.g., compare Fig. 10.9, 10.10 with Fig. 17.4).
The larger of the cranidia (Fig. 17.1–17.3) has sculpture of rounded tubercles that are more widely spaced than on cranidia of P. occulta (Fig. 15) and has fewer terrace ridges on the frontal area. The palpebral lobes are broken off but are almost certainly smaller and more anteriorly positioned than in P. occulta, and the portion of the glabella in front of S2 is relatively shorter. Finally, the anterior branches of the facial sutures of this cranidium are not as strongly convergent as in P. occulta so that the anterior cranidial margin in anterior view is more transversely oriented. Compared with similarly sized specimens of P. bullasa (Figs. 9.1–9.6, 11.1–11.3), tuberculate sculpture extends farther back over the fixigenae, reaching the posterior border furrow, and includes a row of conspicuous tubercles along the posterior border. Further comparisons are hindered by preservation, but palpebral lobes seem to have been in an anterior position, as in P. bullasa.
Genus Wilbernia Walcott, Reference Walcott1924
Type species
Ptychoparia pero (Walcott, Reference Walcott1890) from the Wilberns Formation of central Texas (by original designation).
Wilbernia cf. W. diademata (Hall, Reference Hall1863)
Figure 18
- cf. Reference Hall1863
Conocephalites diadematus Hall, p. 167, pl. 7, fig. 36, pl. 8, fig. 21 (only).
- cf. Reference Bell and Ellinwood1962
Wilbernia diademata; Bell and Ellinwood, p. 365, pl. 34, figs. 9, 10 (synonymy to date).
- cf. Reference Longacre1970
Wilbernia diademata; Longacre, p. 32 (synonymy to date).
- cf. Reference Stitt1971
Wilbernia diademata; Stitt, p. 33, pl. 3, fig. 2.
- cf. Reference Westrop1986
Wilbernia diademata; Westrop, p. 44, pl. 13, figs. 13, 14.

Figure 18. Wilbernia cf. W. diademata (Hall, Reference Hall1863) from the Honey Creek Formation, Bally Mountain, Kiowa County. Both cranidia from collection BM 79.4T. (1) OU 238382, dorsal view. (2–4) OU 238170: (2) dorsal view; (3) lateral view; (4) anterior view. Scale bars = 2 mm.
Occurrence
Honey Creek Formation, section BM, Bally Mountain, Kiowa County, Oklahoma, collection BM 79.4T, Monocheilus reginae fauna.
Remarks
Two nearly complete cranidia possess large palpebral lobes that are equal to about 36% of glabellar length with firmly impressed palpebral furrows. The frontal area consists of a weakly inflated preglabellar field that is slightly shorter than the weakly convex anterior border. They are most like W. diademata (Hall, Reference Hall1863; e.g., Nelson, Reference Nelson1951, pl. 109, figs. 8, 11) but have a shorter border and, consequently, a somewhat shorter frontal area. Cranidia identified as W. diademata by Bell and Ellinwood (Reference Bell and Ellinwood1962, pl. 54, fig. 9) have shorter palpebral lobes than Wilbernia cf. W. diademata. Small cranidia attributed to the poorly known W. halli Resser, Reference Resser1937 by both Bell et al. (Reference Bell, Feniak and Kurtz1952, pl. 32, fig. 5a) and Bell and Ellinwood (Reference Bell and Ellinwood1962, fig. 14) approach Wilbernia cf. W. diademata in frontal area morphology but also have relatively short palpebral lobes. Wilbernia explanata (Whitfield, Reference Whitfield1880) has a longer frontal area (Bell et al., Reference Bell, Feniak and Kurtz1952, pl. 34, figs. 4b–d; Westrop, Reference Westrop1986, pl. 12, figs. 1, 3), whereas W. pero (Walcott, Reference Walcott1890) possesses a preglabellar field that is very short (e.g., Bell and Ellinwood, Reference Bell and Ellinwood1962, pl. 54, fig. 19).
Acknowledgments
This paper is revised and expanded from a master's thesis by S.R.B. that was supervised by S.R.W. Order of authorship is alphabetical. We thank R. Burkhalter for invaluable help in the field and the lab. S.J. Wernette and J.P. Westrop participated in field work at Bally Mountain. C. Labandeira and D. Levin arranged loans of type material from the National Museum of Natural History. J.F. Taylor and an anonymous reviewer contributed many helpful comments that improved the final draft of the manuscript. The project was funded by NSF grants EAR 9973065 and 0308685.
Declaration of competing interests
The authors declare none.